Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sabrin R. M. Ibrahim | -- | 5038 | 2023-02-20 08:46:29 | | | |
| 2 | Camila Xu | Meta information modification | 5038 | 2023-02-20 09:41:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khayat, M.T.; Alharbi, M.; Ghazawi, K.F.; Mohamed, G.A.; Ibrahim, S.R.M. Ferula sinkiangensis (Chou-AWei, Chinese Ferula). Encyclopedia. Available online: https://encyclopedia.pub/entry/41412 (accessed on 07 February 2026).
Khayat MT, Alharbi M, Ghazawi KF, Mohamed GA, Ibrahim SRM. Ferula sinkiangensis (Chou-AWei, Chinese Ferula). Encyclopedia. Available at: https://encyclopedia.pub/entry/41412. Accessed February 07, 2026.
Khayat, Maan T., Majed Alharbi, Kholoud F. Ghazawi, Gamal A. Mohamed, Sabrin R. M. Ibrahim. "Ferula sinkiangensis (Chou-AWei, Chinese Ferula)" Encyclopedia, https://encyclopedia.pub/entry/41412 (accessed February 07, 2026).
Khayat, M.T., Alharbi, M., Ghazawi, K.F., Mohamed, G.A., & Ibrahim, S.R.M. (2023, February 20). Ferula sinkiangensis (Chou-AWei, Chinese Ferula). In Encyclopedia. https://encyclopedia.pub/entry/41412
Khayat, Maan T., et al. "Ferula sinkiangensis (Chou-AWei, Chinese Ferula)." Encyclopedia. Web. 20 February, 2023.
Copy Citation
F. sinkiangensis K.M. Shen (Chou-AWei, Chinese Ferula, (Xinjiang’awei)) is an important member of this genus. F. sinkiangensis is a perennial plant endemic in Xinjiang Uygur Autonomous Region, China.
Ferula sinkiangensis
Apiaceae
traditional uses
sesquiterpene coumarins
1. Traditional Uses of F. sinkiangensis
F. sinkiangensis is mainly found in Xinjiang, which is a region with various minorities. The plant has been described in the Chinese Pharmacopoeia and in “Medica of the Tang Dynasty” for a long time as a folk medicine for gastric disorders and rheumatoid arthritis [1].
The resin of the roots or stems of F. sinkiangensis (Ferulae Resina, “AWei” in China) is a folk medicine recorded in Chinese Pharmacopoeia [2]. It is often utilized for reducing the symptoms of lumps, indigestion, joint pain, wound infection, baldness, bronchitis, and ovarian cysts by Uygur people in Xinjiang [2][3][4][5]. It also is efficient in killing intestinal worms, as well as treating parasite-caused malnutrition, abdominal and stomachic swelling pain, diarrhea, malaria, abdominal mass, cold, and measles. However, its powerful odor has restricted its usage [6][7]. The resin is indicated in treating animal stagnation and food accumulation, concertions and conglomerations because of blood stasis, abdominal syndrome, and abdominal pain due to accumulation of worms, also for malaria and dysentery [8] at doses 1–1.5 g in the form of pills or oral powder. The resin should not be decocted with H2O [8]. Its use is prohibited for patients with spleen and stomach weakness, as well as for pregnant women [6][8].
2. Phytoconstituents of F. sinkiangensis
The phytochemical investigation of different parts of F. sinkiangensis, including gum resin, aerial parts, seed, roots, oleo-gum-resin, and resins led to the separation of different classes of phytoconstituents by the mean of diverse chromatographic tools. Their structure characterization was performed using various spectral techniques (e.g., UV, NMR, MS), as well as CD, [α]D, and Xray analyses and chemical means. A total of 194 metabolites were separated from F. sinkiangensis (excluding polysaccharides). These metabolites were highlighted below.
2.1. Sesquiterpene Coumarins
The reported studies showed that sesquiterpene coumarins represent the major metabolites produced by this plant. They represent 60 metabolites (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6) of the total compounds reported from this plant that were mainly separated from gum resin, seed, roots, and resins. It was noted that no sesquiterpene coumarin derivatives were reported from the aerial parts.
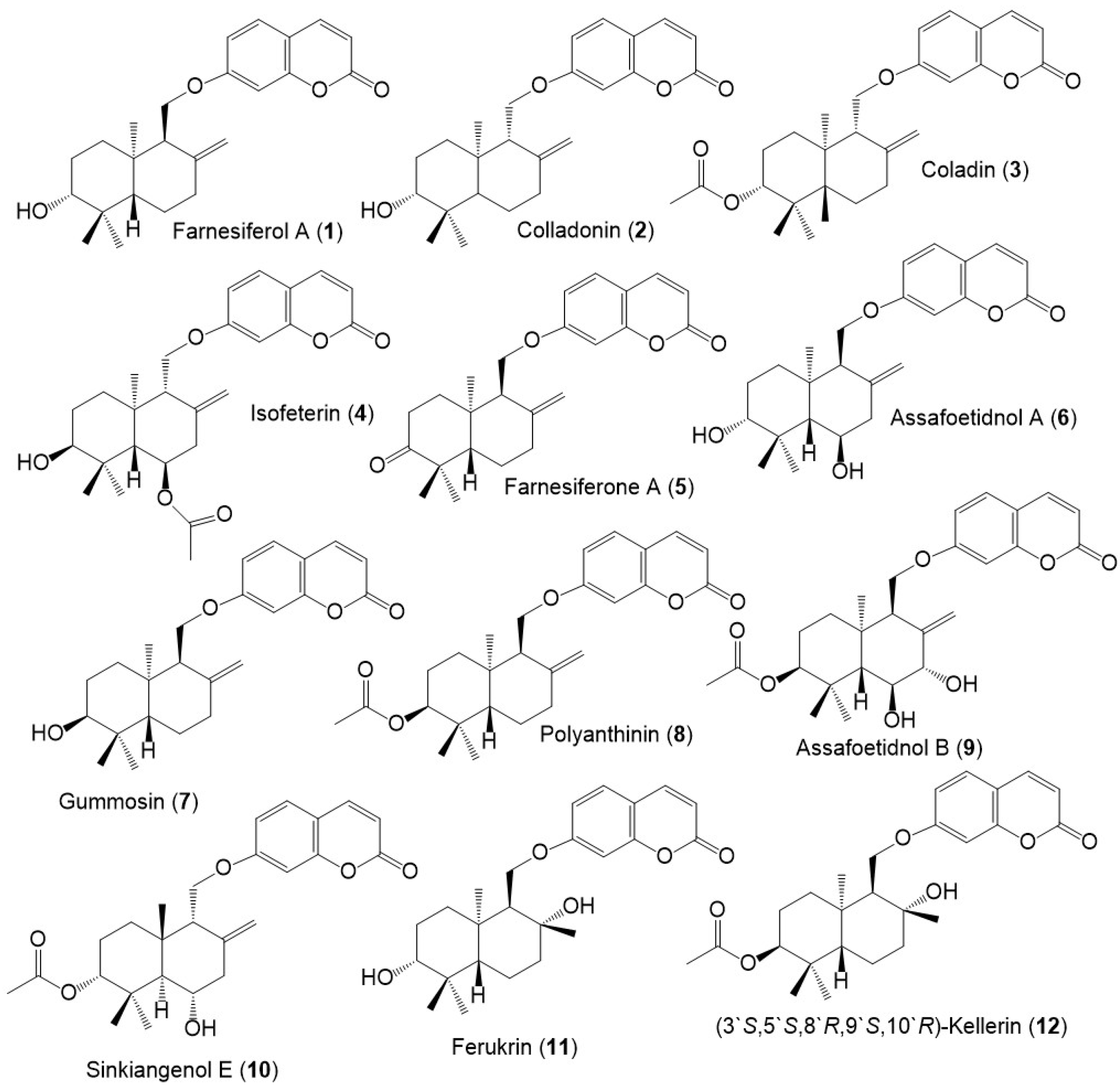
Figure 1. Structures of sesquiterpene coumarins (1–12) reported from F. sinkiangensis.
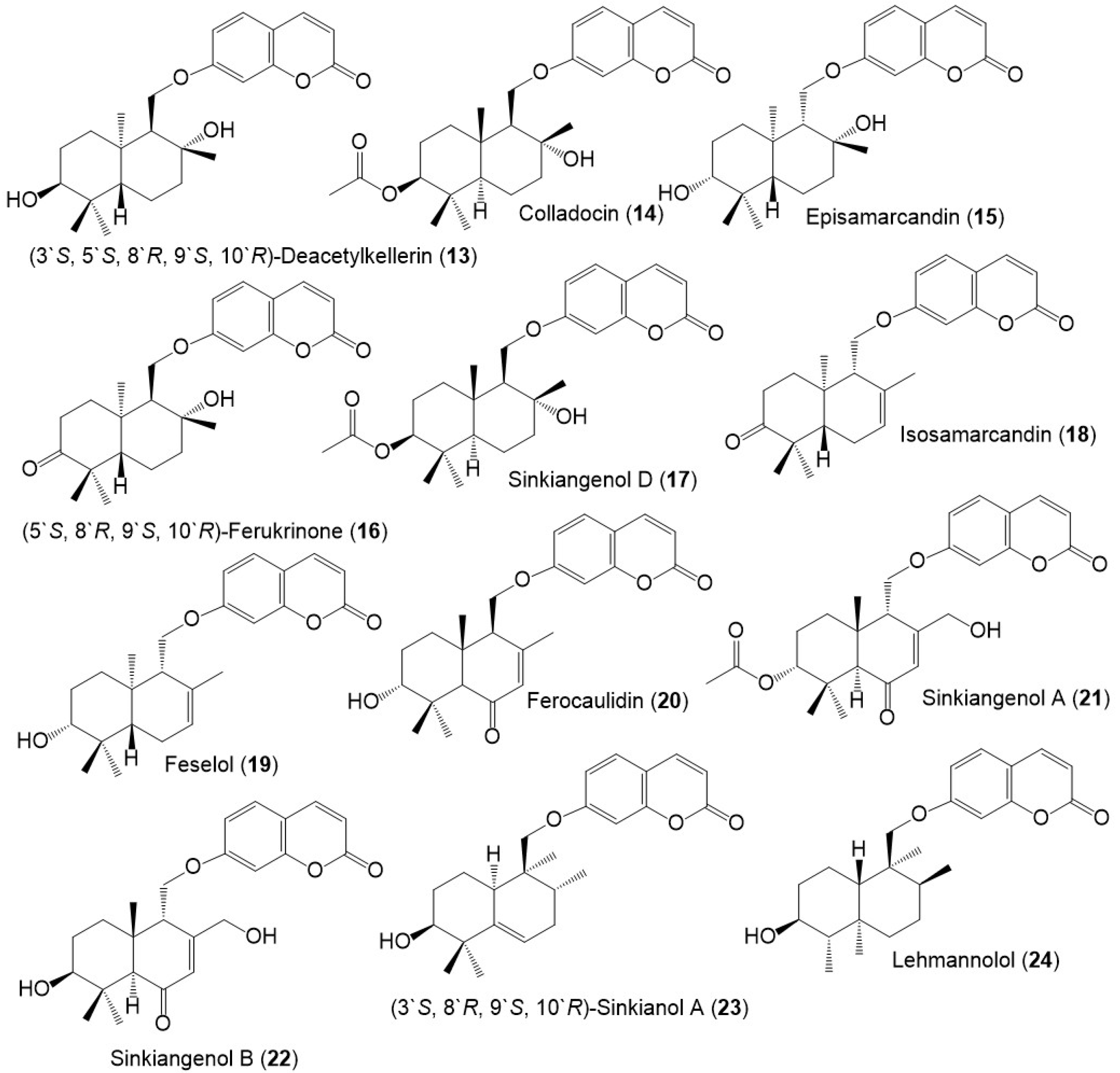
Figure 2. Structures of sesquiterpene coumarins (13–24) reported from F. sinkiangensis.
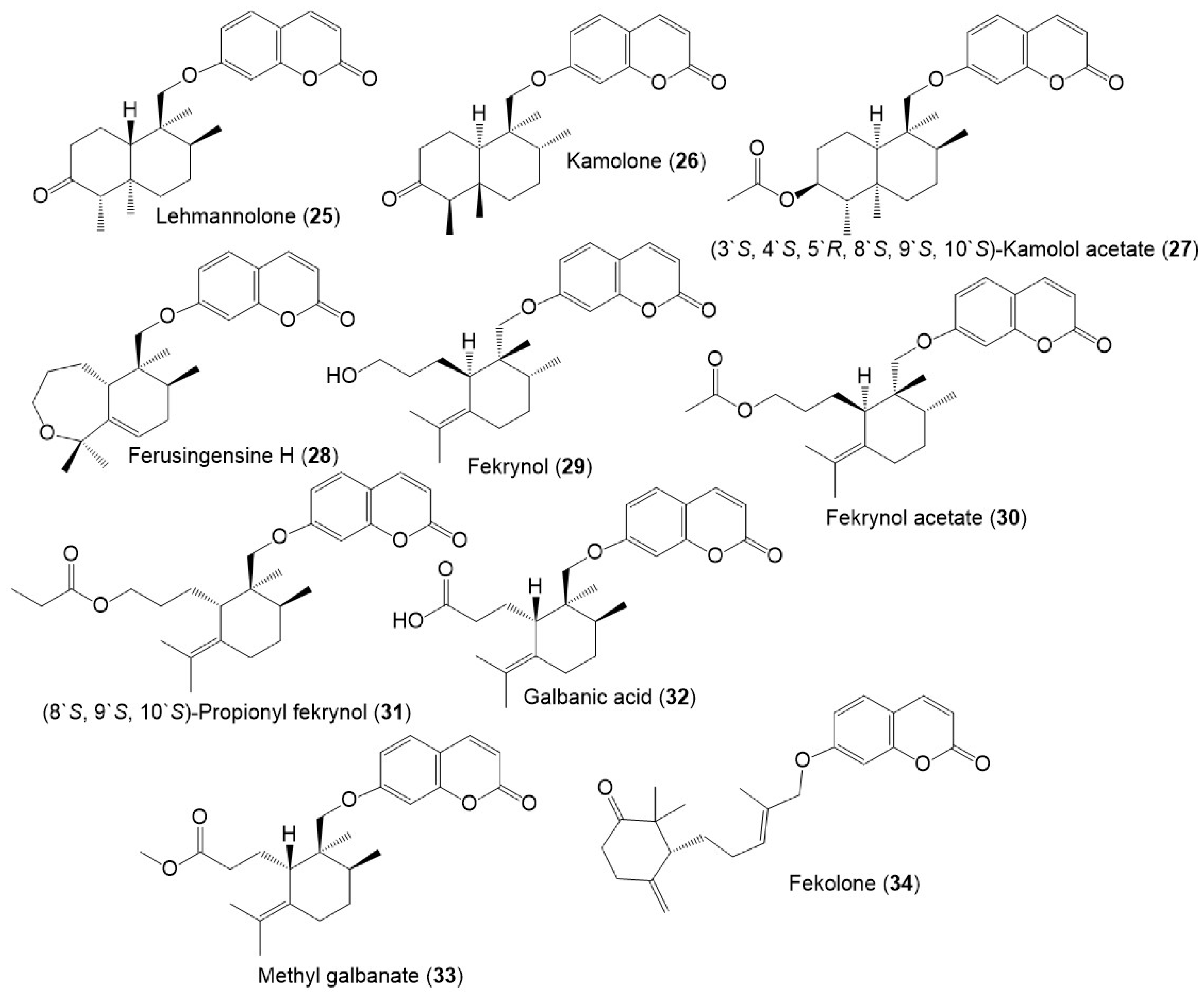
Figure 3. Structures of sesquiterpene coumarins (25–34) reported from F. sinkiangensis.
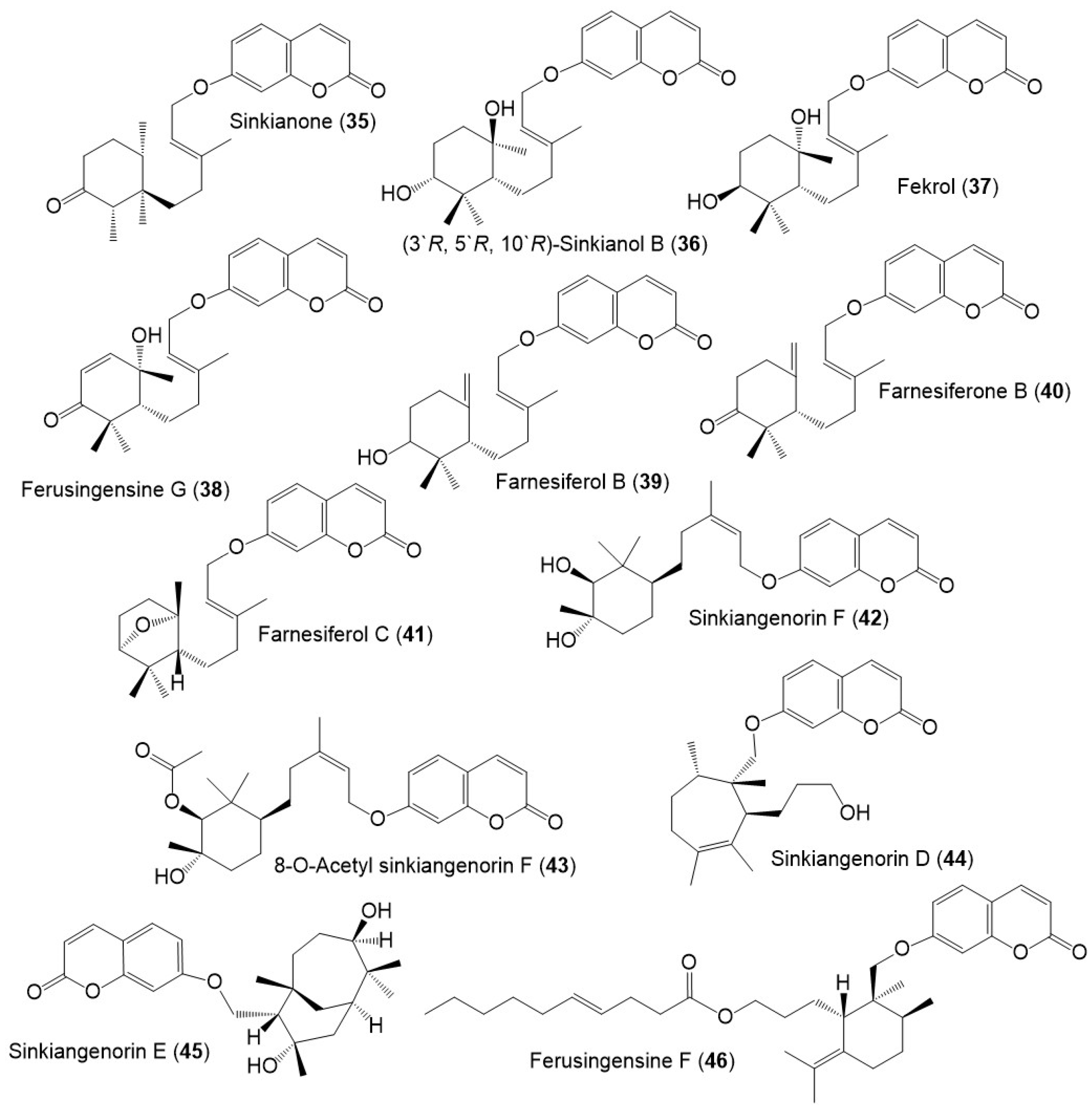
Figure 4. Structures of sesquiterpene coumarins (35–46) reported from F. sinkiangensis.
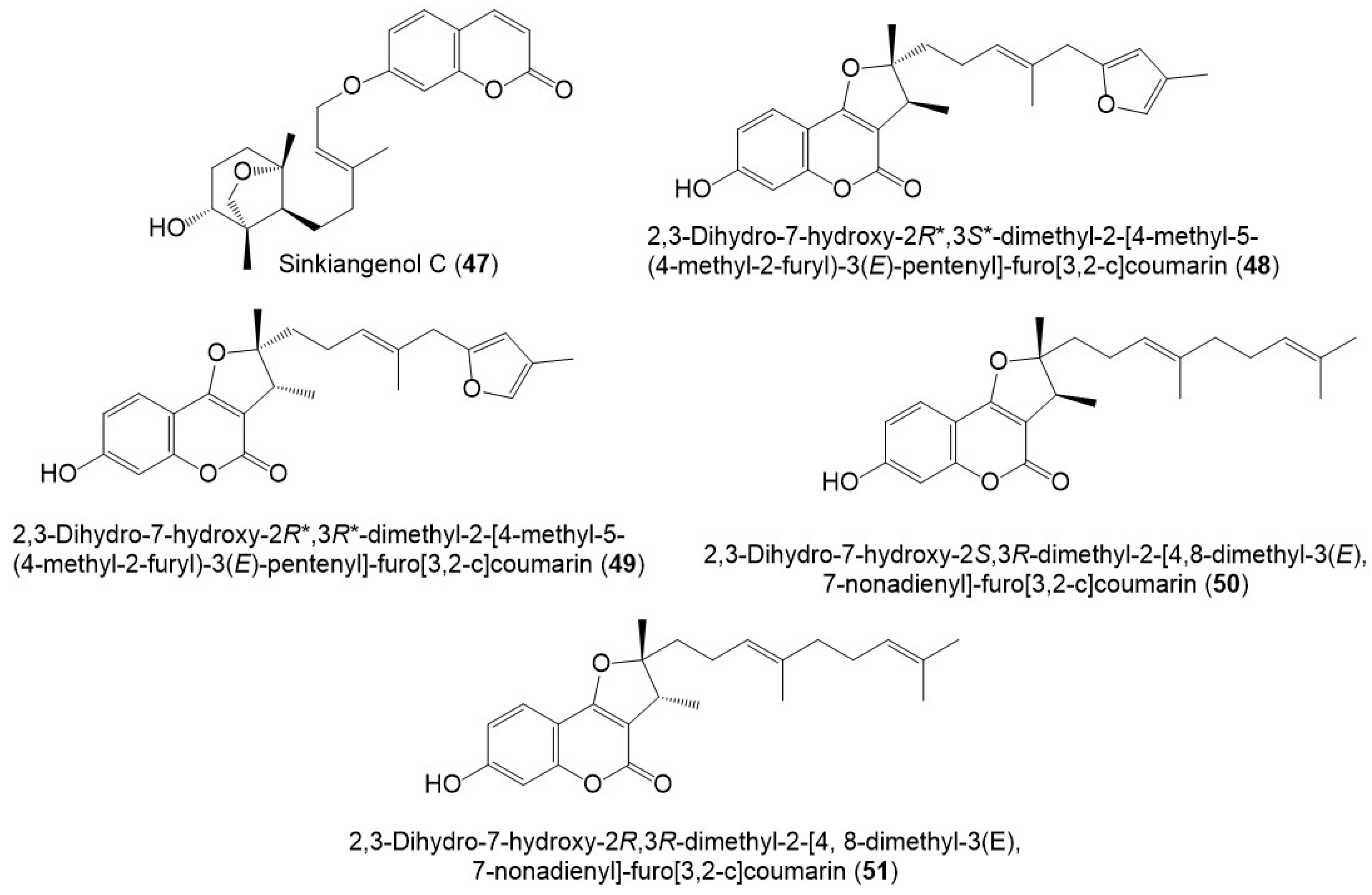
Figure 5. Structures of sesquiterpene coumarins (47–51) reported from F. sinkiangensis.
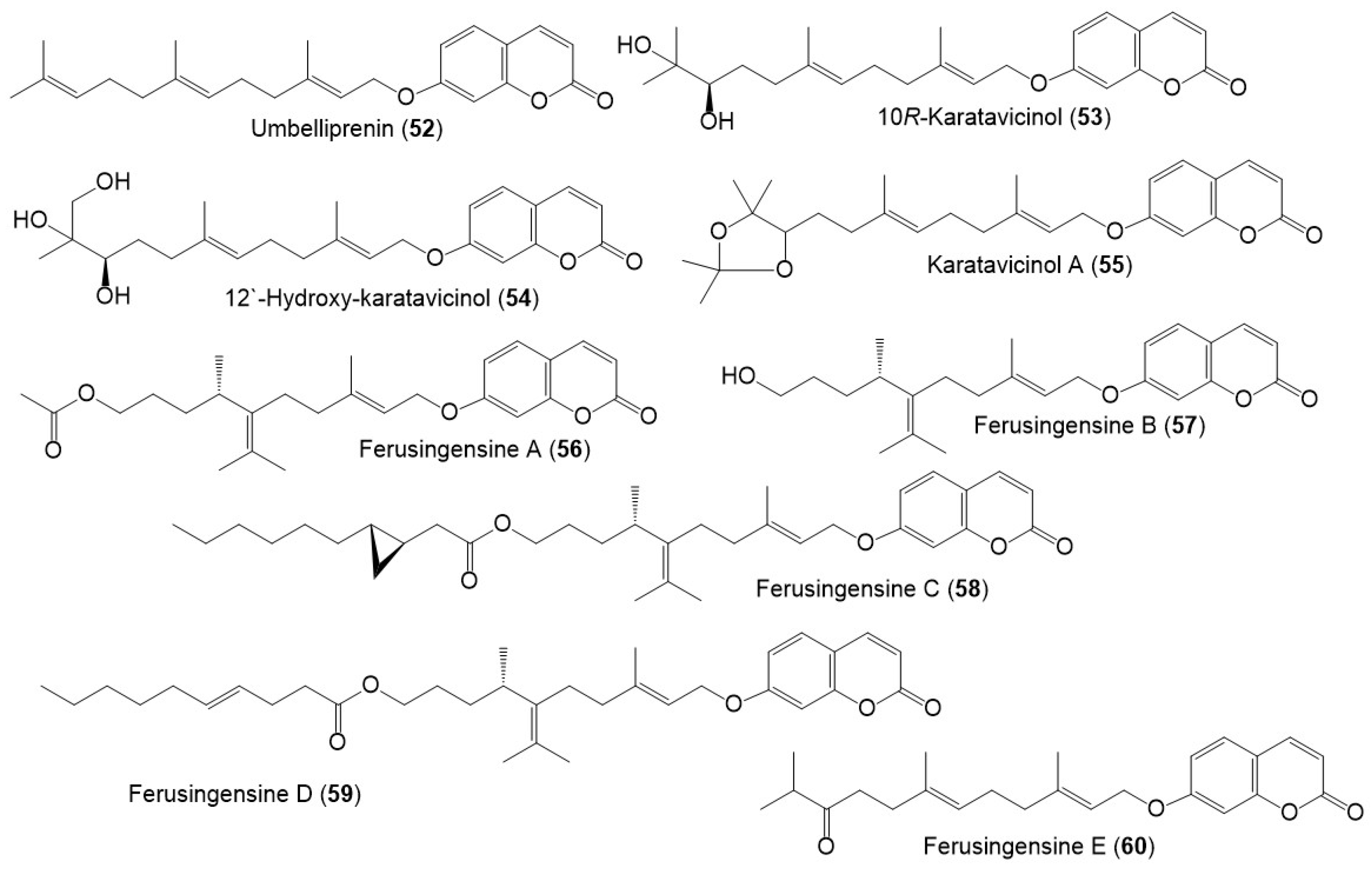
Figure 6. Structures of sesquiterpene coumarins (52–60) reported from F. sinkiangensis.
These compounds featured linked coumarin and sesquiterpene units through C–O–C ether bridge. These metabolites include monocyclic, bicyclic, or chain derivatives. Also, they could be accountable for many of the stated bioactivities of this plant.
Their separation was performed by different chromatographic techniques, including SiO2/RP-18/Sephadex LH-20/HPLC, whereas the identification and configuration were accomplished using assort spectral tools (e.g., UV, NMR, MS), as well as CD, [α]D and Xray analyses. They had UV absorbance at 320–330 nm and a common fragment at m/z 185 in MS [9].
Among these metabolites, karatavicinol A (55), a new sesquiterpene coumarin along with 32, 39, 41, 52, and 53 were purified from the antiulcer resin CHCl3 extract [10] (Figure 3).
2.2. Sesquiterpene Chromones and Monoterpene Coumarins
Sesquiterpene chromones possessing a 24-carbon skeleton consisting of sesquiterpene and chromone were reported from F. sinkiangensis roots. In 2022, Wang et al., reported the purification of new derivatives, (±)-ferulasin from the roots MeOH extract that was established by diverse spectral, Xray, and ECD analyses. Ferulasins (61 and 62) showed an unusual oxygen-bearing macrocyclic skeleton with a tri-oxaspiro unit and a new backbone in which the C-10` and C-11` of the sesquiterpene side chain form an oxygen-including 13-membered ring with C-2 of chromone (Figure 7). It was obtained as an enantiomeric mixture that was chiral-separated by HPLC to (+)-61 and (-)-62 with 2R/3R/10`R and 2S/3S/10`S configurations, respectively based on Xray and ECD data [11]. Wang et al., assumed the biosynthesis of 61 and 62 from ferulaeone A (71). The reduction of the 71-side chain C-3 produces 6-membered ring containing oxygen (Scheme 1). After that, the side chain C2`-C3` is oxidized and yields a 6-membered ring having oxygen with C-2 of chromone. After the six-membered ring fission, C10`-C11` bond reacts with ozone. Lastly, attack of oxygen-atoms to C2-C3 the double bond from below or above the plane with removal of H2O a molecule to afford 61 and 62 (Scheme 1).
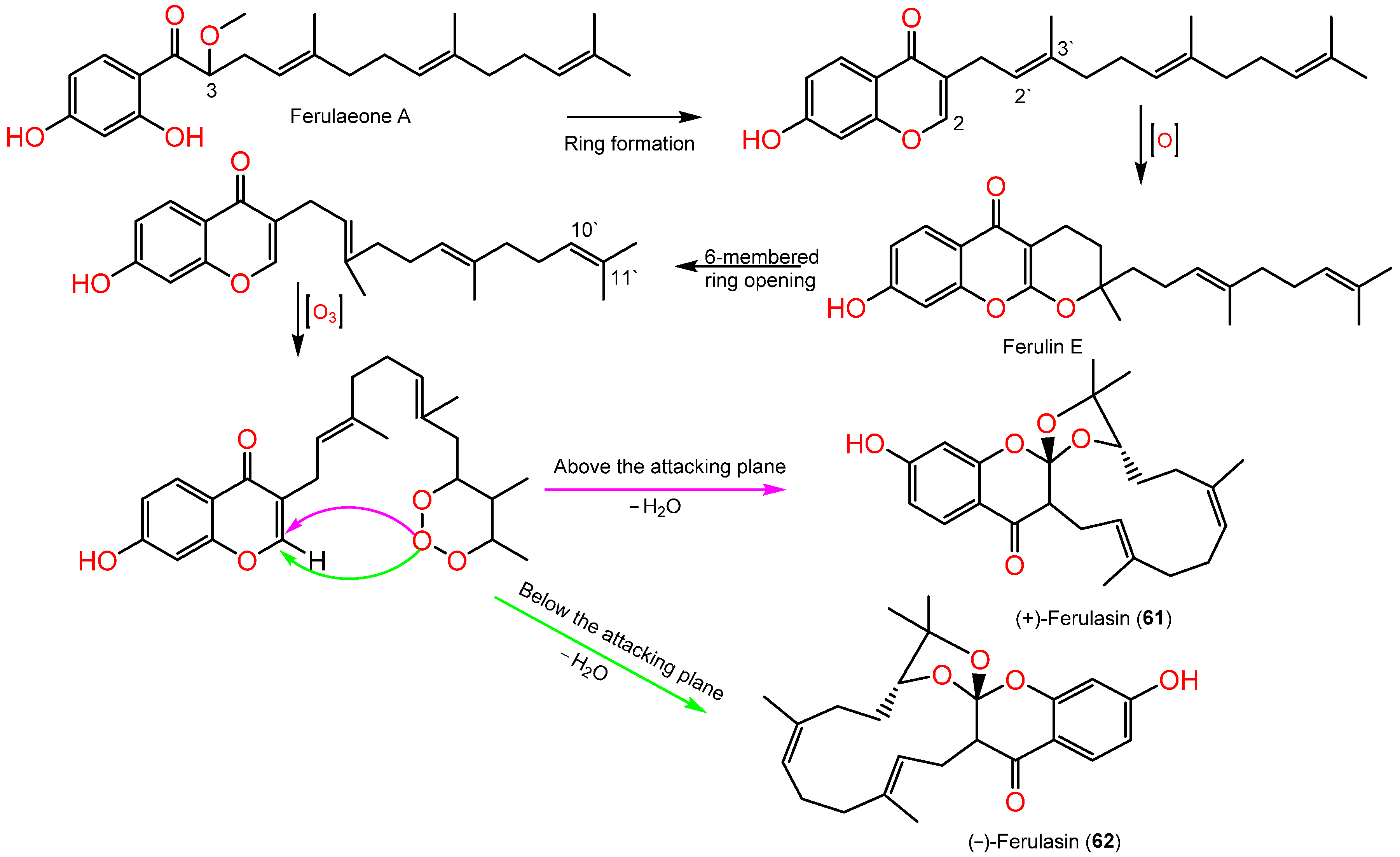
Scheme 1. Biosynthetic pathway of (±)-ferulasin [11].
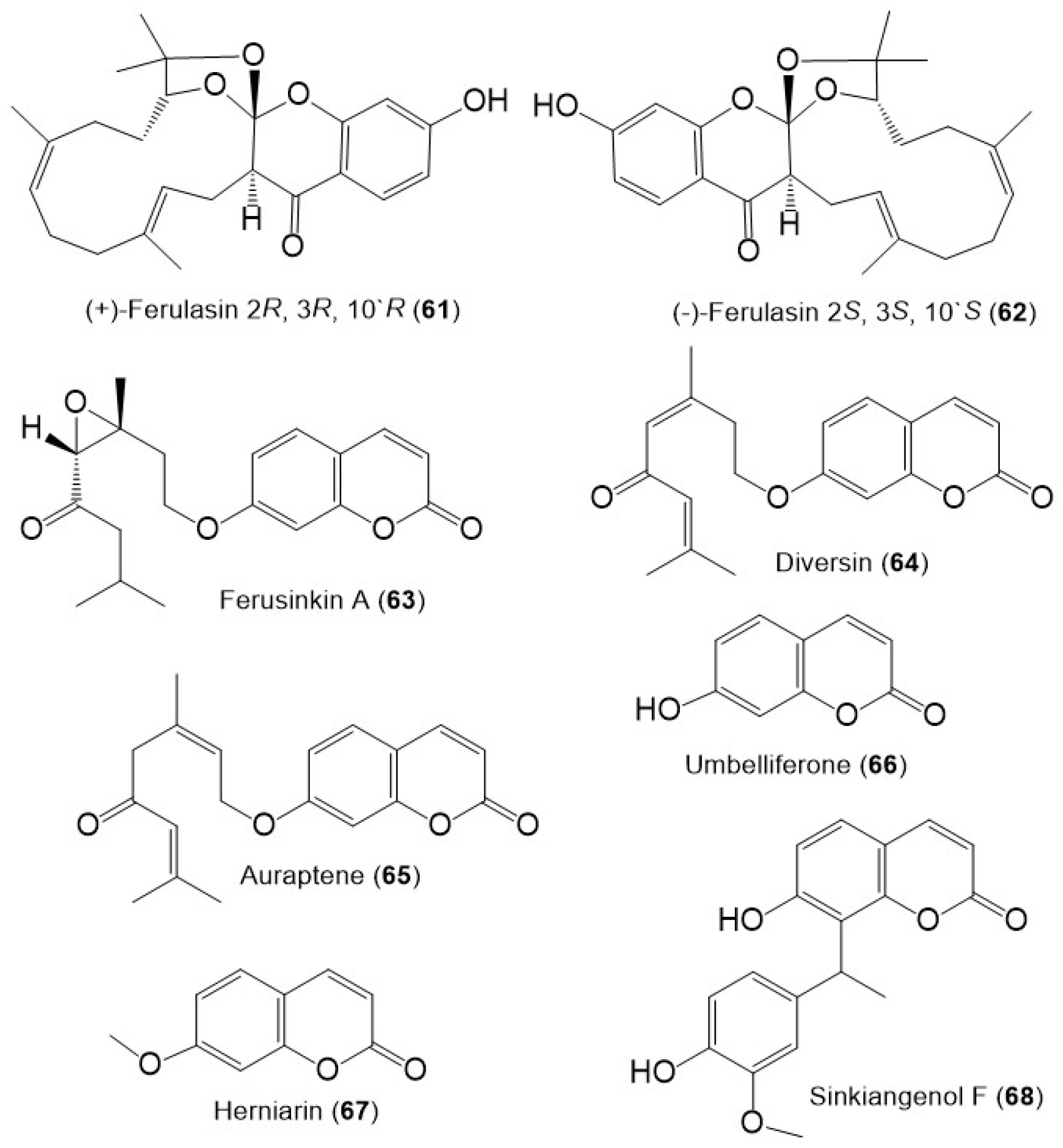
Figure 7. Structures of sesquiterpene chromones (61 and 62), monoterpene coumarins (63–65), and coumarins (66–68) from F. sinkiangensis.
Additionally, ferusinkin A (63), a rare new monoterpene coumarin and known analogs 64 and 65 were purified and identified by Liu et al. in 2020 from the aerial parts MeOH extract (Figure 9) [12].
2.3. Coumarins
The coumarins; 66 and 67 were purified from the F. sinkiangensis aerial parts and characterized based on spectral and physical data [12]. Additionally, sinkiangenol F (68) a new coumarin was purified from the resin EtOH extract. This compound is rare coumarin derivative having a coumarin unit connected to phenylethane moiety by C–C linkage at C-8 [9].
2.4. Sesquiterpene Phenylpropanoids
Sesquiterpene phenylpropanoid derivatives were commonly separated from Ferula genus [13]. In 2018, Wang et al., stated the separation of new sesquiterpene phenylpropanoids, sinkiangenones A (69), and B (70), along with 72 from the resin 95% EtOH extract, which were specified utilizing spectral and CD analyses (Figure 8) [14].
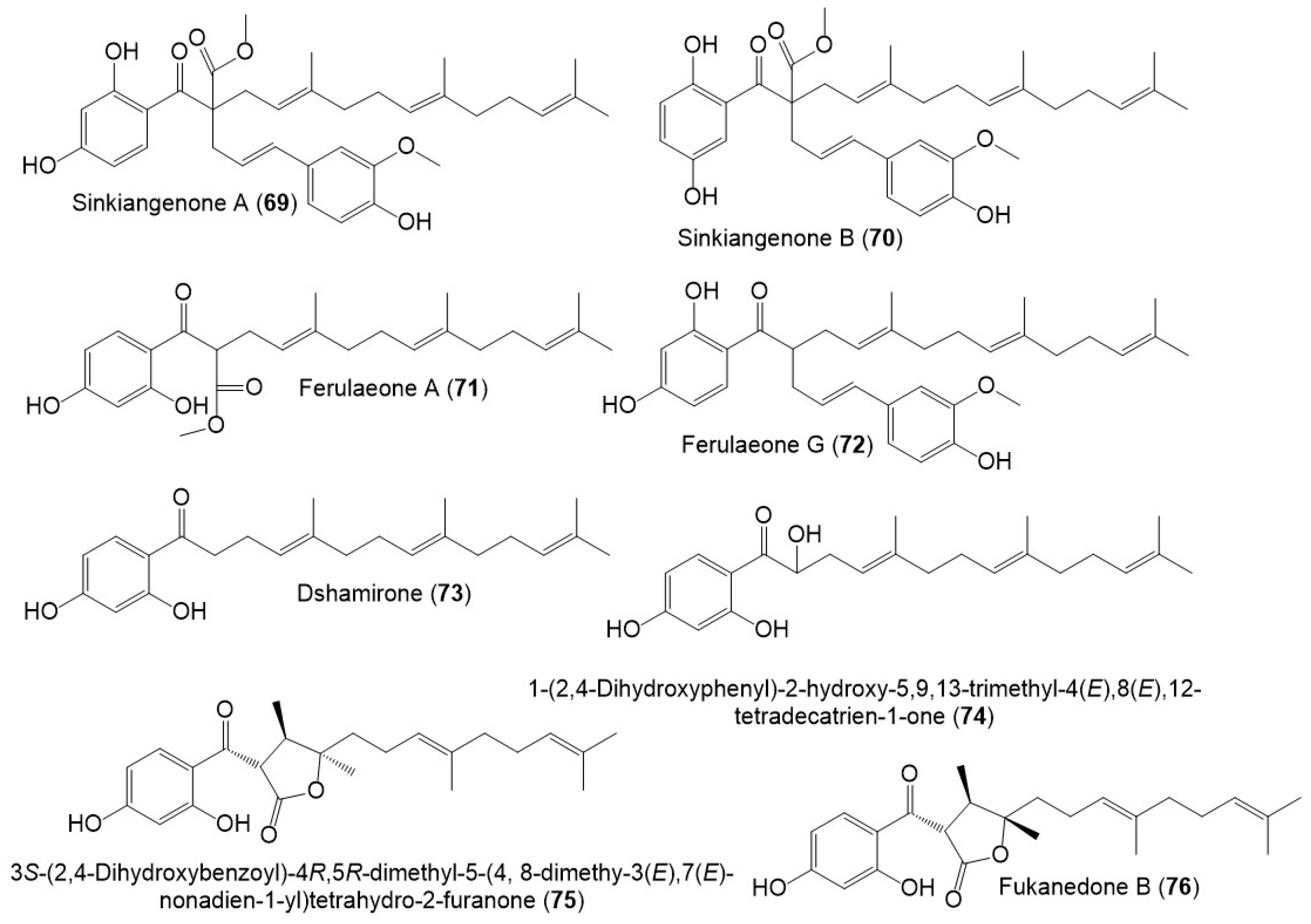
Figure 8. Structures of sesquiterpene phenylpropanoids (69–76) from F. sinkiangensis.
Also, Wang et al., separated sesquiterpene phenylpropanoid derivatives (71 and 73–76) from F. sinkiangensis roots MeOH extract [15]. It is noteworthy that these derivatives were previously reported from other Ferula species: 71 from F. ferulioides roots; 73 from the underground parts of F. heuffelii and roots of F. ferulioides, F. fukanensis, F. dubjanskyi, and F. mongolica; 74 from F. ferulioides roots; 75 from F. heuffelii, F. fukanensis, and F. ferulioides roots, and 76 from F. heuffelii and F. ferulioides roots [13][15], suggesting the close chemotaxonomic relation of F. sinkiangensis and the other Ferula species, therefore, they could share the biosynthetic pathways of these metabolites [15].
2.5. Lignans
Lignans, norlignans, and sesquilignans were reported mainly from F. sinkiangensis seeds and resins. (±)-Ferulasinkins A–D (82–89) (Figure 9), new norlignans characterized by tetrahydrofuran rings were separated as racemic mixtures from the EtOAc fraction of the resins 95% EtOH extract by MCI gel CHP 20P/RP-18/YMC gel ODS-A-HG/SiO2/Sephadex LH-20/preparative TLC.
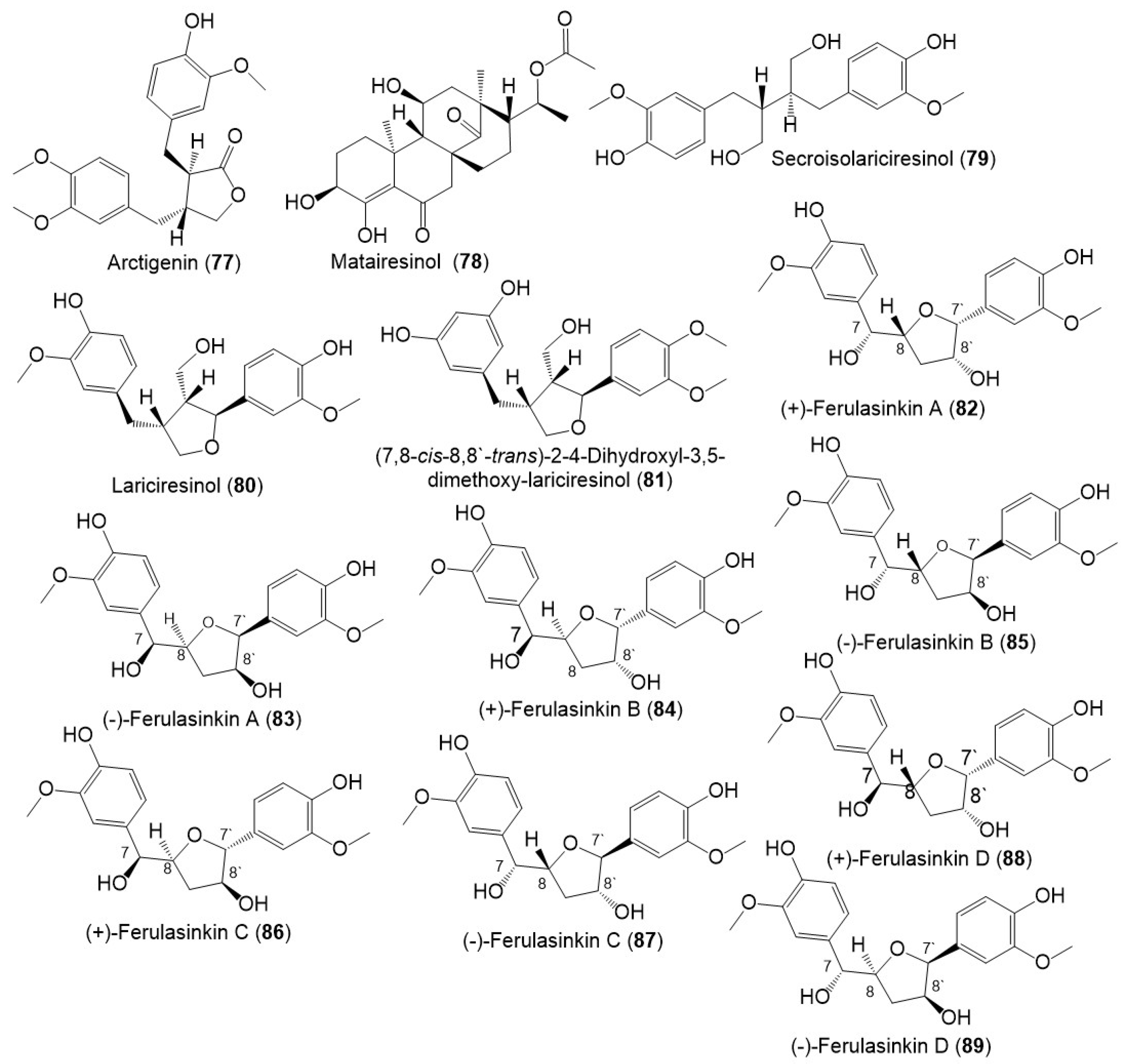
Figure 9. Structures of lignans (77–89) reported from F. sinkiangensis.
The chiral column HPLC separation afforded their (-)- and (+)-antipodes. Their structures and configurations were specified by spectral tools and computational methods [16].
Additionally, Li et al., purified new racemic sesquilignans; sinkianlignans (±)-A–D (90–97) characterized by a rare α-γ′, β-γ′, and γ-γ′ linkage pattern, and new lignans; sinkianlignans (±)-E–F (98–101) from the resin 95% EtOH extract using SiO2/RP-18/MCI gel CHP 20P/YMC gel ODS-A-HG/Sephadex LH-20 CC/preparative TLC and chiral HPLC and elucidated by spectral and computational tools (Figure 10 and Figure 11) [17].
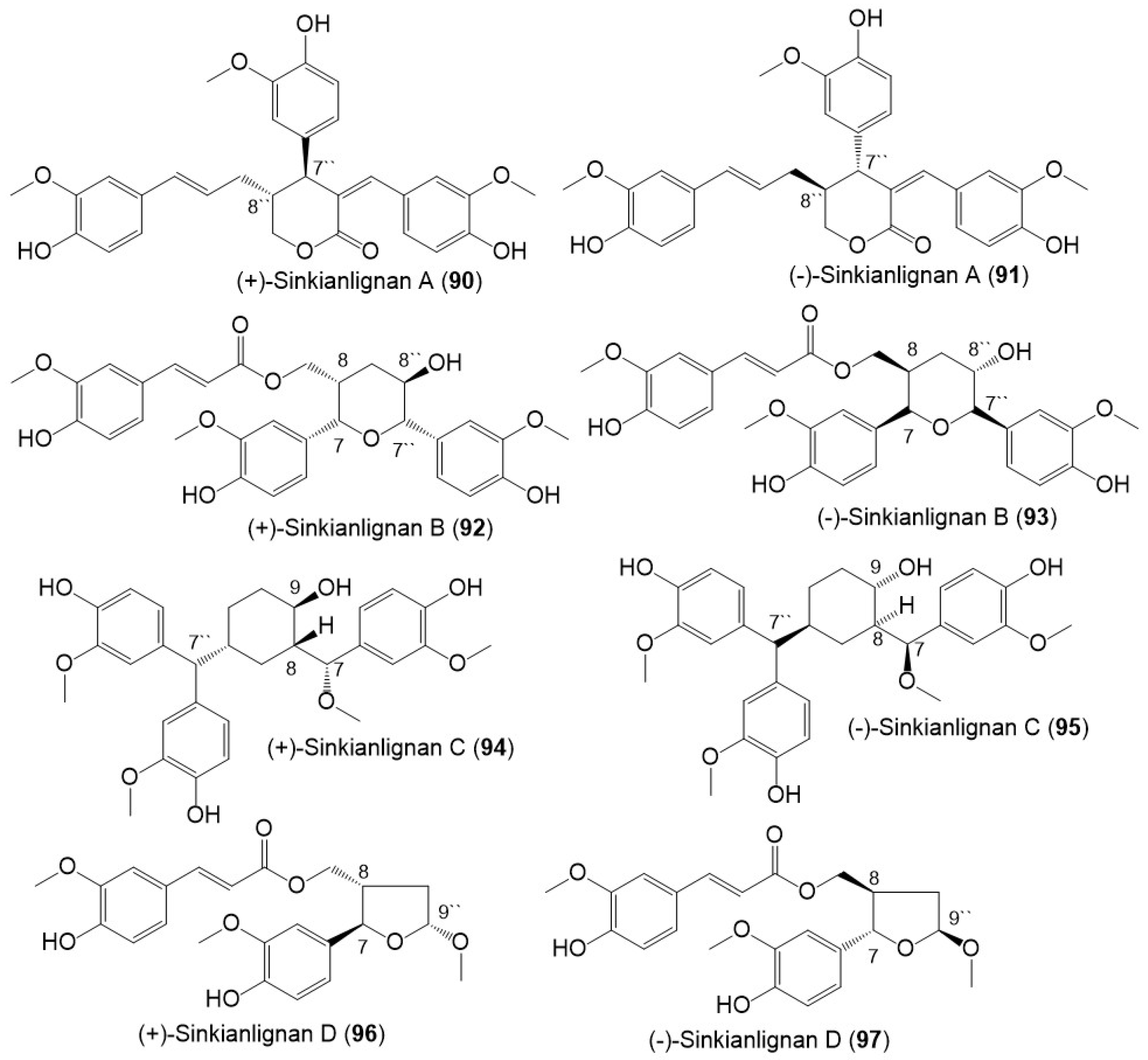
Figure 10. Structures of lignans (90–97) reported from F. sinkiangensis.
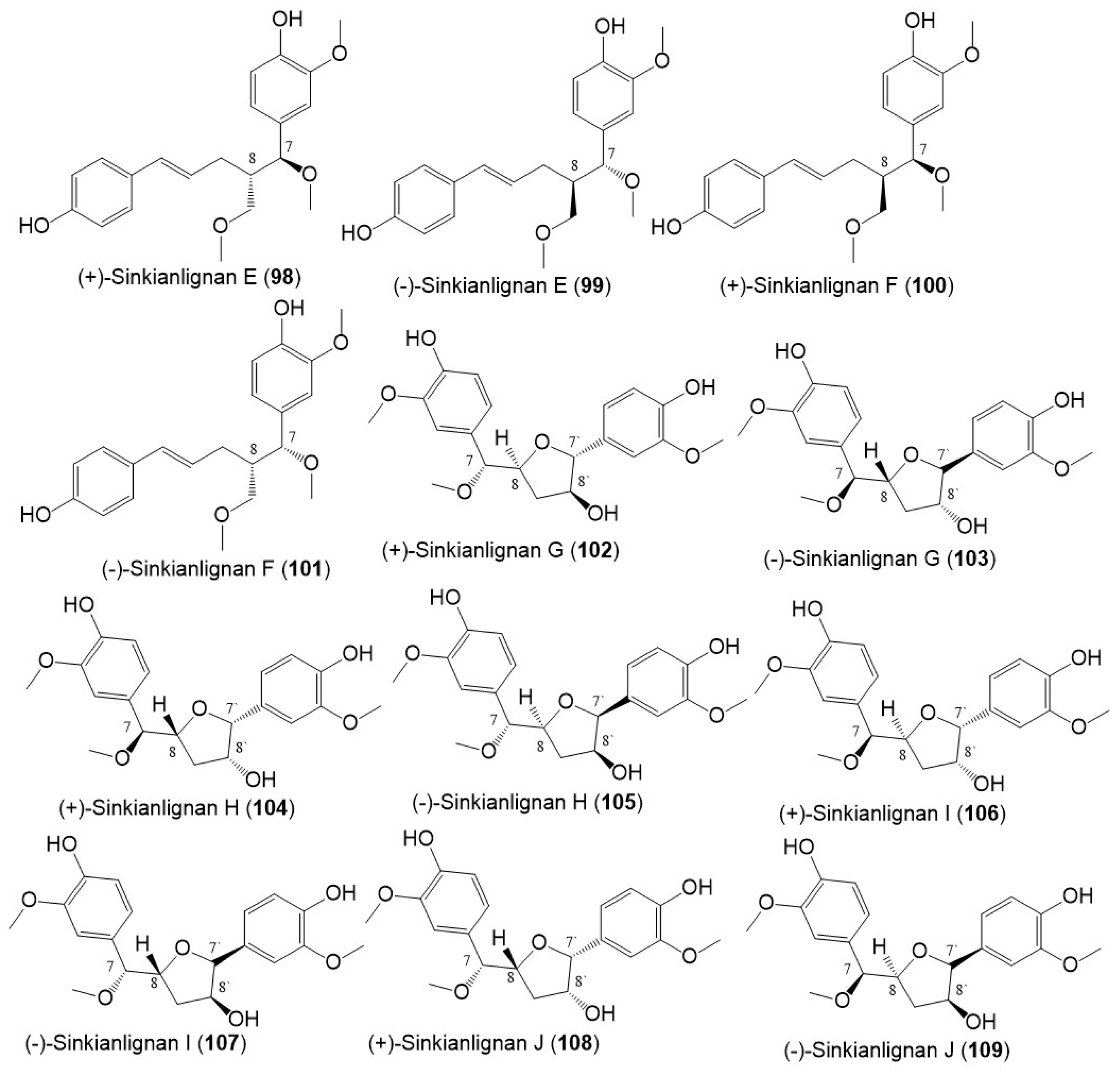
Figure 11. Structures of lignans (98–109) reported from F. sinkiangensis.
Sesquilignans are type of lignans that consist of 3 phenylpropanoid units. Compound 94 was assumed to be biosynthesized by the shikimate pathway (Scheme 2). First, phenylpropanoid is formed by a shikimic acid pathway that undergoes polymerization to produce intermediate A (aryltetralin lignan). In addition, intermediate C with a new six-membered ring skeleton is yielded from the intermediates A and B by the Diesel-Alder cycloaddition reaction. Moreover, C produces D by opening the ring at C1−C7. Subsequent oxidization and decarboxylation of D yields E. After a set of redox reactions, intermediate E gives 94 [17].
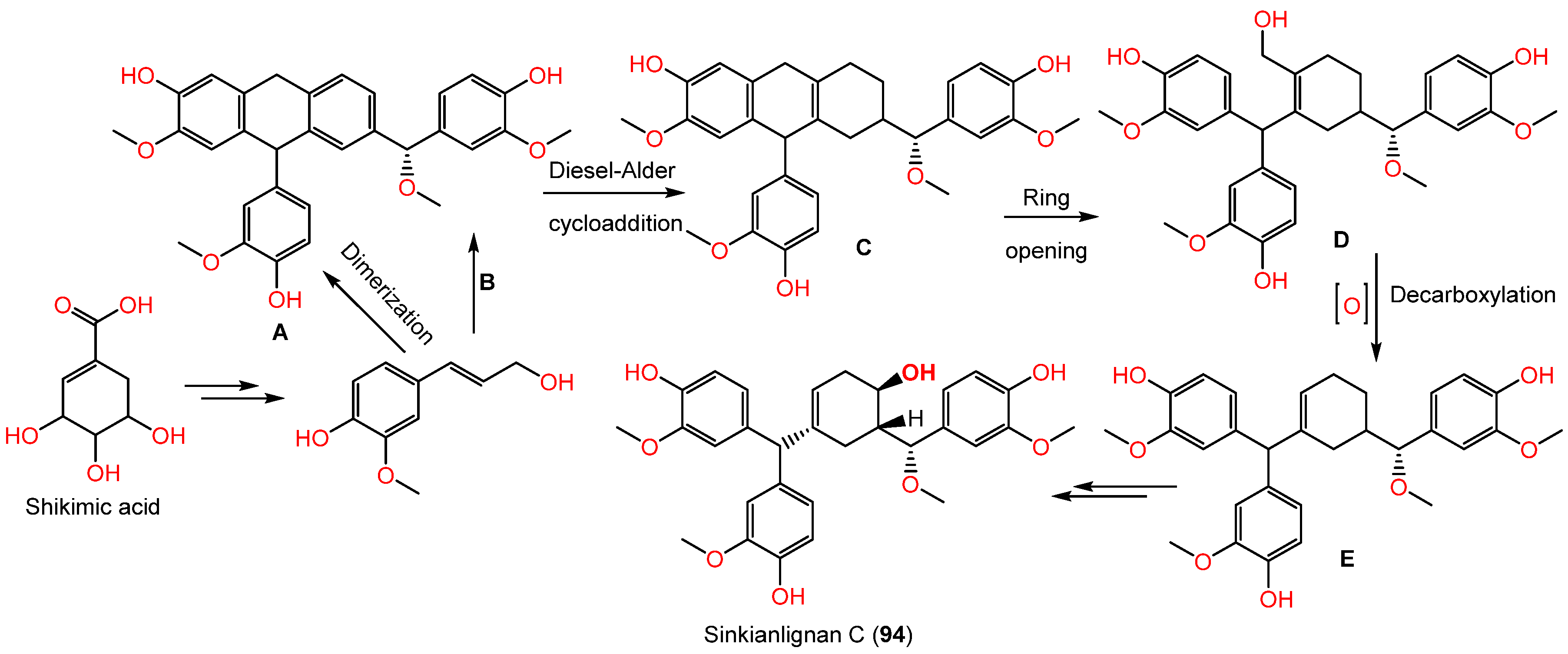
Scheme 2. Biosynthesis of sinkianlignan C (94) via shikimate pathway [17].
Sinkianlignans G–K (102–111) new norneolignans were purified from 95% resin EtOH extract utilizing SiO2/RP-18/MCI gel CHP 20P/Sephadex LH-20/preparative TLC (Figure 12). Compounds 102–111 were obtained as racemic mixtures that were separated by chiral HPLC and characterized by spectral and computational means [18].
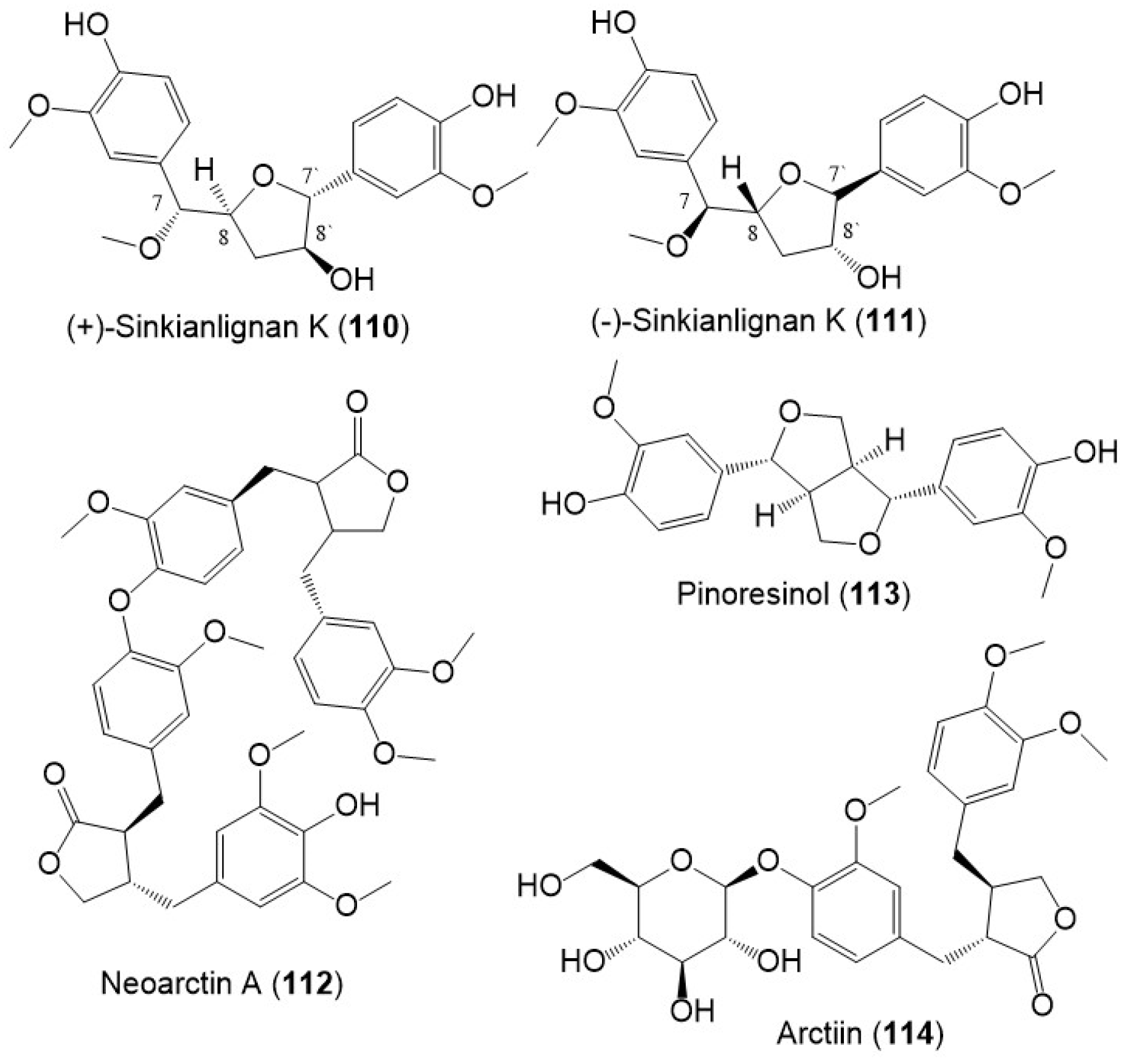
Figure 12. Structures of lignans (110–114) reported from F. sinkiangensis.
2.6. Sesquiterpenes and Monoterpenes
Besides, monoterpenes (e.g., carene (115), camphene (116), β-pinene (117), α- pinene (118), and p-cymene (119)) were encountered in the F. sinkiangensis oleo-gum resins` volatile oil (Figure 13) [19]. Further, Wang et al., purified a new sesquiterpenoid 125, along with 120, 122, and 124 from F. sinkiangensis roots. Compounds 124 and 125 were isomeric cyclic-endoperoxy-nerlildol sesquiterpene derivatives, whereas 120 and 122 were chain and guaiane-type sesquiterpenoid, respectively [11].
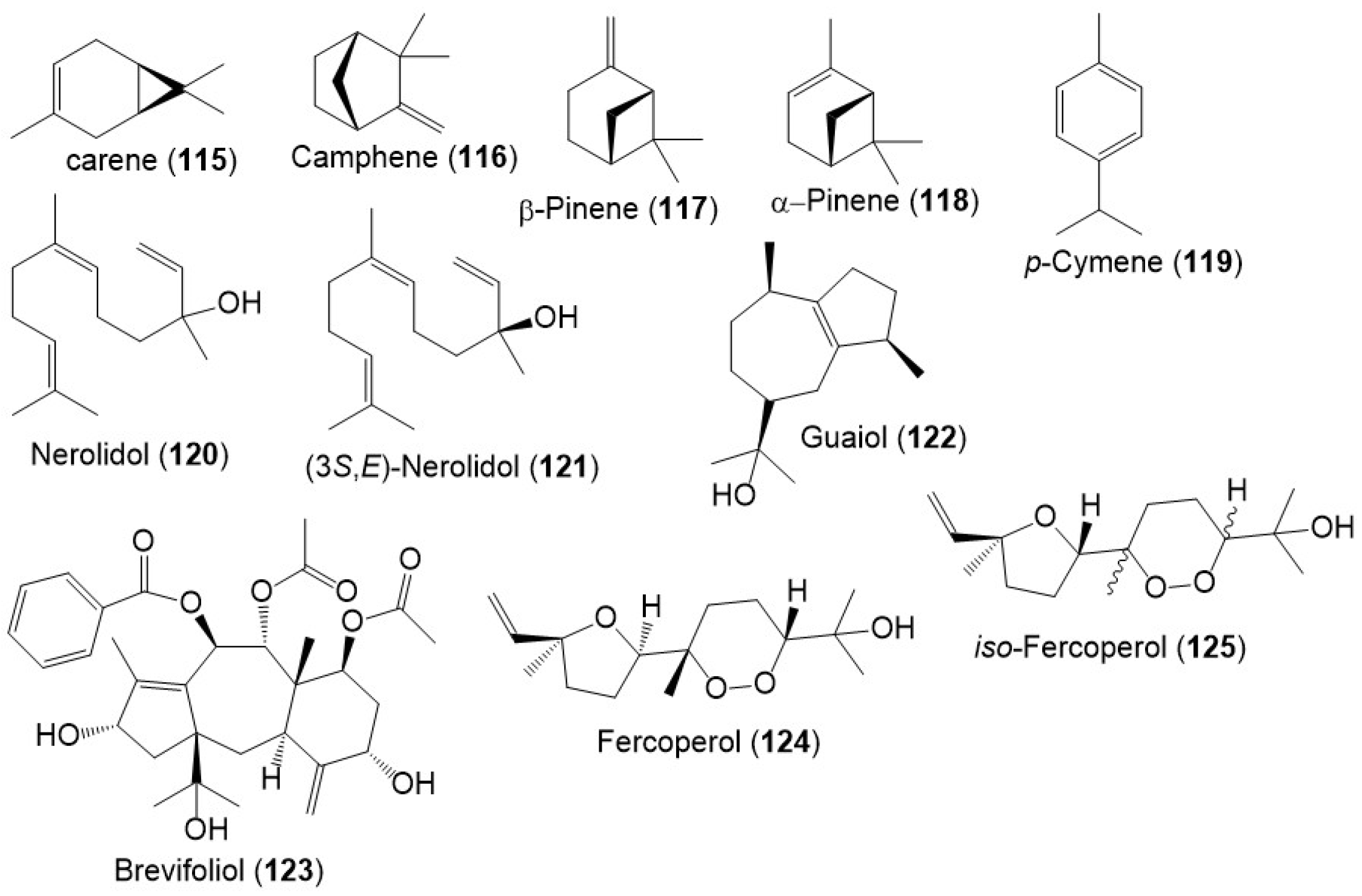
Figure 13. Structures of monoterpenes (115–119) and sesquiterpenes (120–125) reported from F. sinkiangensis.
2.7. Sulfanes
It was reported that polysulfides including disulfanes, trisulfanes, di-disulfanes, and thio-disulfanes are the predominant constituents of the F. sinkiangensis volatile oil oleo-gum resins. The oil content was 16.7% of which 64.1% were sulfur compounds. The disulfanes were the prime components: 126–130, 134, and 135 (Figure 14) [19]. Further, the GC-MS analysis of essential oil (3.8% yield) of F. sikiangensis seeds obtained from Xinjiang, China that was prepared by hydro-distillation method revealed the existence of 26 metabolites, comprising 99.001% of total oil [3].
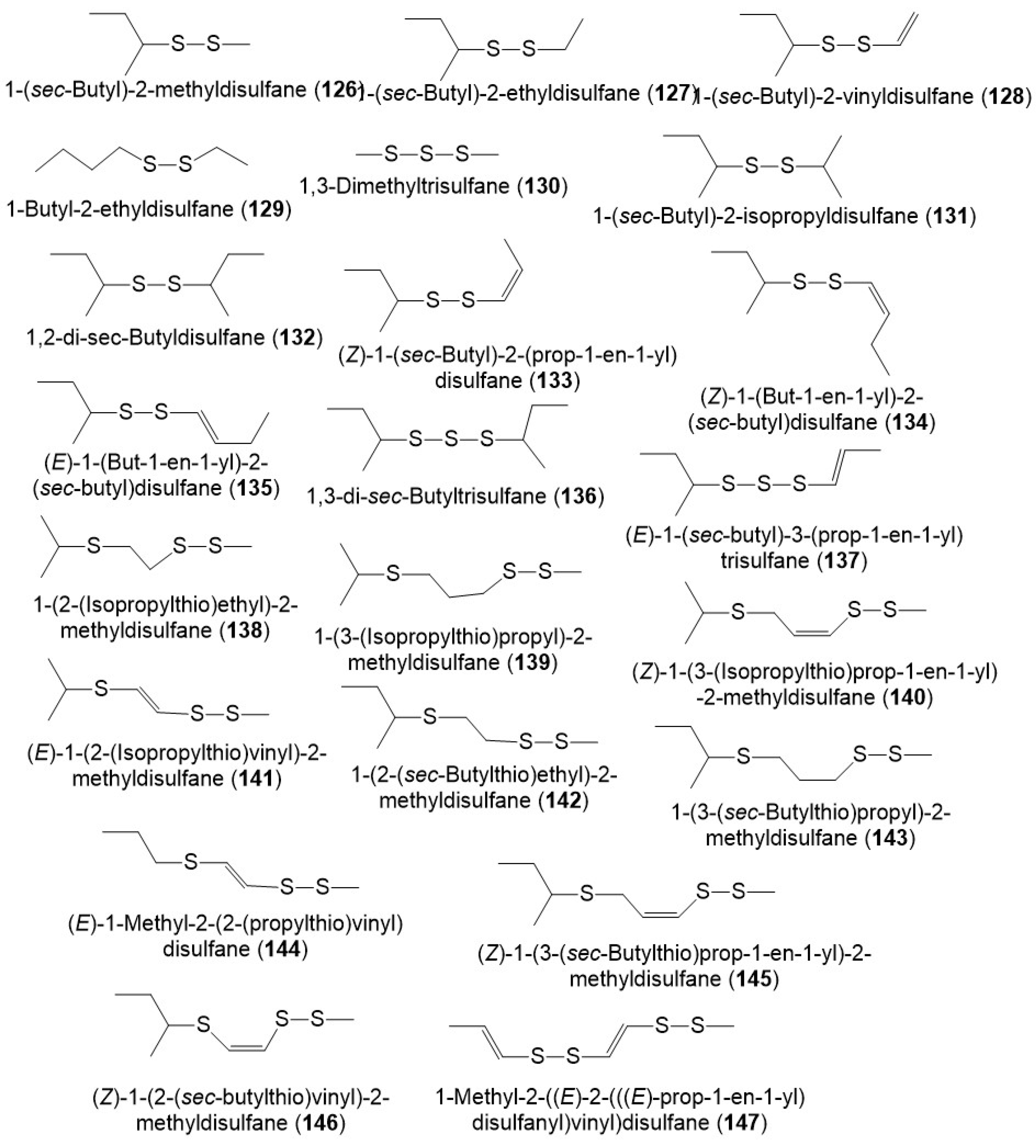
Figure 14. Structures of sulfanes (126–147) reported from F. sinkiangensis.
2.8. Sterols
Chromatography separation of F. sinkiangensis 95% EtOH seed extract using SiO2, Sephadex LH-20, and HPLC yielded new steroidal esters: sinkiangenrins A (148) and B (149) that were characterized by NMR and Xray analyses [20]. These compounds are related to oleagenin-cardenolide with different C-13/C-10/C-9/C-8 configuration, having 3S/8R/9S/10S/11S/13S/17R/18R and 8S/9S/10S/12R/13R/17R/18R-configurations, respectively (Figure 15).
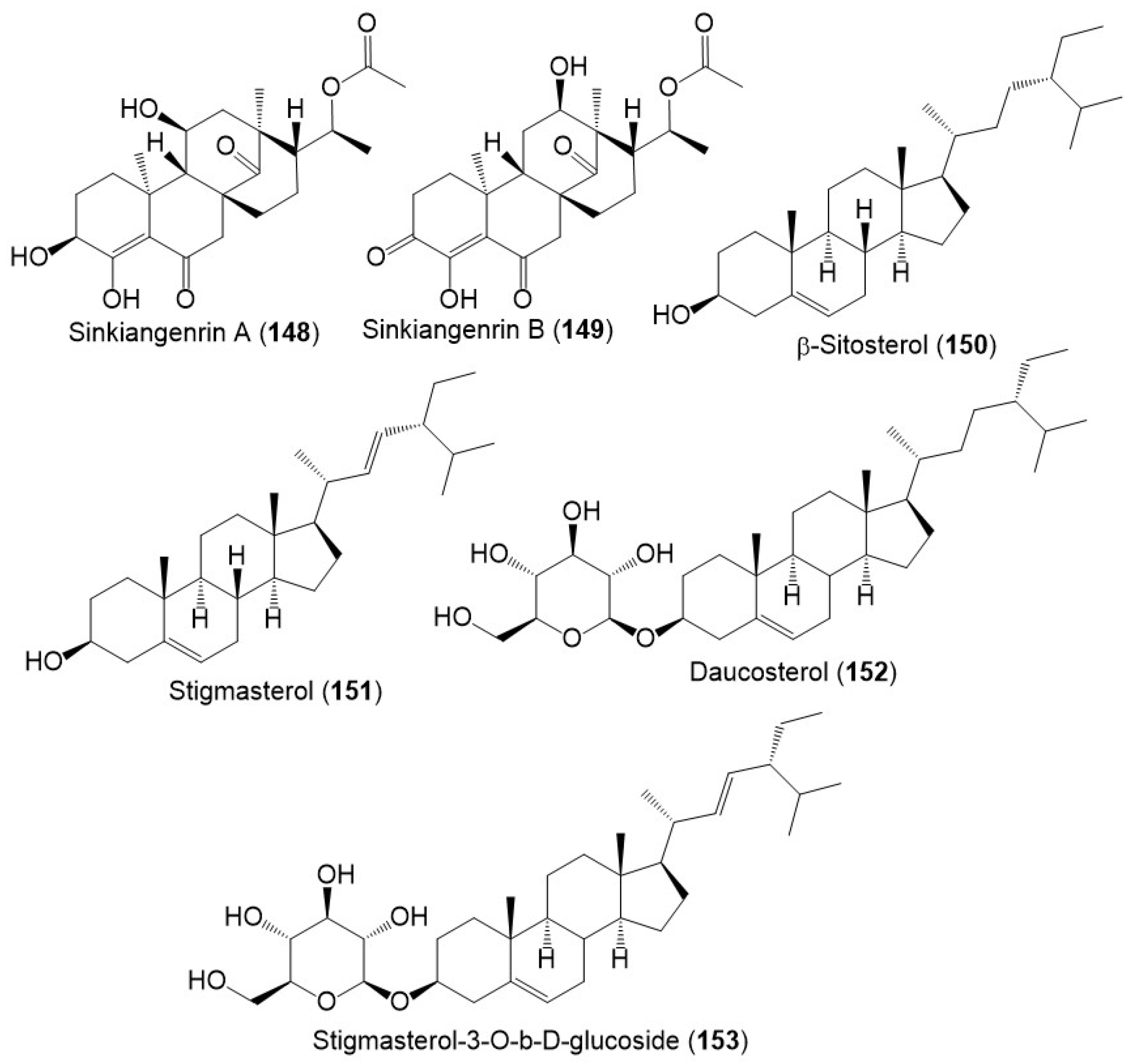
Figure 15. Structures of sterols (148–153) reported from F. sinkiangensis.
These metabolites have an unparallel carbon framework that originates from C21-steroids (Scheme 3). Firstly, the initiation of D-ring rearrangement by C8–C14 pregnane epoxide formation, then C-8 carbocation formation. After that, Wagner–Meerwein rearrangement results in a C14–15 bond migration to C-8 and the creation of a C-14 protonated carbonyl that is deprotonated [21]. Following that set of enzyme-catalyzed reactions produce 148 and 149 [20].
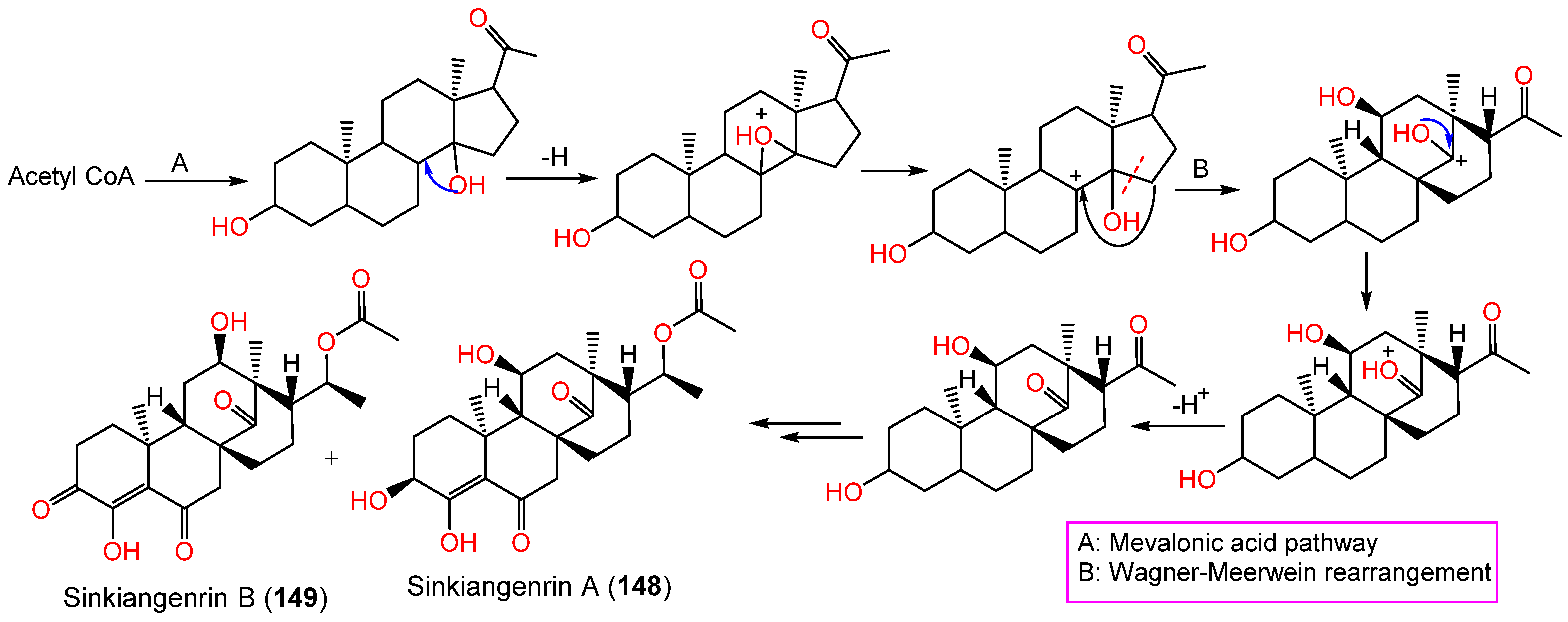
Scheme 3. Biosynthesis of sinkiangenrins A (148) and B (149) [20].
2.9. Phenolic Compounds and Other Metabolites
Several studies reported the separation of phenolics metabolites such as flavonoids, phenylpropanoids, and acids from resin and seed extracts (Figure 16 and Figure 17) [1][14][18][22].
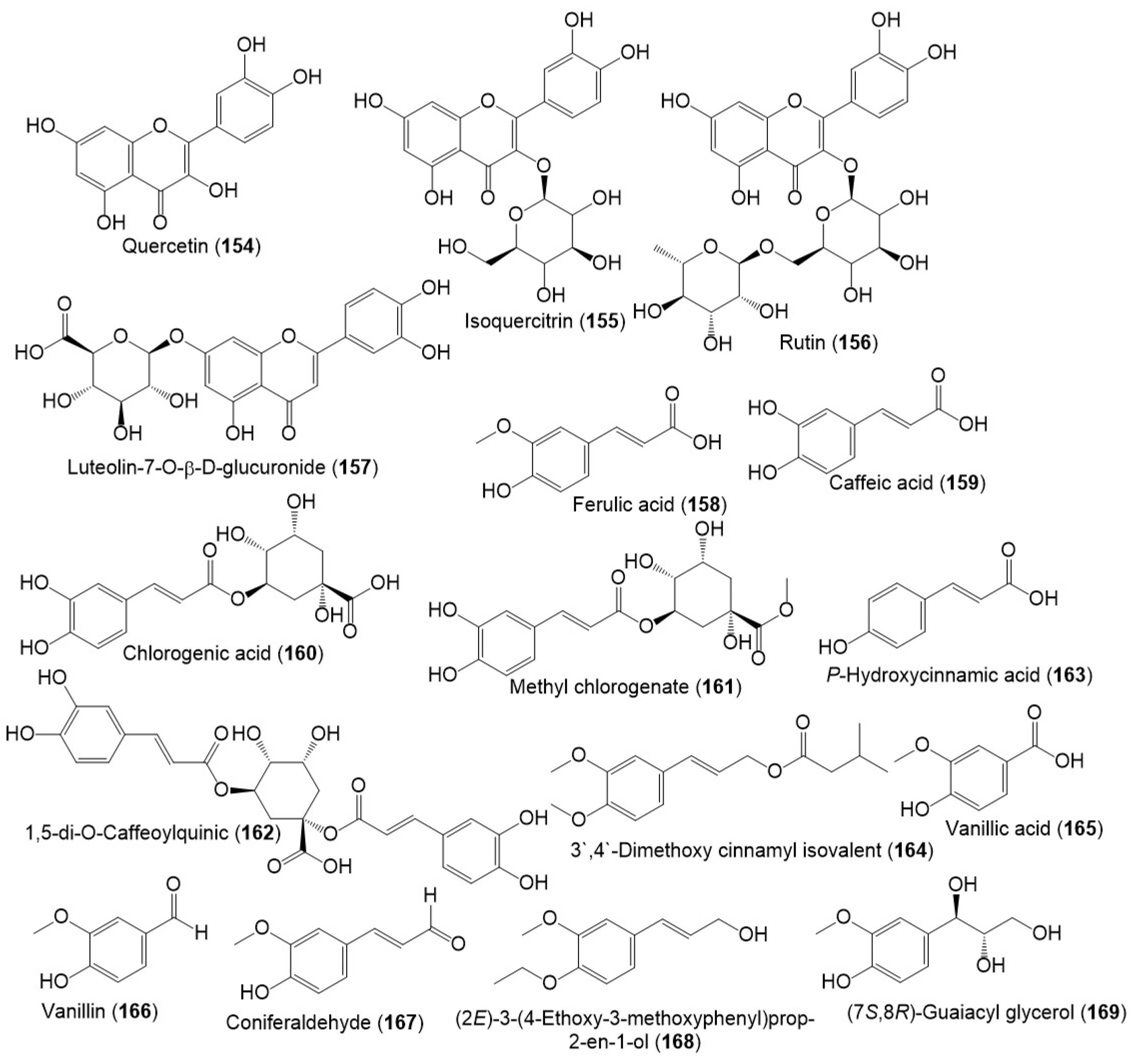
Figure 16. Structures of phenolic compounds (154–169) reported from F. sinkiangensis.
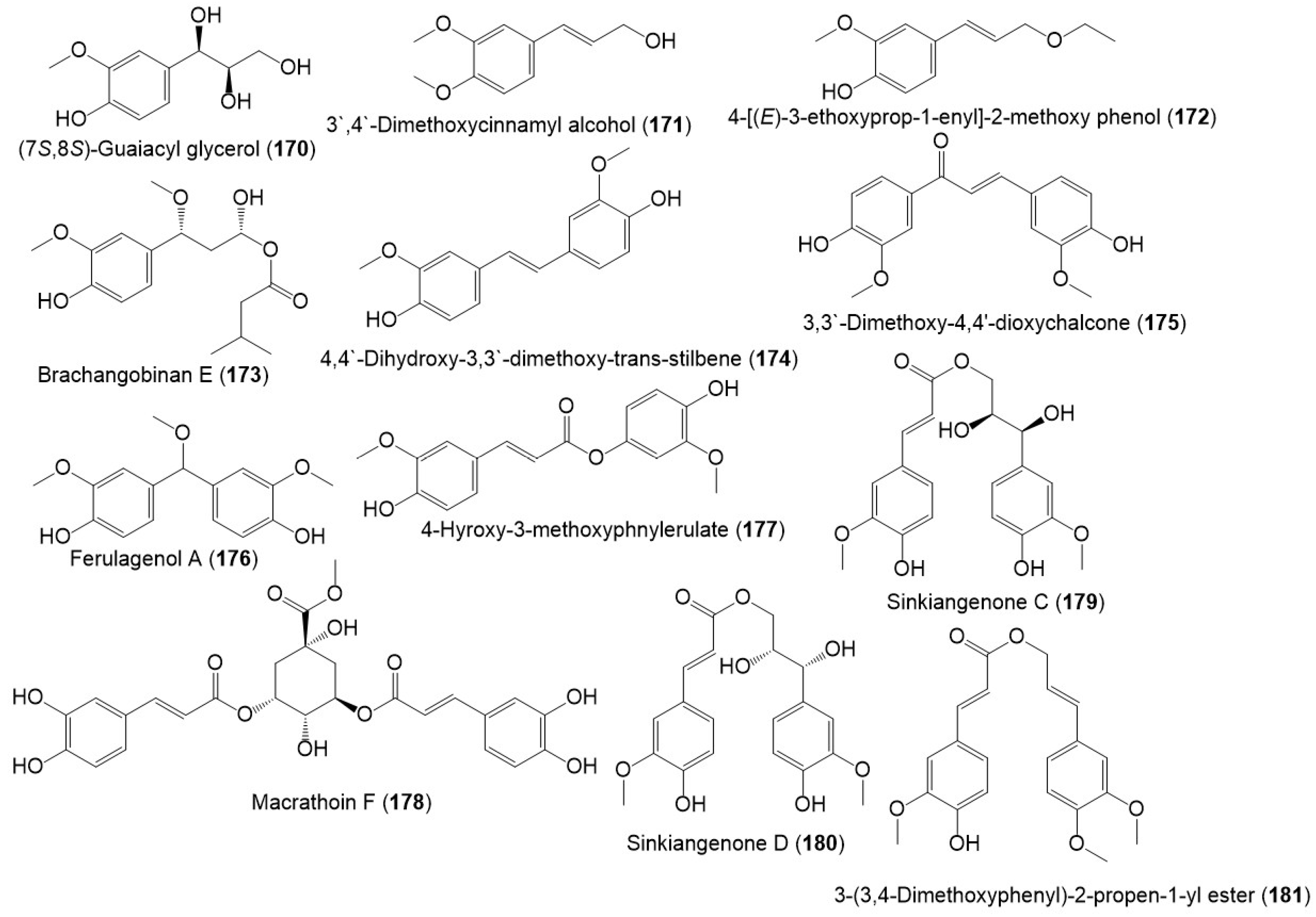
Figure 17. Structures of phenolic compounds (170–181) reported from F. sinkiangensis.
From the resin extract, new tetrahydrobenzofuran derivatives: sinkiangensis A–C (182–184) were purified using SiO2 CC/HPLC and elucidated by spectral and ECD analyses (Figure 18) [23]. Besides, sinkiangenrin C (192), a new organic acid glycoside was purified from seeds 95%EtOH extract. It is a 2-(1-hydroxyethyl)-4-methyl pentanoic acid 4-O-β-D-glucopyranoside [20].
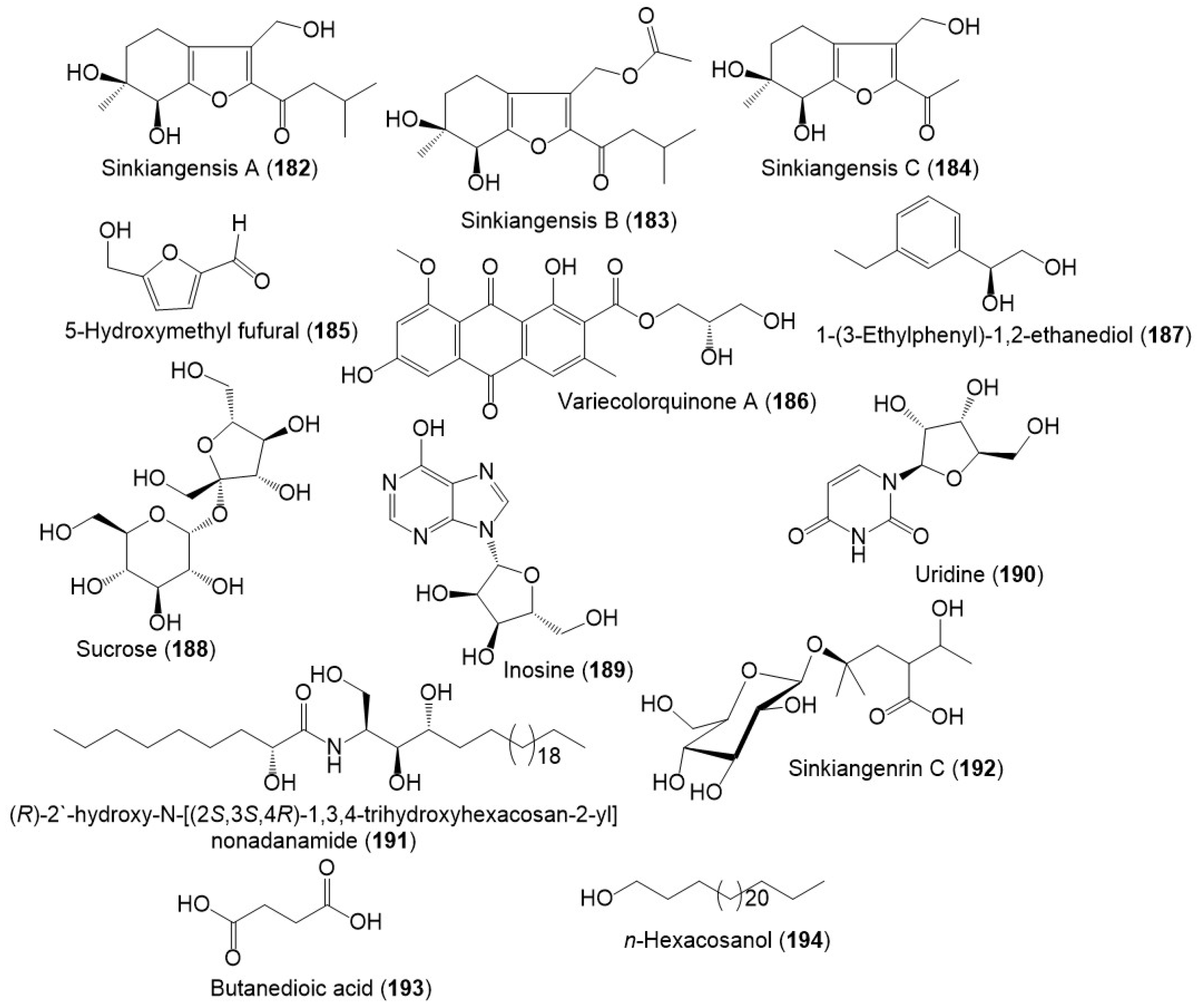
Figure 18. Structures of other metabolites (182–194) reported from F. sinkiangensis.
2.10. Polysaccharides
Ghulameden et al., reported the separation of water-soluble polysaccharide from F. sinkiangensis roots gathered from Yili, Xinjiang Region, China using DEAE-cellulose-52 column (distilled H2O, as eluent) that was assigned by IR and HPLC analyses. The FSPs (crude polysaccharides) had ribose/arabinose/glucose/fucose/galactose (ratios 8.9/3.3/2.1/1.5/0.3), while FSPs-n (neutral polysaccharides) and FSPs-a (acidic polysaccharides) contained glucose/xylose/arabinose/galactose/mannose (ratios 3.9/4.0/1.8/1.4/0.8) and glucose/xylose/mannose/arabinose (ratios 6.5/4.0/1.7/1.0), respectively [4].
In another study, the sequential extraction of F. sinkiangensis roots yielded 28.86 wt% total polysaccharides. The polysaccharide fractions are heteropolysaccharides, containing galacturonic and glucuronic acids, galactose, xylose, rhamnose, fructose, and arabinose [24].
3. Biological Activities of F. sinkiangensis Extracts and Metabolites
3.1. Anti-Inflammatory and Anti-Neuroinflammatory Activity
From the Chinese medicine Awei (F. sinkiangensis gum resin) CHCl3 extract, new metabolites; 23 and 36, in addition to formerly reported 1, 5, 7, 8, 11–13, 16, 32, 33, 39, 41, 52, and 57 (Table 1)were assessed for their anti-neuroinflammatory activity against LPS (lipopolysaccharide)-stimulated NO production in BV-2 microglial cells using the nitrite and MTT (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazoliumbromide) assays. It was noted that 7, 12, 32, and 33 possessed marked activity (IC50s 6.93, 4.96, 10.5, and 5.95 µM, respectively) compared to minocycline (IC50 37.04 µM), whereas 5, 8, 11, 13, 39, 41, and 57 greatly lessened NO production (IC50s ranged 19.88–47.43 µM). Compound 12 (Conc. 1–10 µM) remarkably suppressed IL-6, TNF-α, and IL-1β expression, as well as COX-2 (Conc. 3–10 µM) caused by LPS in BV2 cells. Thus, this plant might have the potential as anti-Alzheimer′s disease therapy [25]. Structure-activity relationship revealed that the substitution at C-3` in the bicyclic derivatives that possess 8`R-CH3 and 8`R-OH had a substantial role in the activity. The capability of C-3`-substitutents to boost the effect followed this order: acetoxy, α-OH, β-OH, and C=O, however, this order varied in bicyclic derivatives with C-8` terminal olefinic bond. In the mono-cyclic derivatives, the rings` breaking position of sesquiterpene moiety could influence the efficacy e.g., 32 and 33 with broken A-ring were more active than 39 and 41 with broken B-ring. Besides, the O-bridge in ring A in monocyclic derivatives improved the efficacy. On the other hand, the chain derivatives (e.g., 57 and 52) had weak activity [25]. In 2020, Zhang et al., evaluated the potential of 12 on ischemic stroke utilizing BCCAO (bilateral common carotid artery occlusion) and LPS-invigorated microglia models. It was found that 12 relieved cognitive weakness, lowered neuronal forfeiture, repressed microglial stimulation, and converted microglia from the proinflammation M1 type to the anti-inflammation M2 type in the BCCAO-mice model. Moreover, it organized microglial polarization and suppressed the MAPK (mitogen-activated protein kinase) and NLRP3 signaling pathways subsequent to LPS-treatment in vitro. These findings highlighted the possible activity of 12 for treating ischemic stroke [26].
Table 1. Biological activity of reported metabolites from Ferula sinkiangensis.
| Compound Name | Biological Activity | Assay, Organism or Cell Line | Biological Results | Ref. | |
|---|---|---|---|---|---|
| Compound | Positive Control | ||||
| Farnesiferol A (1) | Anticancer | MTT/HeLa | 20.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] |
| Colladonin (2) | Anticancer | MTT/AGS | 85.5 µM (IC50) | Taxol 3.5 µM (IC50) | [27] |
| Coladin (3) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 60.5 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Farnesiferone A (5) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 37.88 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Griess reaction/NO production/LPS-activated BV-2 cells | 37.9 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] | ||
| Anticancer | MTT/HeLa | 23.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] | |
| MTT/MGC-803 | 49.0 µM (IC50) | Taxol 3.4 µM (IC50) | [9] | ||
| MTT/AGS | 32.0 µM (IC50) | Taxol 1.8 µM (IC50) | [9] | ||
| Gummosin (7) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 6.93 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Polyanthinin (8) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 19.88 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Griess reaction/NO production/LPS-activated BV-2 cells | 37.3 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] | ||
| Anticancer | MTT/HeLa | 28.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] | |
| Anticancer | MTT/MGC-803 | 45.0 µM (IC50) | Taxol 3.4 µM (IC50) | [9] | |
| Anticancer | MTT/AGS | 24.0 µM (IC50) | Taxol 1.8 µM (IC50) | [9] | |
| Sinkiangenol E (10) | Anticancer | MTT/HeLa | 16.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] |
| Ferukrin (11) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 21.34 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| (3`S, 5`S, 8`R, 9`S, 10`R)-Kellerin (12) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 4.96 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Anticancer | MTT/HeLa | 37.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] | |
| (3`S, 5`S, 8`R, 9`S, 10`R)-Deacetylkellerin (13) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 31.61 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Episamarcandin (15) | Anticancer | MTT/AGS | 83.8 µM (IC50) | Taxol 3.5 µM (IC50) | [27] |
| Lehmannolol (24) | Anticancer | MTT/AGS | 26.0 µM (IC50) | Taxol 3.5 µM (IC50) | [27] |
| Anticancer | MTT/HeLa | 42.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] | |
| Lehmannolone (25) | Anticancer | MTT/HeLa | 81.1 µM (IC50) | Taxol 5.6 µM (IC50) | [27] |
| Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 93.8 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] | |
| Ferusingensine H (28) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 18.6 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Fekrynol (29) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 13.0 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Anticancer | MTT/HeLa | 35.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] | |
| MTT/MGC-803 | 49.0 µM (IC50) | Taxol 3.4 µM (IC50) | [9] | ||
| MTT/AGS | 20.0 µM (IC50) | Taxol 1.8 µM (IC50) | [9] | ||
| Fekrynol acetate (30) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 15.7 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Anticancer | MTT/HeLa | 25.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] | |
| MTT/MGC-803 | 28.0 µM (IC50) | Taxol 3.4 µM (IC50) | [9] | ||
| (8`S, 9`S, 10`S)-Propionyl fekrynol (31) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 21.3 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Galbanic acid (32) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 10.50 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Anticancer | MTT/HeLa | 43.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] | |
| Methyl galbanate (33) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 5.95 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Fekolone (34) | Anticancer | MTT/AGS | 75.4 µM (IC50) | Taxol 3.5 µM (IC50) | [27] |
| Sinkianone (35) | Anticancer | MTT/HeLa | 77.9 µM (IC50) | Taxol 5.6 µM (IC50) | [27] |
| Ferusingensine G (38) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 1.2 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Farnesiferol B (39) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 45.37 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Farnesiferone B (40) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 18.3 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Farnesiferol C (41) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 31.43 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| Griess reaction/NO production/LPS-activated BV-2 cells | 22.6 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] | ||
| Anticancer | MTT/HeLa | 86.9 µM (IC50) | Taxol 5.6 µM (IC50) | [27] | |
| Anticancer | MTT/AGS | 101.6 µM (IC50) | Taxol 3.5 µM (IC50) | [27] | |
| Anticancer | MTT/HeLa | 25.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] | |
| Sinkiangenorin F (42) | Anticancer | MTT/AGS | 27.1 µM (IC50) | Taxol 3.5 µM (IC50) | [28] |
| 8-O-Acetyl sinkiangenorin F (43) | Anticancer | MTT/AGS | 62.78 µM (IC50) | Taxol 3.5 µM (IC50) | [28] |
| Sinkiangenorin D (44) | Anticancer | MTT/HeLa | 20.4 µM (IC50) | Taxol 5.6 µM (IC50) | [27] |
| Anticancer | MTT/K562 | 81.1 µM (IC50) | Taxol 8.5 µM (IC50) | [27] | |
| Anticancer | MTT/AGS | 104.8 µM (IC50) | Taxol 3.5 µM (IC50) | [27] | |
| Sinkiangenorin E (45) | Anticancer | MTT/AGS | 12.7 µM (IC50) | Taxol 3.5 µM (IC50) | [29] |
| MTT/HeLa | 82.9 µM (IC50) | Taxol 5.6 µM (IC50) | [29] | ||
| Antiviral | A/Beijing/7/2009 H1N1 (BJ09/H1N1) | 4.0 µM (IC50) | - | [29] | |
| Ferusingensine F (46) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 29.0 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Umbelliprenin (52) | Anticancer | MTT/AGS | 12.7 µM (IC50) | Taxol 3.5 µM (IC50) | [27] |
| 10R-Karatavicinol (53) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 47.43 µM (IC50) | Minocycline 37.04 µM (IC50) | [25] |
| 12′-Hydroxy-karatavicinol (54) | Anticancer | MTT/HeLa | 48.0 µM (IC50) | Taxol 7.5 µM (IC50) | [9] |
| Ferusingensine A (56) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 31.8 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| Ferusingensine E (60) | Anti-neuroinflammatory | Griess reaction/NO production/LPS-activated BV-2 cells | 65.4 µM (IC50) | Minocycline 65.5 µM (IC50) | [2] |
| (+)-Ferulasin 2R, 3R, 10`R (61) | Anticancer | MTT/SW1990 | 11.77 µM (IC50) | Taxol 1.70 µM (IC50) | [11] |
| Anticancer | MTT/PANC-1 | 2.24 µM (IC50) | Taxol 0.22 µM (IC50) | [11] | |
| Anticancer | MTT/CFPAC-1 | 6.12 µM (IC50) | Taxol 0.38 µM (IC50) | [11] | |
| Anticancer | MTT/Capan-2 | 8.57 µM (IC50) | Taxol 0.51 µM (IC50) | [11] | |
| (-)-Ferulasin 2S, 3S, 10`S (62) | Anticancer | MTT/PANC-1 | 0.92 µM (IC50) | Taxol 0.22 µM (IC50) | [11] |
| Anticancer | MTT/CFPAC-1 | 19.13 µM (IC50) | Taxol 0.38 µM (IC50) | [11] | |
| Ferusinkin A (63) | Anticancer | Spectrophotometrically/IOZCAS-Spex-II | 65.38 µM (EC50) | Camptothecin 51.27 µM (EC50) | [12] |
| Diversin (64) | Anticancer | Spectrophotometrically/IOZCAS-Spex-II | 52.67 µM (EC50) | Camptothecin 51.27 µM (EC50) | [12] |
| Auraptene (65) | Anticancer | Spectrophotometrically/IOZCAS-Spex-II | 22.78 µM (EC50) | Camptothecin 51.27 µM (EC50) | [12] |
| Sinkiangenone A (69) | Anticancer | MTT/MGC-803 | 45.05 µM (IC50) | Taxol 3.35 µM (IC50) | [14] |
| Anticancer | MTT/AGS | 48.13 µM (IC50) | Taxol 1.82 µM (IC50) | [14] | |
| Anticancer | MTT/GES-1 | 32.37 µM (IC50) | Taxol 2.67 µM (IC50) | [14] | |
| Sinkiangenone B (70) | Anticancer | MTT/MGC-803 | 18.89 µM (IC50) | Taxol 3.35 µM (IC50) | [14] |
| Anticancer | MTT/AGS | 16.15 µM (IC50) | Taxol 1.82 µM (IC50) | [14] | |
| Anticancer | MTT/GES-1 | 36.73 µM (IC50) | Taxol 2.67 µM (IC50) | [14] | |
| Ferulaeone G (72) | Anticancer | MTT/MGC-803 | 35.15 µM (IC50) | Taxol 3.35 µM (IC50) | [14] |
| Anticancer | MTT/GES-1 | 35.23 µM (IC50) | Taxol 2.67 µM (IC50) | [14] | |
| Arctigenin (77) | Anticancer | MTT/AGS | 78.3 µM (IC50) | Taxol 3.5 µM (IC50) | [20] |
| Anticancer | MTT/HeLa | 105.1 µM (IC50) | Taxol 5.6 µM (IC50) | [20] | |
| Matairesinol (78) | Anticancer | MTT/AGS | 99.4 µM (IC50) | Taxol 3.5 µM (IC50) | [20] |
| Nerolidol (120) | Anticancer | Spectrophotometrically/IOZCAS-Spex-II | 14.64 µM (EC50) | Camptothecin 51.27 µM (EC50) | [12] |
| Antifungal | Microdilution/Alternaria alternata | 32.0 μg/mL (MIC) | Carbendazim 16.0 μg/mL (MIC) | [12] | |
| Microdilution/Pyricularia grisea | 16.0 μg/mL (MIC) | Carbendazim 32.0 μg/mL (MIC) | [12] | ||
| Microdilution/Botrytis cinerea | 32.0 μg/mL (MIC) | Carbendazim 32.0 μg/mL (MIC) | [12] | ||
| Guaiol (122) | Anticancer | Spectrophotometrically/IOZCAS-Spex-II | 88.92 µM (EC50) | Camptothecin 51.27 µM (EC50) | [12] |
| Coniferaldehyde (167) | Anticancer | MTT/MGC-803 | 69.65 µM (IC50) | Taxol 3.35 µM (IC50) | [14] |
| Sinkiangensis A (182) | Anticancer | MTT/AGS | 87.1 µM (IC50) | Taxol 4.69 µM (IC50) | [23] |
| Sinkiangensis B (183) | Anticancer | MTT/AGS | 72.7 µM (IC50) | Taxol 4.69 µM (IC50) | [23] |
| Sinkiangensis C (184) | Anticancer | MTT/AGS | 15.6 µM (IC50) | Taxol 4.69 µM (IC50) | [23] |
| Sinkiangenrin C (192) | Anticancerity | MTT/AGS | 36.9 µM (IC50) | Taxol 3.5 µM (IC50) | [20] |
Further, in 2021, Mi et al., explored the potential of 12 on cerebral ischemia utilizing MCAO (middle-cerebral-artery occlusion) and LPS-boosted microglia models. In the MCAO model, 12 amended neurological outcomes and decreased infarct size and edema of the brain in rats. It also mitigated neuron injury and restrained microglial activation. Moreover, 12 guarded neuronal cells against damage by repressing microglial activation in LPS-invigorated BV2 cells. It also diminished the proinflammatory cytokines levels, NADPH oxidase activity, and ROS generation, along with the NF-κB signaling pathway repression [16].
Chemical investigation of F. sinkiangensis resin 95% EtOH extract resulted in new sesquiterpene coumarins: 27, 28, 31, 38, 46, and 56–60, along with early reported 3, 5, 8, 23, 25, 29, 30, 40, 41, and 52. They were characterized spectral/ECD/[α]D analyses. Their inhibitory potential on NO production induced by LPS using nitrite and MTT assays in BV2 cells. Compounds 3, 5, 8, 25, 28–31, 38, 40, 41, 46, and 56 possessed noticeable inhibition of NO production in over-activated BV2 cells, (IC50s 1.2–93.8 μM) compared to minocycline (IC50 65.5 μM), while 27, 23, 52, and 57–59 had weak inhibitory capacity (IC50 > 00 μM). It was noted that 28 (IC50 1.2 μM) revealed the potent in vitro anti-neuroinflammatory that was confirmed by docking to TLR4/MD-2. Structure-activity relation showed that chain sesquiterpene coumarins with C-10` acetoxy group (e.g., 56) had powerful activity than the ones with C-10`-OH, an unsaturated fatty chain or 4-decylenic acid ester (e.g., 57, 58, and 59, respectively). A-ring substitution pattern affected the potential of monocyclic derivatives with opened B-ring. The α,β-unsaturated ketone (e.g., 38) increased the effect, while the seven-membered ring-A resulted from C-4′-O–C–3` linkage (e.g., 28) remarkably repressed NO producing relative to six-membered ring-A derivatives. In the compounds with opened A-ring (e.g., 29–31 and 46), increase the length of oxyacyl side chain at C-3` weakened the anti-neuroinflammatory activity [2].
Among the lignan derivatives, 90–101, 92 and 93 possessed anti-inflammatory potential via inhibiting TNF-α and IL-6 production mediated by LPS in RAW264.7 cells without affecting the RAW264.7 viability. Besides 92 and 93 notably suppressed LPS-produced iNOS and COX-2 expression in RAW264.7 cells [17], whereas 102–111 had COX-2 inhibition capacity (IC50s 4.47–21.96 μM) [18]. This evidence supported the role of F. sinkiangensis in treating inflammation [17][18].
In 2022, ferulagenol A (176) a new phenolic metabolite, in addition to 158, 171–173, 175, and 177 were reported by Yan et al., Compounds 176 and 177 possessed notable COX-2 inhibitory capacity (IC50s 3.63 and 3.0 μM, respectively) [18].
On the other hand, F. sinkiangensis gum resin CHCl3 extract considerably prohibited production of NO in LPS-boosted BV-2 microglial cells (IC50 1.66 µg/mL) [25].
3.2. Anticancer Activity
New sesquiterpene coumarins, 10, 17, 21, and 22 and related analogs 1, 5–9, 11–14, 20, 24, 26, 29, 30, 32, 37, 39, 41, 52, 54, and 57 were reported from the 95% EtOH extract of the resin [9]. These compounds displayed moderate to weak anticancer potential against AGS, HeLa, and MGC-803 cell lines. Compounds 5, 8, and 29 demonstrated anticancer potential against AGS, HeLa, and MGC-803 cell lines (IC50s 20.0–49.0 μM), compared to taxol (IC50 1.8, 7.5, and 3.4, respectively). Compound 10 had (IC5016.0 μM) selective activity against HeLa cells. The mechanistic study demonstrated that 10 caused G0/G1 cell cycle arrest and apoptosis in HeLa cells. It induced its effect by affecting the expression of cell cycle regulation- and apoptosis-related proteins by stimulating the MAPK pathway [9]. Additional work by Li et al., reported the separation of another new sesquiterpene coumarins, sinkiangenorin F (42) and its 8-acetyl derivative, and 8-O-acetyl sinkiangenorin F (43) from the seeds EtOH extract. They feature ether-linked coumarin and sesquiterpene moieties with 6`S/8`S/9`S. They exhibited anticancer potential (IC50s 27.1 and 62.7 µM, respectively) against AGS cell lines in the MTT assay [28].
Li et al. in 2015 purified and characterized sinkiangenorin D (44), a novel sesquiterpene coumarin having fekrynol- sesquiterpene skeleton, along with 2, 15, 19, 24, 25, 29, 34, 35, 41, and 52 from the seeds’ EtOH extracts. Compound 44 is sesquiterpene coumarin involving a monocyclic cycloheptene sesquiterpene unit. These metabolites (IC50s 12.7–226.6 μM) demonstrated anticancer capacity on HeLa, AGS, and K562 in the MTT assay. Compounds 24, 25, 29, 35, 41, and 44 had activity on HeLa cells (IC50s 20.4–226.2 μM), whereas 2, 15, 24, 34, 41, 44, and 52 were active against AGS cells (IC50s 12.7–104.8 μM) compared to taxol (IC50s 3.5–8.5 μM) [27]. Sinkiangenorin D (44) was proposed to be biosynthesized from fekrynol-kind sesquiterpene [27]. Firstly, the formation of II is accomplished by C-4 protonation and the olefinic bond electron-transport reaction. Thereafter, the C4–C5 electrons would transmit to C11, resulting in a seven-member ring formation. The C-5′ methyl transmission and successive loss of proton form C4′–C5′ double bond result in this novel framework formation [27] (Scheme 4).
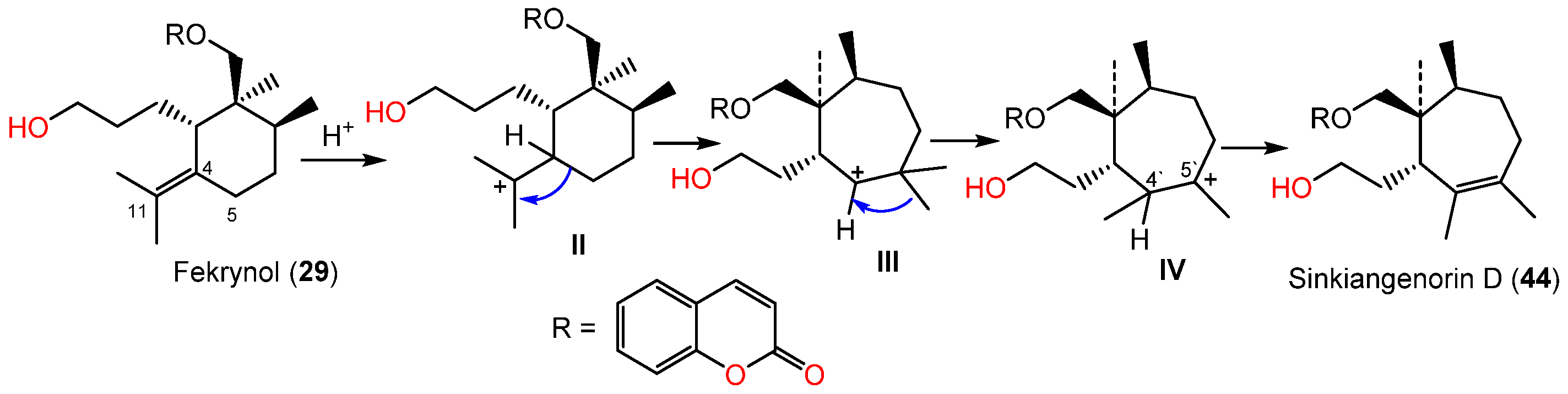
Scheme 4. Biosynthetic pathway of sinkiangenorin D (44) [29].
A novel metabolite (45) having unrivaled bicyclo[4.3.1]decane-type sesquiterpene skeleton was purified from the 95% EtOH extract of the seeds. Its 2′S/3′R/5′R/7′R/10′R configuration was determined based on NMR and CD analyses. It had moderate and weak anticancer activity against AGS (IC50 12.7 μM) and Hela (IC50 82.9 μM) cells, respectively compared to taxol (IC50 3.5 and 5.6 μM, respectively) [29].
Umbelliprenin (52) possessed dose-dependent and time-dependent apoptosis in CLL (chronic lymphocytic leukemia) that was more sensitive to 52 than PBMCs. It is noteworthy that IL-4 could not decline 52-caused apoptosis in CLL (Figure 7). Thus, 52 oral administration as foods or folk medicines, might stimulate the protection against CLL development with few side effects, however, additional clinical investigations are needed [30]. Gholami et al., reported that 52 potentiated apoptosis intrinsic/extrinsic pathways in Jurkat cells by activating caspase-9 and -8, as well as Bcl-2 prohibition [31]. Another study by Zhang et al., revealed that 52 had a notable anticancer activity (IC50s 13.67 and 20.82 µM, respectively) against AGS cells with less anticancer to GES-1 (normal human gastric epithelial cell line). It boosted AGS cells apoptosis with elevated Bax/Bcl-2 ratios, ROS generation, lessened mitochondrial-membrane potential, and PARP and caspase 3 activation resulting in mitochondrial apoptosis pathway activation. It also arrested the G0/G1 phase of the cell cycle, increased P27, P53, and P16, expression, and diminished cyclin E, cyclin D, Cdk2, and Cdk4 expression in cancer cells. Therefore, it could be developed into anti-cancer therapy [1]. In 2019, Zhang et al., also reported that 52 also demonstrated anticancer capacity against BGC-823 and AGS, with less toxic influence on the normal GES-1 gastric cells. It was proven to prevent gastric cancer cell invasion, growth, and migration by disconcerting the Wnt signaling pathway. Additionally, it exhibited no harm in the in vivo BGC-823 xenograft model as evidenced by no observed abnormality in daily diet, body weight, liver function, and histological features of the spleen, liver, lung, kidney, and heart tissue. This further supported the previous evidence of its promising potential for treating gastric cancer [32].
The anticancer investigation against SW1990, CFPAC-1, Capan-2, and PANC-1 revealed that (+)-61 and (-)-62 had marked proliferation inhibition capacity on PANC-1 cells (IC50s 2.24 and 0.92 μM, respectively) and moderate activity against CFPAC-1 cells (IC50s 6.12 and 19.13 μM, respectively) compared to taxol (IC50s 0.22 and 0.38 μM, respectively). It was noted that the anticancer potential of (+)-61 was more powerful than (-)-62 [11]. Also, 63–65 had anticancer activity (EC50s 22.78–65.38 μM) against IOZCAS-Spex-II, where 65 was the most potent (EC50 22.78 μM) relative to camptothecin (EC50 51.27 μM) [12].
Wang et al., examined the activity of 69 and 70 against AGS, MGC-803, and GES-1 using MTT assay. Only 70 possessed the notable anticancer potential against MGC-803 and AGS (IC50s 18.89 and 16.15, respectively) with less toxicity against GES-1 (IC50 36.73 μM) comparing with taxol (IC50 3.35, 1.82, and 2.67 μM, respectively), whereas the other metabolites had no notable potential. The mechanistic study revealed that 70 was found to elevate Bax/Bcl-2 ratios, as well as RB, P16, and P27 expression and decrease cyclins (D1 and E), Cdk4, P53, and Cdk2, resulting in apoptosis and G0/G1 cell cycle arrest in AGS cells. This compound could be a potential candidate for gastric cancer therapy [14].
F. sinkiangensis seeds afforded 77–80 that displayed weak to moderate anticancer activity on AGS and Hela cells in the MTT assay [20] (Figure 10), whereas 77, 80, and 81 had a weak anticancer capacity against AGS, HeLa, A549, and PC3 cell lines in the MTT assay (IC50s 54.4–167.3 µM) [1].
In the CCK-8 assay, 84 and 86 were found to significantly prohibit the migration and invasion of TNBC) cell lines. On the other hand, 88 and 89 promoted the HUVECs) proliferation which was more remarkable than bFGF (basic-fibroblast-growth factor, positive control) in the wound-healing assay [5].
New phenylpropanoid derivative; sinkiangenones C (179) and D (180), along with 158, 163, 164, 166–170, 174, and 181 were separated from the resin 95% EtOH extract and specified by spectral and CD analyses. In the MTT assay against AGS, MGC-803, and GES-1, they had no or weak potential (IC50s 18.89 to 182.46 μM) [14].
Sinkiangensis A–C (182–184) possessed anticancer activity on the AGS cell line (IC50s 87.1, 72.7, and 15.6 μM, respectively), whereas 184 was the most active in comparison to taxol (IC50 4.69 μM) and induced AGS cell apoptosis in the MTT assay. Unfortunately, they (IC50 ˃100 μM) had no effect against HeLa and K562 cells [23]. Whilst 192 exhibited anticancer activity on AGS cells (IC50 36.9 μM) [20].
The petroleum ether, EtOAc, n-BuOH, and MeOH fraction possessed of F. sinkiangensis resin anticancer activity against Caco-2, HC-T116, MFC, and HepG2 cells in the SRB assay. EtOAc fraction was found to have the potent anti-proliferative and apoptotic effects against all tested cell lines. This was correlated to its content of sesquiterpene coumarins [33].
3.3. Antiviral, Insecticidal, and Antimicrobial Activities
Besides, 45 prohibited (IC50 4.0 μM) BJ09/H1N1 (influenza A/Beijing/7/2009 H1N1) infection in MDCK cells [29]. The monoterpene coumarins; 63–65 and the coumarins: 66 and 67 displayed insecticidal potential (Conc. 10 μg/larva, 24 h) against S. exigua 3rd instar larvae (%mortality ranging from 26.67–52.22%) compared to camptothecin (18.89% mortality) [12]. From the aerial parts, sesquiterpenes; nerolidol (120) and guaiol (122) (58.89 and 41.11% mortality, respectively) possessed toxic potential on the S. exigua insect 3rd instar larvae [12]. Additionally, 120 exhibited antifungal effect against plant pathogens: inhibitory effects on Pyricularia grisea, Alternaria alternata, and Botrytis cinereal (MICs 16, 32, and 32 μg/mL, respectively) compared to carbendazim (MICs 32, 16, and 32 μg/mL, respectively) [12].
3.4. Anti-Drug Addiction Activity
Drug addiction is a prime health concern that influences a growing number of persons and gives rise to severe economic and social burdens to economy society [34][35]. Despite the fact that diverse remedial strategies for drug addiction and abuse are developed, including psychological, sociological, and pharmacological interventions, their activity is yet restricted [36][37].
A mixture of 133 and 135 was obtained from the F. sinkiangensis crude essential oil. A mixture of 133 and 135 (1:3 ratio, doses: 20, 40, and 60 mg/kg, ip) significantly repressed the morphine abstinence syndrome and physiological addiction in rats and mice [38]. At the same doses, this mixture (1:3 ratio, i.p.) reduced morphine-induced bodyweight loss. While the mixture declined the abdominal writhing movements number and automatic activity (doses 10.73, 21.45, and 43.55 mg/kg, i.p.) revealing its analgesic and sedative potential. Its acute toxicity evaluation showed the LD50 values of its iv and ip injections were 1.42 g/kg and 1.66 g/kg, respectively [38].
In 2006, Wang reported in his patent that the resin extract in the form of capsules, powder, injection, drop pills, granules, tablets, or oral liquid) ameliorated the influences of serious and moderate long-time drug addictions in addicted patients, indicating its potential for treating subjects addicted to morphine, opioid, diamorphine, and marijuana [6][8]. It is noteworthy that 0.1–20 g/kg was found to be the therapeutically effective amount of the extract for producing an effect of abstinence of morphine, whereas the preferable dose was 1–3 g/kg [6].
3.5. Protein-Tyrosine Phosphatase 1B Inhibition Activity
FSPs-a (acidic polysaccharides) fraction of F. sinkiangensis roots water-soluble polysaccharides revealed in vitro PTP1B (protein-tyrosine phosphatase 1B) competitive inhibition (IC50 0.29 µg/mL, % inhibition 91.23%) [4]. In another study, the PTP1B inhibitory potential of the different polysaccharides fractions was estimated. It was noted that the inhibitory capacity of the tested fractions elevated with raising their galactose content, therefore, galactose may be a ligand for blocking PTP1B catalytic site [24].
3.6. Antiulcer and Antioxidant Activities
In the in vivo antiulcer assay, different F. sinkiangensis resin extracts possessed antiulcer capacity, whereas CHCl3 extract (%inhibition 48.43) had comparatively better antiulcer potential than the n-BuOH and EtOAC (%inhibition 37.07 and 46.06%, respectively) extracts comparing to famotidine (%inhibition 45.37) [10]. In the DPPH assay, the F. sinkiangensis resin n-BuOH, EtOAc, and MeOH fractions significantly scavenged DPPH, whereas the EtOAc fraction was the most effective and the petroleum ether fraction was weakly active in the DPPH assay [33].
3.7. Feed Attraction Activity
In 2020, Xu et al., reported that feeding Lateolabrax japonicus (commercial fish) with F. sinkiangensis was found to notably promote L. japonicus foraging and better digestive enzyme activity and fish growth performance, where 10 g/kg was appropriate in the fish diet. Thus, it had an efficient role in L. japonicus farming and could have potential in the aquaculture industry as aquafeed formulation [39].
References
- Zhang, L.; Si, J.; Li, G.; Li, X.; Zhang, L.; Gao, L.; Huo, X.; Liu, D.; Sun, X.; Cao, L. Umbelliprenin and lariciresinol isolated from a long-term-used herb medicine Ferula sinkiangensis induce apoptosis and G0/G1 arresting in gastric cancer cells. RSC Adv. 2015, 5, 91006–91017.
- Guo, T.; Zhou, D.; Yang, Y.; Zhang, X.; Chen, G.; Lin, B.; Sun, Y.; Ni, H.; Liu, J.; Hou, Y. Bioactive sesquiterpene coumarins from the resin of Ferula sinkiangensis targeted on over-activation of microglia. Bioorg. Chem. 2020, 104, 104338.
- Li, X.; Wang, Y.; Zhu, J.; Xiao, Q. Essential oil composition analysis of three cultivars seeds of Resina Ferulae from Xinjiang, China. Pharmacogn. Mag. 2011, 7, 116.
- Ghulameden, S.; Yili, A.; Zhao, H.Q.; Gao, Y.H.; Aisa, H.A. Polysaccharides from Ferula sinkiangensis and potent inhibition of protein tyrosine phosphatase 1B. Chem. Nat. Compd. 2014, 50, 515–517.
- Li, Y.; Yang, B.; Zheng, S.; Cheng, Y.; Cui, H. Racemic norlignans as diastereoisomers from Ferula sinkiangensis resins with antitumor and wound-healing promotion activities. Molecules 2022, 27, 3907.
- Wang, Z. Asafetida Extract as Medicine for Abstinence of Drugs. U.S. Patent 7,288,269, 30 October 2007.
- Zhu, W.; Zhang, Y.; Huang, Y.; Lu, L. Chinese Herbal Medicine for The Treatment of Drug Addiction. Int. Rev. Neurobiol. 2017, 135, 279–295.
- Xi, S.; Gong, Y. Essentials of Chinese Materia Medica and Medical Formulas: New Century Traditional Chinese Medicine; Academic Press: Cambridge, MA, USA, 2017.
- Wang, J.; Wang, H.; Zhang, M.; Li, X.; Zhao, Y.; Chen, G.; Si, J.; Jiang, L. Sesquiterpene coumarins from Ferula sinkiangensis KM Shen and their cytotoxic activities. Phytochemistry 2020, 180, 112531.
- Teng, L.; Ma, G.Z.; Li, L.; Ma, L.Y.; Xu, X.Q. Karatavicinol a, a new anti-ulcer sesquiterpene coumarin from Ferula Sinkiangensis. Chem. Nat. Compd. 2013, 49, 606–609.
- Wang, J.; Meng, X.; Zheng, Y.; Sang, C.; Wang, W.; Ma, J.; Zhao, Y.; Yang, J. (±)-Ferulasin, unusual sesquiterpene chromones from Ferula sinkiangensis. Tetrahedron 2022, 122, 132953.
- Liu, T.; Wang, L.; Zhang, L.; Jiang, H.; Zhang, Y.; Mao, L. Insecticidal, cytotoxic and anti-phytopathogenic fungal activities of chemical constituents from the aerial parts of Ferula sinkiangensis. Nat. Prod. Res. 2020, 34, 1430–1436.
- Mohammadhosseini, M.; Venditti, A.; Sarker, S.D.; Nahar, L.; Akbarzadeh, A. The genus Ferula: Ethnobotany, phytochemistry and bioactivities—A review. Indust. Crops Prod. 2019, 129, 350–394.
- Wang, J.; Gao, Y.; Wang, H.; Chen, L.; Cao, L.; Xu, J.; Li, X.; Zhao, Y.; Zhu, J.; Si, J. Apoptosis induction and cell cycle arrest induced by sinkiangenone B, a novel phenylpropanoid derivative from the resin of Ferula sinkiangensis KM Shen. RSC Adv. 2018, 8, 4093–4103.
- Wang, J.; Zhao, Y.; Qiang, Y. Chemical constituents from Ferula sinkiangensis and their chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 105, 104519.
- Mi, Y.; Jiao, K.; Xu, J.; Wei, K.; Liu, J.; Meng, Q.; Guo, T.; Zhang, X.; Zhou, D.; Qing, D. Kellerin from Ferula sinkiangensis exerts neuroprotective effects after focal cerebral ischemia in rats by inhibiting microglia-mediated inflammatory responses. J. Ethnopharmacol. 2021, 269, 113718.
- Li, Q.; Li, J.; Bao, X.; Zhang, S.; Luo, Q.; Li, K.; Jiao, Y.; Cheng, Y.; Yan, Y. Unusual sesquilignans with anti-inflammatory activities from the resin of Ferula sinkiangensis. Bioorg. Chem. 2022, 127, 105986.
- Yan, Y.; Bao, X.; Li, Y.; Li, Q.; Cheng, Y.; Jiao, Y.; Zhang, H. Racemic Norneolignans from the resin of Ferula sinkiangensis and their Cox-2 inhibitory activity. Fitoterapia 2023, 164, 105341.
- Min, Z.; Mai, Q.; Mizuno, M.; Tanaka, T.; Iinuma, M. Polysulfanes in the volatile oils of Ferula species. Planta Med. 1987, 53, 300–302.
- Li, G.; Li, X.; Cao, L.; Shen, L.; Zhu, J.; Zhang, J.; Wang, J.; Zhang, L.; Si, J. Steroidal esters from Ferula sinkiangensis. Fitoterapia 2014, 97, 247–252.
- Zhang, M.; Zhu, Y.; Zhan, G.; Shu, P.; Sa, R.; Lei, L.; Xiang, M.; Xue, Y.; Luo, Z.; Wan, Q.; et al. Micranthanone A, a new diterpene with an unprecedented carbon skeleton from Rhododendron micranthum. Org. Lett. 2013, 15, 3094–3097.
- Li, G. Chemical constituents from seeds of Ferula sinkiangensis. Chin. Tradit. Herb. Drugs. 2015, 24, 1730–1736.
- Yi, X.; Li, Z.; Zheng, Q.; Sang, R.; Li, H.; Gao, G.; Qin, Q.; Zhu, N. Three new tetrahydrobenzofuran derivatives from Ferula sinkiangensis KM shen and their cytotoxic activities. Nat. Prod. Res. 2022, 1–5.
- Wulamu, S.; Yimamu, H.; Abuduwaili, A.; Mutailifu, P.; Maksimov, V.V.; Gao, Y.H.; Yili, A.; Aisa, H.A. Determination of the inhibitory activity of Ferula sinkiangensis polysaccharides for protein tyrosine phosphatase 1B. Chem. Nat. Compd. 2019, 55, 235–238.
- Xing, Y.; Li, N.; Zhou, D.; Chen, G.; Jiao, K.; Wang, W.; Si, Y.; Hou, Y. Sesquiterpene coumarins from Ferula sinkiangensis act as neuroinflammation inhibitors. Planta Med. 2017, 83, 135–142.
- Zhang, W.; Mi, Y.; Jiao, K.; Xu, J.; Guo, T.; Zhou, D.; Zhang, X.; Ni, H.; Sun, Y.; Wei, K. Kellerin alleviates cognitive impairment in mice after ischemic stroke by multiple mechanisms. Phytother. Res. 2020, 34, 2258–2274.
- Li, G.; Wang, J.; Li, X.; Cao, L.; Gao, L.; Lv, N.; Si, J. An unusual sesquiterpene coumarin from the seeds of Ferula sinkiangensis. J. Asian Nat. Prod. Res. 2016, 18, 891–896.
- Li, G.; Li, X.; Cao, L.; Zhang, L.; Shen, L.; Zhu, J.; Wang, J.; Si, J. Sesquiterpene coumarins from seeds of Ferula sinkiangensis. Fitoterapia 2015, 103, 222–226.
- Li, G.; Wang, J.; Li, X.; Cao, L.; Lv, N.; Chen, G.; Zhu, J.; Si, J. Two New sesquiterpene coumarins from the seeds of Ferula sinkiangensis. Phytochem. Lett. 2015, 13, 123–126.
- Ziai, S.A.; Gholami, O.; Iranshahi, M.; Zamani, A.H.; Jeddi-Tehrani, M. Umbelliprenin induces apoptosis in cll cell lines. Iran. J. Pharm. Res. 2012, 11, 653.
- Gholami, O.; Jeddi-Tehrani, M.; Iranshahi, M.; Zarnani, A.H.; Ziai, S.A. Umbelliprenin from Ferula szowitsiana activates both intrinsic and extrinsic pathways of apoptosis in jurkat T-CLL cell line. Iran. J. Pharm. Res. 2013, 12, 371.
- Zhang, L.; Sun, X.; Si, J.; Li, G.; Cao, L. Umbelliprenin isolated from Ferula sinkiangensis inhibits tumor growth and migration through the disturbance of Wnt signaling pathway in gastric cancer. PLoS ONE 2019, 14, e0207169.
- Zhang, H.; Lu, J.; Zhou, L.; Jiang, L.; Zhou, M. Antioxidant and antitumor effects of Ferula sinkiangensis KM Shen. Int. J. Clin. Exp. Med. 2015, 8, 20845.
- Florence, C.; Luo, F.; Xu, L.; Zhou, C. The economic burden of prescription opioid overdose, abuse and dependence in the United States, 2013. Med. Care 2016, 54, 901.
- Kenna, G.A.; Nielsen, D.M.; Mello, P.; Schiesl, A.; Swift, R.M. Pharmacotherapy of dual substance abuse and dependence. CNS Drugs 2007, 21, 213–237.
- Murphy, S.A.; Lynch, K.G.; Oslin, D.; McKay, J.R.; TenHave, T. Developing adaptive treatment strategies in substance abuse research. Drug Alcohol Depend. 2007, 88, S24–S30.
- Volkow, N.D.; Jones, E.B.; Einstein, E.B.; Wargo, E.M. Prevention and treatment of opioid misuse and addiction: A review. JAMA Psychiatry 2019, 76, 208–216.
- Ye, B.; Wang, S.; Zhang, L. Studies on the detoxification effects and acute toxicity of a mixture of cis-sec-butyl-1-propoenyl disulphide and trans-sec-butyl-1-propoenyl disulphide isolated from crude essential oil of Ferula sinkiangensis KM Shen, a Chinese traditional herbal medicine. Nat. Prod. Res. 2011, 25, 1161–1170.
- Xu, A.; Shang-Guan, J.; Li, Z.; Gao, Z.; Huang, Y.C.; Chen, Q. Effects of dietary asafoetida (Ferula sinkiangensis KM Shen) levels on feeding attraction activity, growth performance, healthiness, and digestive enzyme activity in juvenile Lateolabrax japonicus. Fish Physiol. Biochem. 2020, 46, 1991–2003.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
21 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




