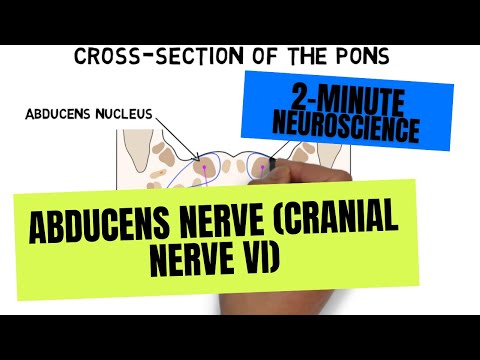
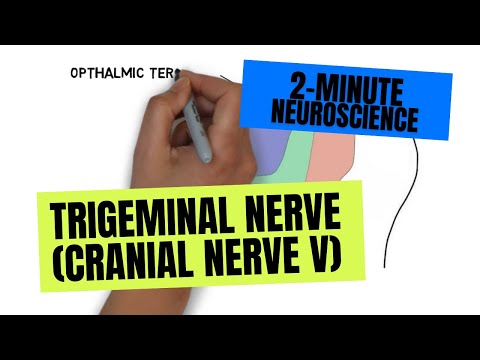
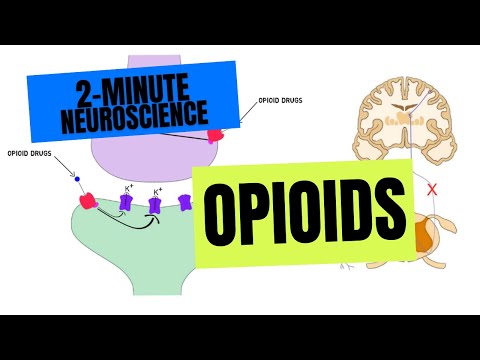
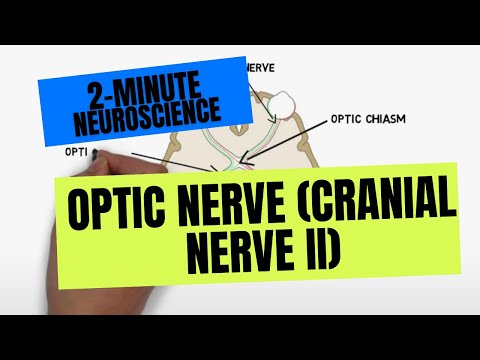
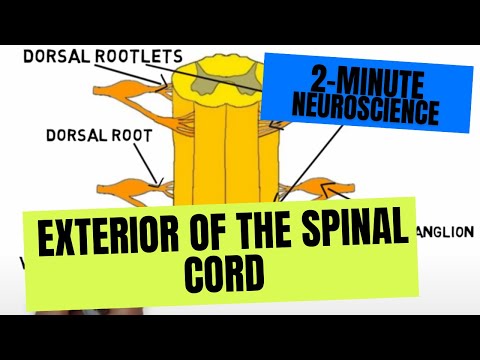
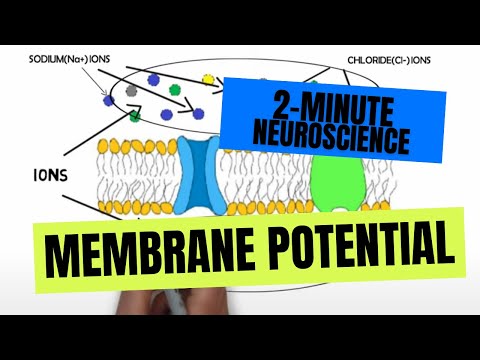
- Subjects: Neurosciences
- |
- Contributor:
- Neuroscientifically Challenged
- neurotransmitter
- synaptic vesicle
- ion channel
- synapsin
- cell membrane
This video is adapted from: https://youtu.be/FIIK2Gp5WzU
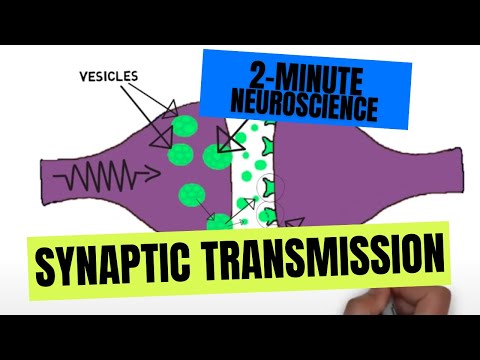
Neurotransmitters are stored in the axon terminals of a neuron in small sac-like structures called synaptic vesicles. When an action potential travels down the neuron and reaches the axon terminal, it causes depolarization of the neuron. This change in membrane potential causes voltage-gated ion channels, which are ion channels that open in response to changes in membrane potential, to open and allow calcium to enter the cell. Calcium seems to be involved with mobilizing vesicles to prepare them for neurotransmitter release. One way this occurs is through an interaction between calcium and a protein called synapsin, which attaches vesicles to the cytoskeleton of the cell. Calcium activates an enzyme that causes synapsin to separate from the vesicles, mobilizing them for release.
After mobilization, a family of proteins called SNARE proteins are involved with getting the vesicle ready to fuse with the cell membrane of the neuron. Synaptobrevin (also called VAMP) is a SNARE protein found in the membrane of vesicles, while syntaxin and SNAP-25 are two SNARE proteins found in the cell membrane. These three proteins are thought to form a complex, which helps to bring vesicles in contact with the cell membrane, allowing the two membranes to fuse together. This process is thought to be facilitated by another protein called munc18. The role of munc18 in vesicle fusion is not completely understood, but it seems to bind to syntaxin and be necessary for fusion to occur. Another protein found in synaptic vesicles known as synaptotagmin is thought to act as a calcium sensor, which aims to promote vesicle fusion only when calcium levels in the cell are high. When the vesicle fuses with the cell membrane, it empties its contents into the synaptic cleft. After neurotransmitter release, the SNARE complex is disassembled with the help of proteins called NSF and SNAP, and the vesicle is recycled so it can be used again. [1][2]
- Südhof TC. A molecular machine for neurotransmitter release: synaptotagmin and beyond. Nat Med. 2013 Oct;19(10):1227-31. doi: 10.1038/nm.3338.
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009 Jan 23;323(5913):474-7. doi: 10.1126/science.1161748.