
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Díaz Díaz | + 2308 word(s) | 2308 | 2021-09-08 08:12:48 |
Video Upload Options
Cerebral ischemia represents the third cause of death and the first cause of disability in adults. This process results from decreasing cerebral blood flow levels as a result of the occlusion of a major cerebral artery. This restriction in blood supply generates low levels of oxygen and glucose, which leads to a decrease in the energy metabolism of the cell, producing inflammation, and finally, neurological deterioration. Currently, blood restoration of flow is the only effective approach as a therapy in terms of ischemic stroke. However, a significant number of patients still have a poor prognosis, probably owing to the increase in the generation of reactive oxygen species (ROS) during the reperfusion of damaged tissue. Oxidative stress and inflammation can be avoided by modulating mitochondrial function and have been identified as potential targets for the treatment of cerebral ischemia. In recent years, the beneficial actions of flavonoids and polyphenols against cerebrovascular diseases have been extensively investigated. The use of resveratrol (RSV) has been shown to markedly decrease brain damage caused by ischemia in numerous studies. According to in vitro and in vivo experiments, there is growing evidence that RSV is involved in several pathways, including cAMP/AMPK/SIRT1 regulation, JAK/ERK/STAT signaling pathway modulation, TLR4 signal transduction regulation, gut/brain axis modulation, GLUT3 up-regulation inhibition, neuronal autophagy activation, and de novo SUR1 expression inhibition.
1. Introduction
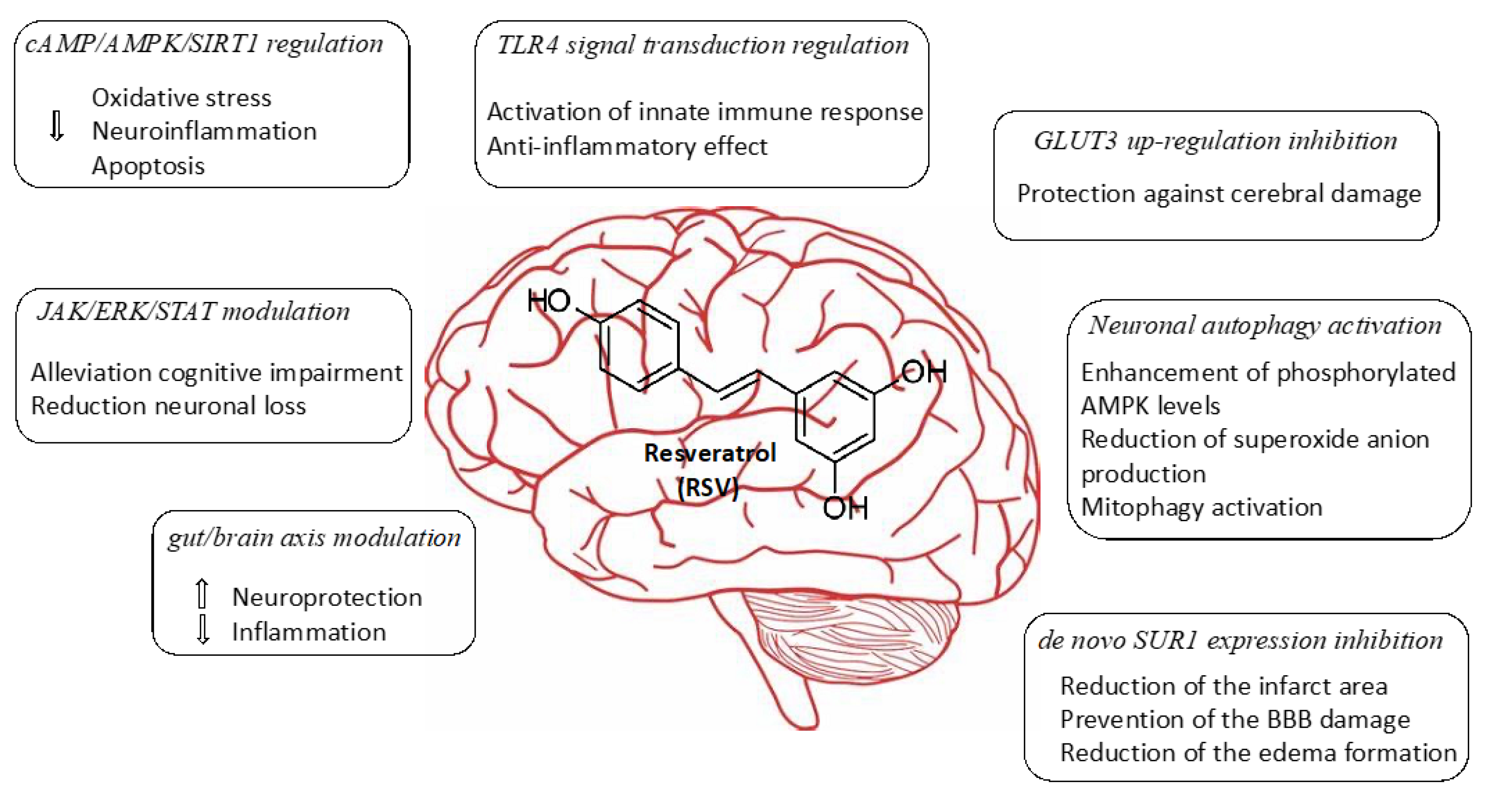
2. Neuroprotective Mechanism Associated with Treatments Involving Resveratrol
2.1. Regulation of cAMP/AMPK/SIRT1 Pathway
2.2. Modulating JAK/ERK/STAT Signaling Pathway
2.3. Regulation of TLR4 Signal Transduction
2.4. Mechanism Targeting Gut/Brain Axis
2.5. Inhibition of GLUT3 Up-Regulation
2.6. Activation of Neuronal Autophagy
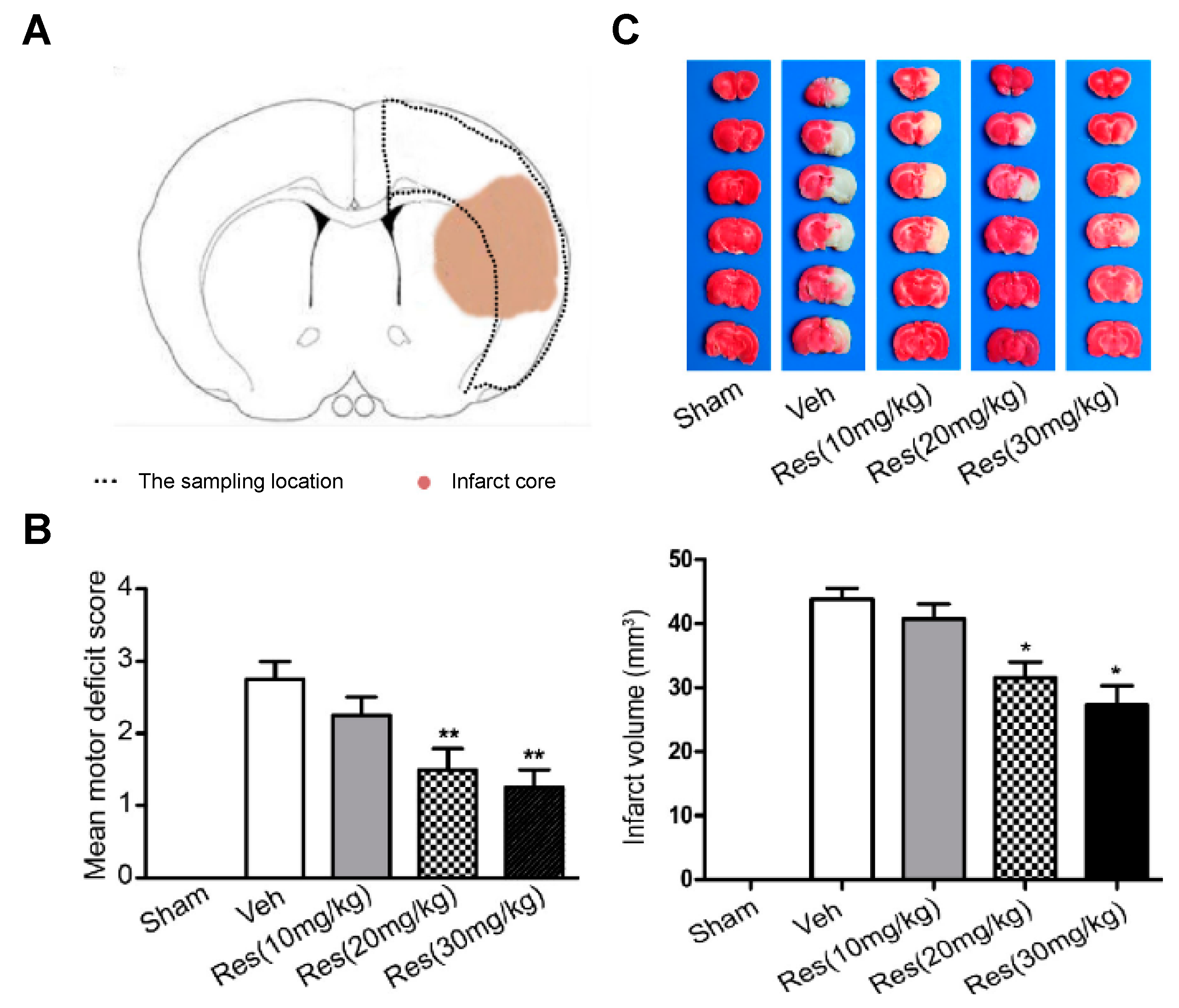
2.7. Via De Novo SUR1 Expression Inhibition
2.8. Administration of Resveratrol-Loaded Nanoparticles (NPs)
3. Conclusions
Herein we collected various recent studies describing the evaluation of the RSV effectiveness in the treatment of ischemic brain injury in different rodent animal models. RSV itself shares common properties with other polyphenol derivatives such as neuroprotection, anti-apoptotic, anti-inflammatory, and antioxidant characteristics. This has allowed RSV to be considered a promising small molecule drug for the treatment of age-related disorders. In this regard, data experiments collected from recent studies have shown different mechanisms of action in which RSV plays important roles in the management of the disease, such as cAMP/AMPK/SIRT1 regulation, JAK/ERK/STAT signaling pathway modulation, TLR4 signal transduction regulation gut/brain axis modulation, GLUT3 up-regulation inhibition, neuronal autophagy activation, and de novo SUR1 expression inhibition. The in vivo experiments that have been selected for this review may open novel perspectives and challenges for ischemic brain injuries with the potential to be translated from bench to bedside. While RSV has exhibited remarkable activities when it has been systemically administered, its poor bioavailability makes this drug unsuitable for clinical use. Interestingly, novel encapsulation strategies and the use of nanotechnology have emerged to overcome these drawbacks and might facilitate the transport of RSV at a specific site of action. Despite the fact that there are still no effective drugs approved for the treatment of cerebral ischemia, additional and detailed preclinical experiments should be performed involving a range of animal models with the aim to afford the most efficient therapy strategies, including new encapsulation techniques and the use of RSV-load NPs.
References
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290.
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178.
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891.
- Raj, P.; Lieben Louis, X.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71.
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349.
- Delmas, D.; Cornebise, C.; Courtaut, F.; Xiao, J.; Aires, V. New highlights of resveratrol: A review of properties against ocular diseases. Int. J. Mol. Sci. 2021, 22, 1295.
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123.
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Petersf, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305.
- Huang, J.; Huang, N.; Xu, S.; Luo, Y.; Li, Y.; Jin, H.; Yu, C.; Shi, J.; Jin, F. Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J. Nutr. Biochem. 2021, 88, 108552.
- Ding, Z.; Cao, J.; Shen, Y.; Zou, Y.; Yang, X.; Zhou, W.; Guo, Q.; Huang, C. Resveratrol promotes nerve regeneration via activation of p300 acetyltransferase-mediated VEGF signaling in a rat model of sciatic nerve crush injury. Front. Neurosci. 2018, 12, 341.
- Zhang, S.; Botchway, B.O.A.; Zhang, Y.; Liu, X. Resveratrol can inhibit Notch signaling pathway to improve spinal cord injury. Ann. Anat. 2019, 223, 100–107.
- Tang, K.S.; Tan, J.S. The protective mechanisms of polydatin in cerebral ischemia. Eur. J. Pharmacol. 2019, 842, 133–138.
- Tressera-Rimbau, A.; Arranz, S.; Eder, M.; Vallverdú-Queralt, A. Dietary polyphenols in the prevention of stroke. Oxid. Med. Cell. Longev. 2017, 2017, 7467962.
- Zou, L.; Gong, Y.; Liu, S.; Liang, S. Natural compounds acting at P2 receptors alleviate peripheral neuropathy. Brain Res. Bull. 2019, 151, 125–131.
- Cheng, Y.C.; Sheen, J.M.; Hu, W.L.; Hung, J.C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 8526438.
- Yao, Y.; Zhang, Y.; Liao, X.; Yang, R.; Lei, Y.; Luo, J. Potential therapies for cerebral edema after ischemic stroke: A mini review. Front. Aging Neurosci. 2021, 12, 618819.
- Shao, A.; Lin, D.; Wang, L.; Tu, S.; Lenahan, C.; Zhang, J. Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis. 2020, 11, 1537–1566.
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795.
- Parrella, E.; Gussago, C.; Porrini, V.; Benarese, M.; Pizzi, M. From preclinical stroke models to humans: Polyphenols in the prevention and treatment of stroke. Nutrients 2021, 13, 85.
- López, M.; Dempsey, R.J.; Vemuganti, R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem. Int. 2015, 89, 75–82.
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: Potential application of natural compounds. Front Physiol. 2020, 11, 433.
- Mohsenpour, H.; Pesce, M.; Patruno, A.; Bahrami, A.; Pour, P.M.; Farzaei, M.H. A review of plant extracts and plant-derived natural compounds in the prevention/treatment of neonatal hypoxic-ischemic brain injury. Int. J. Mol. Sci 2021, 22, 833.
- Reyes-Corral, M.; Sola-Idígora, N.; de la Puerta, R.; Montaner, J.; Ybot-González, P. Nutraceuticals in the prevention of neonatal hypoxia–ischemia: A comprehensive review of their neuroprotective properties, mechanisms of action and future directions. Int. J. Mol. Sci. 2021, 22, 2524.
- Subedi, L.; Gaire, B.P. Phytochemicals as regulators of microglia/macrophages activation in cerebral ischemia. Pharmacol. Res. 2021, 165, 105419.
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19.
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195.
- Yu, P.; Wang, L.; Tang, F.; Zeng, L.; Zhou, L.; Song, X.; Jia, W.; Chen, J.; Yang, Q. Resveratrol pretreatment decreases ischemic injury and improves neurological function via Sonic Hedgehog signaling after stroke in rats. Mol. Neurobiol. 2017, 54, 212–226.
- Wan, D.; Zhou, Y.; Wang, K.; Hou, Y.; Hou, R.; Ye, X. Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Res. Bull. 2016, 121, 255–262.
- Chang, C.; Zhao, Y.; Song, G.; She, K. Resveratrol protects hippocampal neurons against cerebral ischemia-reperfusion injury via modulating JAK/ERK/STAT signaling pathways in rats. J. Neuroimmunol. 2018, 315, 9–14.
- Le, K.; Daliv, E.C.; Wu, S.; Qian, F.; Ali, A.I.; Yu, D.; Guo, Y. SIRT1-regulated HMGB1 release is partially involved in TLR4 signal transduction: A possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain injury. Int. Immunopharmacol. 2019, 75, 105779.
- Dou, Z.; Rong, X.; Zhao, E.; Zhang, L.; Lv, Y. Neuroprotection of resveratrol against focal cerebral ischemia/reperfusion injury in mice through a mechanism targeting gust-brain axis. Cell. Mol. Neurobiol. 2019, 39, 883–898.
- Gutiérrez Aguilar, G.F.; Alquisiras-Burgos, I.; Franco-Pérez, I.; Pineda-Ramírez, N.; Ortiz-Plata, A.; Torres, I.; Pedraza-Chaverri, J.; Aguilera, P. Resveratrol prevents GLUT3 up-regulation induced by middle cerebral artery occlusion. Brain Sci. 2020, 10, 651.
- Pineda-Ramírez, N.; Alquisiras-Burgos, I.; Ortiz-Plata, A.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M.; Aguilera, P. Resveratrol activates neuronal autophagy through AMPK in the ischemic brain. Mol. Neurobiol. 2020, 57, 1055–1069.
- Lu, X.; Dong, J.; Zheng, D.; Li, X.; Ding, D.; Xu, H. Reperfusion combined with intraarterial administration of resveratrol-loaded nanoparticles improved cerebral ischemia-reperfusion injury in rats. Nanomed. Nanotech. Biol. Med. 2020, 28, 102208.




