
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Deanna M Minich | -- | 2935 | 2022-08-25 16:36:19 | | | |
| 2 | Peter Tang | -34 word(s) | 2901 | 2022-08-26 03:20:47 | | |
Video Upload Options
Glutathione is a tripeptide that plays a pivotal role in critical physiological processes resulting in effects relevant to diverse disease pathophysiology such as maintenance of redox balance, reduction of oxidative stress, enhancement of metabolic detoxification, and regulation of immune system function. The diverse roles of glutathione in physiology are relevant to a considerable body of evidence suggesting that glutathione status may be an important biomarker and treatment target in various chronic, age-related diseases.
1. Introduction
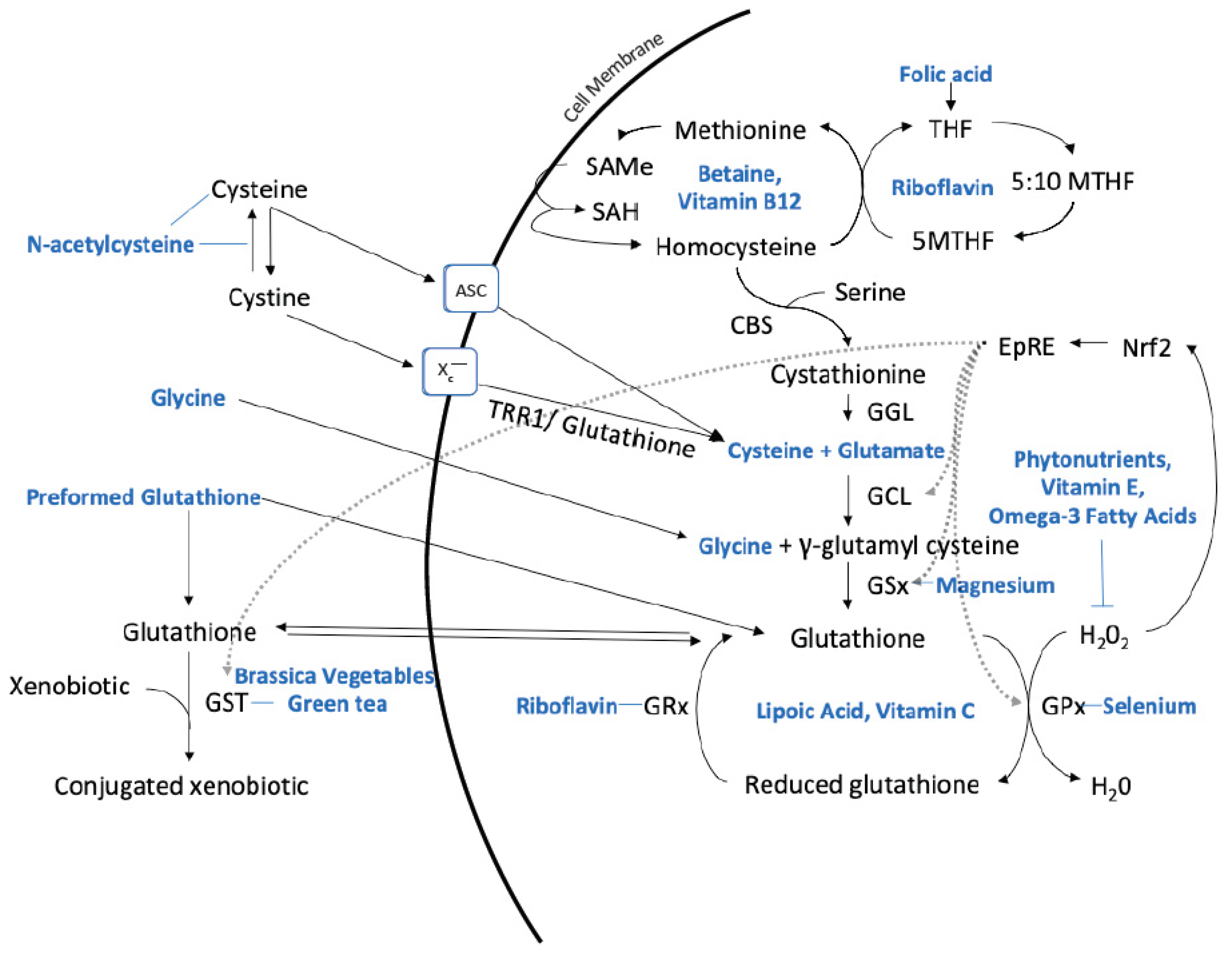
|
Research has found that many chronic diseases are associated with a reduction in glutathione levels, leading to the hypothesis that increasing glutathione levels can help prevent and/or mitigate the progression of these diseases. Below is a list of some of the diseases [2] and issues associated with glutathione dysregulation or deficiency [3]: |
|
• Alzheimer’s disease [16] |
|
• cancer [17] |
|
• chronic liver disease [18] |
|
• cognitive impairment [19] |
|
• cystic fibrosis [20] |
|
• human immunodeficiency virus (HIV)/ acquired immune deficiency syndrome (AIDS) [23] |
|
• hypertension [24] |
|
• infertility in both men and women [25] |
|
• lupus [26] |
|
• mental health disorders [27] |
|
• multiple sclerosis [28] |
|
• neurodegenerative disorders [29] |
|
• Parkinson’s disease [30] |
2. The Role of Gene Deletions and Single Nucleotide Polymorphisms (SNPs)
3. Optimizing Glutathione Production with Nutrients
4. Preformed Glutathione
5. N-Acetylcysteine (NAC)
6. Dietary Protein Considerations
7. Omega-3 Fatty Acids
8. Vitamins
8.1. B Vitamins
8.2. Vitamin C
8.3. Vitamin E
9. Other Nutrients
9.1. Alpha-Lipoic Acid
9.2. Selenium
9.3. Phytonutrients
9.4. Brassica Vegetables
9.5. Green Tea
9.6. Juice Studies
9.7. Herbs and Roots
9.8. Plant Foods that Contain Glutathione
References
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12.
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258.
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214.
- Pompella, A.; Emdin, M.; Franzini, M.; Paolicchi, A. Serum gamma-glutamyltransferase: linking together environmental pollution, redox equilibria and progression of atherosclerosis? Clin. Chem. Lab. Med. 2009, 47, 1583–1584.
- Lee, D.H.; Jacobs, D.R., Jr. Hormesis and public health: can glutathione depletion and mitochondrial dysfunction due to very low-dose chronic exposure to persistent organic pollutants be mitigated? J. Epidemiol. Commun. Health 2015, 69, 294–300.
- Halliwell, B. The antioxidant paradox: less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644.
- Feoli, A.M.; Siqueira, I.; Almeida, L.M.; Tramontina, A.C.; Battu, C.; Wofchuk, S.T.; Gottfried, C.; Perry, M.L.; Gonçalves, C.A. Brain glutathione content and glutamate uptake are reduced in rats exposed to pre- and postnatal protein malnutrition. J. Nutr. 2006, 136, 2357–2361.
- López-López, A.L.; Jaime, H.B.; Escobar Villanueva, M.D.C.; Padilla, M.B.; Palacios, G.V.; Aguilar, F.J.A. Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol. Behav. 2016, 161, 15–23.
- Lee, D.H.; Jacobs, D.R., Jr. Serum gamma-glutamyltransferase: new insights about an old enzyme. J Epidemiol. Commun. Health 2009, 63, 884–886.
- Hall, M.N.; Niedzwiecki, M.; Liu, X.; Harper, K.N.; Alam, S.; Slavkovich, V.; Gamble, M.V. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ. Health Perspect. 2013, 121, 1068–1074.
- Nebraska Redox Biology Center Educational Portal. Available online: http://genomics.unl.edu/RBC_EDU/gp.html (accessed on 7 July 2019).
- Allocati, N.; Masulli, M.; Di Ilio, C.; & Federici, L. Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 8.
- Lu, S.C. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999, 13, 1169–1183.
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492.
- Lang, C.A.; Mills, B.J.; Lang, H.L.; Liu, M.C.; Usui, W.M.; Richie, J., Jr.; Mastropaolo, W.; Murrell, S.A. High blood glutathione levels accompany excellent physical and mental health in women ages 60 to 103 years. J Lab. Clin. Med. 2002, 140, 413–417.
- Saharan, S.; Mandal, P.K. The emerging role of glutathione in Alzheimer’s disease. J. Alzheimers Dis. 2014, 40, 519–529.
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell Longev. 2013, 2013, 972913.
- Czuczejko, J.; Zachara, B.A.; Staubach-Topczewska, E.; Halota, W.; Kedziora, J. Selenium, glutathione and glutathione peroxidases in blood of patients with chronic liver diseases. Acta Biochim. Pol. 2003, 50, 1147–1154.
- Rae, C.D.; Williams, S.R. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal. Biochem. 2017, 529, 127–143.
- Kettle, A.J.; Turner, R.; Gangell, C.L.; Harwood, D.T.; Khalilova, I.S.; Chapman, A.L.; Winterbourn, C.C.; Sly, P.D.; Arest, C.F. Oxidation contributes to low glutathione in the airways of children with cystic fibrosis. Eur. Respir. J. 2014, 44, 122–129.
- Achari, A.E.; Jain, S.K. l-Cysteine supplementation increases insulin sensitivity mediated by upregulation of GSH and adiponectin in high glucose treated 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2017, 630, 54–65.
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011, 34, 162–167.
- Nguyen, D.; Hsu, J.W.; Jahoor, F.; Sekhar, R.V. Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 2014, 99, 169–177.
- Robaczewska, J.; Kedziora-Kornatowska, K.; Kozakiewicz, M.; Zary-Sikorska, E.; Pawluk, H.; Pawliszak, W.; Kedziora, J. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J. Physiol. Pharmacol. 2016, 67, 331–337.
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61–66.
- Shah, D.; Sah, S.; Nath, S.K. Interaction between glutathione and apoptosis in systemic lupus erythematosus. Autoimmun. Rev. 2013, 12, 741–751.
- Nucifora, L.G.; Tanaka, T.; Hayes, L.N.; Kim, M.; Lee, B.J.; Matsuda, T.; Nucifora, F.C., Jr.; Sedlak, T.; Mojtabai, R.; Eaton, W.; et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl. Psychiatry 2017, 7, e1215.
- Carvalho, A.N.; Lim, J.L.; Nijland, P.G.; Witte, M.E.; Van Horssen, J. Glutathione in multiple sclerosis: More than just an antioxidant? Mult. Scler. 2014, 20, 1425–1431.
- Aoyama, K.; Nakaki, T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013, 14, 21021–21044.
- Coles, L.D.; Tuite, P.J.; Öz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-dose oral N-Acetylcysteine in Parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol. 2018, 58, 158–167.
- Lux, O.; Naidoo, D. Biological variability of superoxide dismutase and glutathione peroxidase in blood. Redox Rep. 1995, 1, 331–335.
- Van ‘t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749.
- Nebert, D.W.; Vasiliou, V. Analysis of the glutathione S-transferase (GST) gene family. Hum. Genom. 2004, 1, 460–464.
- Sprenger, R.; Schlagenhaufer, R.; Kerb, R.; Bruhn, C.; Brockmöller, J.; Roots, I.; Brinkmann, U. Characterization of the glutathione S-transferase GSTT1 deletion: discrimination of all genotypes by polymerase chain reaction indicates a trimodular genotype-phenotype correlation. Pharmacogenetics 2000, 10, 557–565.
- Hollman, A.L.; Tchounwou, P.B.; Huang, H.C. The association between gene-environment interactions and diseases involving the human GST superfamily with SNP variants. Int. J. Environ. Res. Public Health 2016, 13, 379.
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760.
- Meister, A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol. Ther. 1991, 51, 155–194.
- Allen, J.; Bradley, R.D. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J. Altern. Complement. Med. 2011, 17, 827–833.
- Witschi, A.; Reddy, S.; Stofer, B.; Lauterburg, B.H. The systemic availability of oral glutathione. Eur. J. Clin. Pharmacol. 1992, 43, 667–669.
- Park, E.Y.; Shimura, N.; Konishi, T.; Sauchi, Y.; Wada, S.; Aoi, W.; Nakamura, Y.; Sato, K. Increase in the protein-bound form of glutathione in human blood after the oral administration of glutathione. J. Agric. Food Chem. 2014, 62, 6183–6189.
- Richie, J.P., Jr.; Nichenametla, S.; Neidig, W.; Calcagnotto, A.; Haley, J.S.; Schell, T.D.; Muscat, J.E. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur. J. Nutr. 2015, 54, 251–263.
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary glycine is rate-limiting for glutathione synthesis and may have broad potential for health protection. Ochsner J. 2018, 18, 81–87.
- U.S. National Library of Medicine. Amino Acids. Available online: https://medlineplus.gov/ency/article/002222.htm (accessed on June 26 2019).
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153.
- Parcell, S. Sulfur in human nutrition and applications in medicine. Altern. Med. Rev. 2002, 7, 22–44.
- Jones, D.P.; Park, Y.; Gletsu-Miller, N.; Liang, Y.; Yu, T.; Accardi, C.J.; Ziegler, T.R. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition 2011, 27, 199–205.
- Skvarc, D.R.; Dean, O.M.; Byrne, L.K.; Gray, L.; Lane, S.; Lewis, M.; Fernanders, B.S.; Berk, M.; Marriott, A. The effect of N-acetylcysteine (NAC) on human cognition—A systematic review. Neurosci. Biobehav. Rev. 2017, 78, 44–56.
- Bhutto, A.; Morley, J.E. The clinical significance of gastrointestinal changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 651–660.
- Corleto, V.D.; Festa, S.; Di Giulio, E.; Annibale, B. Proton pump inhibitor therapy and potential long-term harm. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 3–8.
- Dvorshchenko, K.O.; Bernyk, O.O.; Dranytsyna, A.S.; Senin, S.A.; Ostapchenko, L.I. Influence of oxidative stress on the level of genes expression Tgfb1 and Hgf in rat liver upon long-term gastric hypochlorhydria and administration of multiprobiotic Symbiter; Article in Ukrainian. Ukrains’ kyi Biokhimichnyi Zhurnal (1999) 2013, 85, 114–123.
- Naito, Y.; Yoshikawa, T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic. Biol. Med. 2002, 33, 323–336.
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J. Gastroenterol. 2017, 23, 563–572.
- Jackson, A.A.; Gibson, N.R.; Lu, Y.; Jahoor, F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am. J. Clin. Nutr. 2014, 80, 101–107.
- Rahman, I. Inflammation and the regulation of glutathione level in lung epithelial cells. Antioxid. Redox. Signal 1999, 1, 425–447.
- Duffy, S.L.; Lagopoulos, J.; Cockayne, N.; Lewis, S.J.; Hickie, I.B.; Hermens, D.F.; Naismith, S.L. The effect of 12-wk ω-3 fatty acid supplementation on in vivo thalamus glutathione concentration in patients “at risk” for major depression. Nutrition 2015, 31, 1247–1254.
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189.
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B₂) and oxidative stress: a review. Br. J. Nutr. 2014, 111, 1985–1991.
- Majchrzak, D.; Singer, I.; Männer, M.; Rust, P.; Genser, D.; Wagner, K.H.; Elmadfa, I. B-vitamin status and concentrations of homocysteine in Austrian omnivores, vegetarians and vegans. Ann. Nutr. Metab. 2006, 50, 485–491.
- Moat, S.J.; Ashfield-Watt, P.A.; Powers, H.J.; Newcombe, R.G.; McDowell, I.F. Effect of riboflavin status on the homocysteine-lowering effect of folate in relation to the MTHFR (C677T) genotype. Clin Chem. 2003, 49, 295–302.
- Slyshenkov, V.S.; Dymkowska, D.; Wojtczak, L. Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett. 2004, 569, 169–172.
- Misra, U.K.; Kalita, J.; Singh, S.K.; Rahi, S.K. Oxidative stress markers in Vitamin B12 deficiency. Mol. Neurobiol. 2017, 54, 1278–1284.
- Lenton, K.J.; Sané, A.T.; Therriault, H.; Cantin, A.M.; Payette, H.; Wagner, J.R. Vitamin C augments lymphocyte glutathione in subjects with ascorbate deficiency. Am. J. Clin. Nutr. 2003, 77, 189–195.
- Johnston, C.S.; Meyer, C.G.; Srilakshmi, J.C. Vitamin C elevates red blood cell glutathione in healthy adults. Am. J. Clin. Nutr. 1993, 58, 103–105.
- Chugh, S.N.; Kakkar, R.; Kalra, S.; Sharma, A. An evaluation of oxidative stress in diabetes mellitus during uncontrolled and controlled state and after vitamin E supplementation. J. Assoc. Physic. Ind. 1999, 47, 380–383.
- Sharma, A.; Kharb, S.; Chugh, S.N.; Kakkar, R.; Singh, G.P. Evaluation of oxidative stress before and after control of glycemia and after vitamin E supplementation in diabetic patients. Metabolism 2000, 49, 160–162.
- Jain, S.K.; McVie, R.; Smith, T. Vitamin E supplementation restores glutathione and malondialdehyde to normal concentrations in erythrocytes of type 1 diabetic children. Diabetes Care. 2000, 23, 1389–1394.
- Gupta, S.; Sharma, T.K.; Kaushik, G.G.; Shekhawat, V.P. Vitamin E supplementation may ameliorate oxidative stress in type 1 diabetes mellitus patients. Clin Lab. 2011, 57, 379–386.
- Aghadavod, E.; Soleimani, A.; Hamidi, G.; Keneshlou, F.; Heidari, A.; Asemi, Z. Effects of high-dose vitamin E supplementation on markers of cardiometabolic risk and oxidative stress in patients with diabetic nephropathy: A randomized double-blinded controlled trial. Iran J. Kidney Dis. 2018, 12, 156–162.
- Kolesnichenko, L.S.; Kulinskiĭ, V.I.; Shprakh, V.V.; Bardymov, V.V.; Verlan, N.V.; Gubina, L.P.; Pensionerova, G.A.; Sergeeva, M.P.; Stanevich, L.M.; Filippova, G.T. The blood glutathione system in cerebral vascular diseases and its treatment with alpha-lipoic acid. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova 2008, 108, 36–40.
- Martins, V.D.; Manfredini, V.; Peralba, M.C.; Benfato, M.S. Alpha-lipoic acid modifies oxidative stress parameters in sickle cell trait subjects and sickle cell patients. Clin Nutr. 2009, 28, 192–197.
- Ansar, H.; Mazloom, Z.; Kazemi, F.; Hejazi, N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med. J. 2011, 32, 584–588.
- Georgakouli, K.; Deli, C.K.; Zalavras, A.; Fatouros, I.G.; Kouretas, D.; Koutedakis, Y.; Jamurtas, A.Z. A-lipoic acid supplementation up-regulates antioxidant capacity in adults with G6PD deficiency. Food Chem. Toxicol. 2013, 61, 69–73.
- Khalili, M.; Eghtesadi, S.; Mirshafiey, A.; Eskandari, G.; Sanoobar, M.; Sahraian, M.A.; Motevalian, A.; Norouzi, A.; Moftakhar, S.; Azimi, A. Effect of lipoic acid consumption on oxidative stress among multiple sclerosis patients: a randomized controlled clinical trial. Nutr. Neurosci. 2014, 17, 16–20.
- Zhao, L.; Hu, F.X. α-Lipoic acid treatment of aged type 2 diabetes mellitus complicated with acute cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3715–3719.
- Becker, K.; Pons-Kühnemann, J.; Fechner, A.; Funk, M.; Gromer, S.; Gross, H.J.; Grünert, A.; Schirmer, R.H. Effects of antioxidants on glutathione levels and clinical recovery from the malnutrition syndrome kwashiorkor--a pilot study. Redox Rep. 2005, 10, 215–226.
- Jariwalla, R.; Lalezari, J.; Cenko, D.; Mansour, S.E.; Kumar, A.; Gangapurkar, B.; Nakamura, D. Restoration of blood total glutathione status and lymphocyte function following alpha-lipoic acid supplementation in patients with HIV infection. J. Altern. Complement. Med. 2008, 14, 139–146.
- Song, E.; Su, C.; Fu, J.; Xia, X.; Yang, S.; Xiao, C.; Lu, B.; Chen, H.; Sun, Z.; Wu, S.; et al. Selenium supplementation shows protective effects against patulin-induced brain damage in mice via increases in GSH-related enzyme activity and expression. Life Sci. 2014, 109, 37–43.
- Richie, J.P., Jr.; Muscat, J.E.; Ellison, I.; Calcagnotto, A.; Kleinman, W.; El-Bayoumy, K. Association of selenium status and blood glutathione concentrations in blacks and whites. Nutr. Cancer 2011, 63, 367–375.
- Galan-Chilet, I.; Tellez-Plaza, M.; Guallar, E.; De Marco, G.; Lopez-Izquierdo, R.; Gonzalez-Manzano, I.; Carmen Tormos, M.; Martin-Nuñez, G.M.; Rojo-Martinez, G.; Saez, G.T.; et al. Plasma selenium levels and oxidative stress biomarkers: a gene-environment interaction population-based study. Free Radic. Biol. Med. 2014, 74, 229–236.
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237.
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78 (Suppl. S1), A18–A25.
- Fowke, J.H.; Morrow, J.D.; Motley, S.; Bostick, R.M.; Ness, R.M. Brassica vegetable consumption reduces urinary F2-isoprostane levels independent of micronutrient intake. Carcinogenesis 2006, 27, 2096–2102.
- Visioli, F.; Riso, P.; Grande, S.; Galli, C.; Porrini, M. Protective activity of tomato products on in vivo markers of lipid oxidation. Eur J. Nutr. 2003, 42, 201–206.
- Thompson, H.J.; Heimendinger, J.; Sedlacek, S.; Haegele, A.; Diker, A.; O’Neill, C.; Meinecke, B.; Wolfe, P.; Zhu, Z.; Jiang, W. 8-Isoprostane F2alpha excretion is reduced in women by increased vegetable and fruit intake. Am. J. Clin. Nutr. 2005, 82, 768–776.
- Thompson, H.J.; Heimendinger, J.; Haegele, A.; Sedlacek, S.M.; Gillette, C.; O’Neill, C.; Wolfe, P.; Conry, C. Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis 1999, 20, 2261–2266.
- Rink, S.M.; Mendola, P.; Mumford, S.L.; Poudrier, J.K.; Browne, J.R.; Eactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. Self-report of fruit and vegetable intake that meets the 5 a day recommendation is associated with reduced levels of oxidative stress biomarkers and increased levels of antioxidant defense in premenopausal women. J. Acad. Nutr. Diet. 2013, 113, 776–785.
- Minich, D.M. A Review of the Science of Colorful, Plant-Based Food and Practical Strategies for Eating the Rainbow. J. Nutr. Metab. 2019, 2019, 19.
- Hodges, R.E.; Minich, D.M. Modulation of metabolic detoxification pathways using foods and food-derived components: A scientific Review with clinical application. J. Nutr. Metab. 2015, 2015, 760689.
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126.
- Johnson, I.T. Cruciferous vegetables and risk of cancers of the gastrointestinal tract. Mol Nutr Food Res. 2018, 62, e1701000.
- Saleh, D.O.; Mansour, D.F.; Hashad, I.M.; Bakeer, R.M. Effects of sulforaphane on D-galactose-induced liver aging in rats: Role of keap-1/nrf-2 pathway. Eur. J. Pharmacol. 2019, 855, 40–49.
- Abdull Razis, A.F.; Konsue, N.; Ioannides, C. Isothiocyanates and xenobiotic detoxification. Mol. Nutr. Food Res. 2018, 62, e1700916.
- Clapper, M.L.; Szarka, C.E.; Pfeiffer, G.R.; Graham, T.A.; Balshem, A.M.; Litwin, S.; Goosenberg, E.B.; Frucht, H.; Engstrom, P.F. Preclinical and clinical evaluation of broccoli supplements as inducers of glutathione S-transferase activity. Clin. Cancer Res. 1997, 3, 25–30.
- Liu, P.; Zhang, M.; Xie, X.; Jin, J.; Holman, C.A.J. Green tea consumption and glutathione S-transferases genetic polymorphisms on the risk of adult leukemia. Eur. J. Nutr. 2017, 56, 603–612.
- Pourahmadi, Z.; Mahboob, S.; Saedisomeolia, A.; Reykandeh, M.T. The effect of tomato juice consumption on antioxidant status in overweight and obese females. Women Health 2015, 55, 795–804.
- Rangel-Huerta, O.D.; Aguilera, C.M.; Martin, M.V.; Soto, M.J.; Rico, M.C.; Vallejo, F.; Tomas-Barberan, F.; Perez-de-la-Cruz, A.J.; Gil, A.; Mesa, M.D. Normal or high polyphenol concentration in orange Juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J. Nutr. 2015, 145, 1808–1816.
- Ghavipour, M.; Sotoudeh, G.; Ghorbani, M. Tomato juice consumption improves blood antioxidative biomarkers in overweight and obese females. Clin. Nutr. 2015, 34, 805–809.
- Guo, C.; Wei, J.; Yang, J.; Xu, J.; Pang, W.; Jiang, Y. Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutr. Res. 2008, 28, 72–77.
- Singletary, K.W.; Rokusek, J.T. Tissue-specific enhancement of xenobiotic detoxification enzymes in mice by dietary rosemary extract. Plant Foods Hum. Nutr. 1997, 50, 47–53.
- Singletary, K.W. Rosemary extract and carnosol stimulate rat liver glutathione-S-transferase and quinone reductase activities. Cancer Lett. 1996, 100, 139–144.
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern Med. 2014, 14, 225.
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21.
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna, Y.; González-Rubio, M.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149.
- Sasaki, K.; Hatta, S.; Wada, K.; Ueda, N.; Yoshimura, T.; Endo, T.; Sakata, M.; Tanaka, T.; Haga, M. Effects of extract of Ginkgo biloba leaves and its constituents on carcinogen-metabolizing enzyme activities and glutathione levels in mouse liver. Life Sci. 2002, 70, 1657–1667.
- Lee, H.Y.; Kim, S.W.; Lee, G.H.; Choi, M.K.; Jung, H.W.; Kim, Y.J.; Kwon, H.J.; Chae, H.J. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complement. Altern Med. 2016, 16, 316.




