
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alejandro K. Samhan-Arias | + 2693 word(s) | 2693 | 2020-11-27 03:16:21 |
Video Upload Options
Lipid peroxidation refers to the process in which lipids are oxidized to generate lipid peroxides as a primary product. Cellular lipid peroxidation can occur through different reactions, but they can be categorized into enzyme and non-enzyme dependent reactions. The primary substrates in lipid peroxidation reactions are polyunsaturated lipids since carbon-carbon double bonds are susceptible to reactive oxygen species, such as the hydroxyl radical (HO•), which is a key radical that participates in peroxidation reactions.
1. Introduction
Oxidative stress and inflammation are linked to cancer development [1][2]. Mutations in the DNA, phosphorylation of kinases, or inactivation of phosphatases can alter the cell growth, cellular control of the division, cell death, cell fate, and cell motility, which are altered in angiogenesis, inflammation, and fuel cancer progression [3][4][5][6]. A progressive increase of reactive oxygen species (ROS) marks the transition steps from a healthy tissue towards an invasive carcinoma [7]. This trend is owed to cancer cells' metabolic aberrations to adapt strategies to escape from cell death. It occurs in the presence of compensatory upregulation of the genes coding antioxidant enzymes, preventing ROS induced cell death [2][5]. Therefore, a blockage of the antioxidant cellular defenses or pro-oxidant therapies' stimulation is suggested as potential strategies to fight against cancer [8][9]. In general, lipids peroxides ability to participate in anti-inflammatory and/or pro-inflammatory signaling cascades is defined by the length of the fatty acyl chain, the number of unsaturations, and the place where the oxidation account [10]. During lipid peroxidation, oxygen molecules are added to the unsaturated fatty acyl chain of non-polar lipids, increasing their water solubility and diffusion towards the membrane surface. Cyclooxygenases or lipoxygenases accessibility for their substrates is boosted, the generation of lipid metabolites linked to inflammation is prompted, and the interaction of specific proteins and receptors recognizing lipid oxidation products is promoted [11][12][13][14][15]. All these actions are part of the lipid-dependent inflammatory cascades. Moreover, lipid peroxides can be the substrate for certain enzymes such as phospholipases, which prevent the accumulation of lipid peroxides into the membrane, which might functionally damage membrane components such as proteins by induction of covalent modifications, which might compromise membrane permeability [16][17]. The release of fatty acids and oxidized fatty acids from phospholipids by the action of phospholipases is essential in inflammation [18]. In addition, polyunsaturated fatty acids (PUFA) and their related phospholipids are very well-known signaling molecules with pro-inflammatory and anti-inflammatory functions, but also sensitive substrates for peroxidation [19][20][21][22][23][24][25][26][27][28]. PUFA can promote cell life or cell death through complex signaling cascades related to the fatty acid structure and their oxidation products [29][30]. In cancer, the connection of some of these pathways with inflammation is being unveiled by identifying a group of lipid oxidation products, known as lipid pro-resolving mediators, which can resolve the inflammation [31][32][33]. Their discovery opens the opportunity to identify new potential drugs in cancer therapy [32]. Some lipid oxidation products have also gained attention since they are suggested as biomarkers for cancer development and recurrence [34][35]. In general, lipids' ability to participate in anti-inflammatory or pro-inflammatory signaling cascades depends on the lipid's nature and its degree of oxidation.
2. Non-Enzyme-Dependent Lipid Peroxidation
In the non-enzymatic reactions, the Fenton and Haber–Weiss reactions producing HO• are dependent on transition metals (i.e., iron [36][37]) for the initiation of the radical chain reactions required for lipid peroxidation. In addition to this radical formation, some authors have suggested that for the initiation of lipid peroxidation reactions, the formation of a complex between iron and the lipids is required [38]. In general, it is accepted that the initiation reaction starts when a hydrogen atom is abstracted from lipid, forming an alkyl radical [39]. HO• is preferred over other radicals to performed this abstraction [38][39][40][41][42][43]. Once the alkyl radical is formed, the chain-carrying a carbon radical reacts with oxygen, leading to an alkyl peroxyl radical formation. This radical can abstract hydrogen from an organic substrate, which can be another lipid, to form a hydroperoxide plus an organic radical or be added to alkenes, such as those present in the fatty acyl chains of PUFA present in phospholipids, which provide isolated double bonds [44]. This last reaction leads to the formation of a lipid hydroperoxide with a conjugated double bond. By reaction with metals, lipid radical reactions leading to lipid peroxidation can be reinitiated as part of the propagating radical reactions [45][46]. This process occurs when the hydroperoxides react with an oxidized metal forming an alkoxyl radical. In case the reaction involves a reduced metal, e.g., Fe2+, an alkyl peroxyl radical is generated, which also contributes to the propagation of the reaction. PUFA are lipid molecules priming the Fenton's reaction, as previously indicated. Arachidonic acid (AA) and the phospholipids containing this fatty acid are essential molecules since they are precursors of pro- and anti-inflammatory mediators, sometimes enriched at cellular locations identified as signaling platforms, such as the plasma membrane lipid rafts [47][48]. Lipid oxidation at this location during the inflammation process is relevant since lipid rafts are platforms required for cell activation in the immune system [49].
3. Radiation Inducing Lipid Peroxidation
HO• can also be generated by ionizing radiation [38], which is generally used and applied in patients to treat cancer. As previously indicated, HO• is a very reactive radical, leading to the generation of lipid hydroperoxides and oxidizing other biomolecules, including DNA. Radiation exposition leads to peroxides generation in membranes enriched with PUFA. Indeed, this fact should not be discarded as a relevant factor for cancer therapy's success in these patients [50][51][52][53]. Noteworthy, more efforts are required to shed light on the role that ionizing radiation generating lipid peroxides have in cancer cell death vs. other targets. These investigations could help in the characterization of pharmacological drugs prompting the cancer cell sensitivity to lipid hydroperoxides generated by ionizing radiation (i.e., glutathione peroxidase 4 (GPX4) inhibitors) and, therefore, better define or reduce the patient's exposure to ionizing radiation that can also damage non-tumoral tissues.
4. Enzyme-Dependent Lipid Peroxidation
The enzyme-dependent reactions are executed by peroxidases, which have been elegantly classified by Vlasova [54]. Based on this classification, lipids can be oxidized by proteins that possess a true peroxidase activity, myeloperoxidase, eosinophil peroxidase, lactoperoxidases, or by proteins that do not have a peroxidase activity but acquire a pseudo-peroxidase activity under certain conditions, i.e., cytochrome c [55][56][57][58][59] upon binding to cardiolipin or other hemeproteins in defined conditions [60][61][62][63]. Lipoxygenases (LOX), cyclooxygenases (COX), and some cytochrome P450s present a pseudo-peroxidase activity that generates intermediary lipid peroxides required in their catalytic cycles, which might be a substrate for other activities, i.e.: cyclization reactions in the case of COX. The coordinated iron or iron associated with the heme group is key in peroxidases' catalytic center. Compound I, compound II, and sometimes compound III are generally typical and associated with different iron valences [64]. The function of real peroxidases might depend upon the existence of binding pockets where substrates can settle and interact with the enzyme catalytic center and upon electrons donated by organic molecules, which might be protein amino acids acting as electron donors [54]. In heme-dependent pseudo-peroxidases, substrates accessibility to the enzyme coordinating sphere depends upon the enzyme catalytic center flexibility to swift from a metal hexa- to penta-coordination, a feature that can be influenced by the redox state or the interaction with a ligand, i.e., cytochrome c upon cardiolipin binding to the protein [55].
The main enzymes using AA to generate lipid hydroperoxides and derived metabolites as signaling molecules in mammals and in cancer are cyclooxygenases (COX), lipoxygenases (LOX), and P450 families [29][30][47][65]. COX-1 is constitutively expressed in many tissues and cell types, whereas COX-2 is an inducible cyclooxygenase isoform which activation has been reported in tumoral tissues [66][67][68][69][70][71]. Some studies have also pointed out that other peroxidases (like myeloperoxidase and eosinophil peroxidase) are released from infiltrating neutrophils and eosinophils in the tumor microenvironment [72][73][74][75] or from infiltrated macrophages can also generate lipid hydroperoxide [76]. Although myeloperoxidases are potential sources of lipid peroxides and some myeloperoxidases polymorphisms have been correlated with a higher risk of suffering pulmonary, ovarian, and gastric cancer [77][78][79], there is no correlation between their activity and the lipid peroxides derived from it with disease development. In contrast, anti-inflammatory drugs have been linked to a decreased risk of cancer development and decreased tumor growth rate [28]. Notably, in this context, overexpression of enzymes generating eicosanoids in breast, lung, and pancreas cancer has been reported [28]. In particular, prostaglandins (PGs) can stimulate mitogenesis by directly affecting fibroblasts, osteoblasts, and mammary cells. The production of the proinflammatory PG named prostaglandin E2 (PGE2) (Figure 1A) through COX-2 activity can be found in mutagenesis, angiogenesis, and cell migration processes associated with cancer (Table 1). An activation mechanism for COX-2 using human colorectal HT-29, and the human prostate carcinoma DU145 cell lines has been proposed [80]. A correlation between the production of PGE2 with the resistance of cancer cells to apoptosis has been found through activation of the P2Y2/Src/p38 signaling pathway, which leads to AA release from the membranes by overexpression of some PLA2 isoforms, and the overexpression of COX-2 with the subsequent PGE2 production [80].
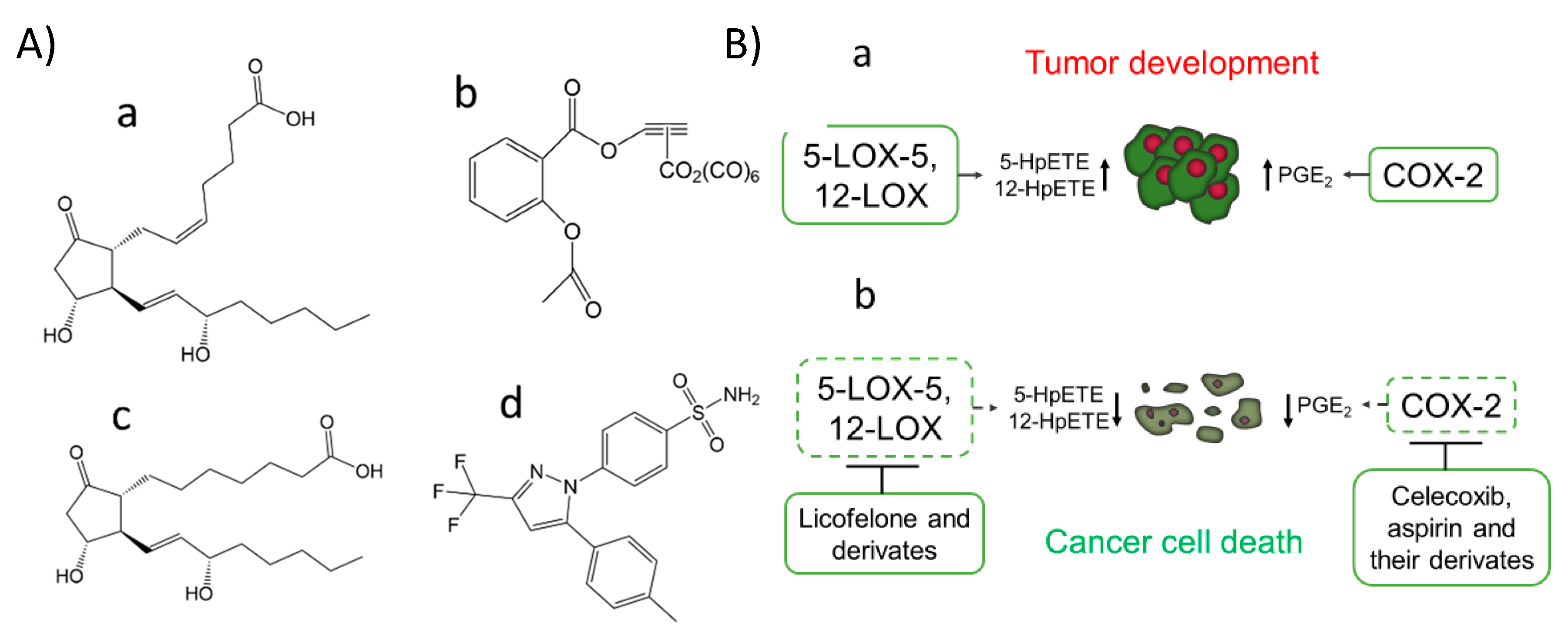
Figure 1. COX-2 inhibitors, PGE1, and PGE2 chemical structure (A). The prostaglandin E2 (PGE2) (pro-cancer) (a), the aspirin derivate named [2-acetoxy-(2-propynyl)benzoate]hexacarbonyldicobalt (Co-ASS) (b), the prostaglandin E1 (PGE1) (anticancer) (c) and the COX-2 selective inhibitor named celecoxib, (p-(5-p-Tolyl-3-(trifluoromethyl) pyrazol-1-yl)benzenesulfonamide) (d). Expression of COX-2, 5-LOX, and 12-LOX in cancer and the effect of inhibitors against these targets (B). Activation of 5-LOX-5, 12-LOX, and COX-2 has been reported in the development and progress of tumors from different tissues associated with the production of specific lipid peroxides and metabolites, such as PGE2 (a). Some inhibitors of these LOX isoforms and COX-2 have emerged as potential therapeutical agents for cancer treatment, modulating the production of previously commented metabolites and by induction of cancer cell death (b).
To better define the role of lipid peroxidation in mammals signaling, the type of lipid hydroperoxide generated should be finely characterized. In the metal-mediated lipid peroxidation based on the Fenton and Haber–Weiss reactions, random lipid peroxides are generated and differentiated from those formed by specialized enzymes that produce specific lipid signatures that can be used as fingerprints of enzymatic activities [58][81][82]. Many efforts are being made to find specific inhibitors that can provide a specific modulation of lipid hydroperoxide production that could act as mediators in signaling cascades [58][83]. For example, aspirin, which has beneficial effects in some cancer types, has been proposed to play that role [82][84][85]. Its implication in cancer has been associated with COX inhibition via acetylation of the active site, where AA binds. This accounts for COX-1 isozyme inhibition, while in COX-2, aspirin binding produces a structural rearrangement shifting the cyclooxygenase towards the lipoxygenase activity [86][87]. Therefore, AA oxygenation and cyclization to form a 15R-Prostaglandin endoperoxide is promoted, which favors the production of Prostaglandin D2 (PGD2) (with has a suggested function in inflammation resolution) instead of PGE2 [88]. Recent studies have also shown the potential therapeutically effect of aspirin organometallic derivatives as anticancer agents targeting COX-2 [89], such as the 4-[5-(4-Chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-benzenesulfonamide (SC-236) and [2-acetoxy-(2-propynyl)benzoate]hexacarbonyldicobalt (Co-ASS) (Figure 1A), which open a promising field in the search for inhibitors derived from aspirin [90][91][92].
Table 1. Correlation between COX-2 level and other biomarkers found in tumoral tissues
|
Tissue Location and Type of Cancer |
Correlation with Other Biomarkers |
|
Colon cancer [66], primary tumors and metastatic lymph nodes resections for colorectal adenocarcinoma [93], stage II and III colorectal cancer patients [94] |
High levels of COX-2 correlates with high levels of MMP-2 and VEGF expression and shorter survival time [93][94]. |
|
Cervical cancer [67] |
Multivariate analysis of COX-2 levels in tumor/stromal compartments. The proportion of CD3+, CD4+, and CD25+ cells was lower in tumors with high tumor/stroma ratios, but in these tumors, mast cells were increased [67]. |
|
No correlation between COX-2 expression and EGFR, and HER-2/neu status [96]. |
|
|
Elevated COX-2 expression associated with a large tumor size, a high histological grade, a negative hormone receptor status, a high proliferation rate, high p53 expression, and the presence of HER-2 oncogene amplification along with axillary node metastases and a ductal type of histology [98]. COX-2 inhibition may potentially prevent the development of ER-positive and ER-negative breast cancers [98]. Expression of PGE2 and IL-8 [101]. COX-2 over-expression induces an oncogenic microRNA (miR655) in human breast cancer cells by activation of EP4 [102]. |
|
|
COX-2 expression stabilizes survivin, an inhibitor of apoptosis (IAP) [103]. CacyBP expression was significantly negatively associated with the COX expression [104]. |
|
|
Correlation between HER-2, EGFR, and COX-2 expression in patients of non-small cell lung cancer at different degrees [69] |
|
|
Laryngeal cancer [71] |
Cox-2 overexpression was significantly associated with radioresistant tumors [71]. |
|
Papillary thyroid cancer [106] |
The expression of COX-2 is increased with age in papillary thyroid cancer [106]. Immunohistochemically, expression of COX-2 and VEGF-C correlated strongly, and both were induced by the tumor promoter phorbol 12-myristate 13-acetate [107]. |
|
No correlation between COX-2 expression with estrogen (ER) or progesterone receptor (PR), p53, and neu [110]. Correlation between COX-2 (59%) and aromatase (65%) expression but not estrogen and progesterone receptor [111]. |
|
|
Invasive gallbladder cancer [112] |
COX-2, c-Met, β-catenin, c-erbB2 and EGFR were over-expressed in 80%, 74%, 71%, 62%, and 11% of invasive gallbladder cancers, respectively [112]. |
|
Prostate cancer Metastatic primary prostate carcinoma compared to non-metastatic cancers [113][114][115][116] |
COX-2 and Ki-67 antigen co-expression in 42.9% and 67% of the prostate cancer patients [113]. Patients with PSA > 7 ng/mL and high COX-2 expression had the highest probability of recurrence [114]. The expressions of COX-2 and E-cadherin are very firmly and inversely correlated as prognostic indicators. [115]. High expression of COX-2, TGF-beta, and Ki67 in metastatic primary prostate carcinoma was associated with death from prostate carcinoma [116]. |
|
A positive correlation between COX-2 and K-ras expression with the depth of invasion and lymph node metastasis in gastric cancer [117]. Epithelial MMP-2 expression in gastric cancer is associated with aggressive forms, COX-2 expression, and poor survival [118]. |
|
|
Cervical cancer [119] |
DNA hypermethylation of the COX-2 gene may be a potential prognostic marker in the early stages of cervical cancer [119]. |
|
Anaplastic pancreatic cancer [122] |
Tumor COX-2 expression portends a poor prognosis for patients with resected adenocarcinoma of the pancreas, particularly in tumors > or = 3 cm [121]. Expression of L1CAM, COX-2, and EGFR in the majority of undifferentiated pancreatic carcinomas [122]. |
5. Lipid Peroxidation Derived Products and Biological Targets
Lipid hydroperoxides generated via enzyme or non-enzyme-dependent reactions can be further oxidized to form highly reactive species and lipid autoxidation products. Acrolein, malonaldehyde, and 4-hydroxynonenal can covalently modify proteins leading to functional and structural changes in proteins [123][124][125]. Lipid autoxidation products mainly react with primary amines and lysines, histidine, and cysteine residues from proteins to induce covalent crosslinking and prompt protein aggregation [125]. The amino acid residues mentioned above are also the primary targets for several protein post-transductional modifications, such as acylation, acetylation, phosphorylation, methylation, glycation, and S-nitrosylation, among other modifications [126][127]. Therefore, it can be presumed that the reaction of essential amino acid residues with lipid autoxidation products will also induce changes in the signaling pathways in which these proteins are involved. The generation of lipid autoxidation products has been reported in cancer development, angiogenesis, and invasiveness [128][129][130][131][132]. Some autoxidation products, such as 4-hydroxynonenal, have been implicated in DNA modifications that generate cancer-linked mutations [1].
6. Antioxidants against Lipid Radical Reactions and Peroxidases
Antioxidants play a central role to counteract lipid peroxidation. In the non-enzyme-dependent reactions, the radical chain reactions can terminate when antioxidants react with the alkyl peroxyl or the alkoxyl radicals. Tocopherol is the main membrane antioxidant in charge of reacting with these radicals and one of the primary membrane antioxidants against reactions generating lipid peroxides, in general [133]. Consequently, a lipid hydroperoxide or the alcohol and the radical antioxidant are products of the reaction between the lipid radical with the antioxidant. In the particular case of the tocopheroxyl radical, it can be reduced back to tocopherol by its reaction with other antioxidants, such as ascorbate and ubiquinol [134], or by enzymes in charge of reducing the antioxidant radical [135][136]. Indirectly or directly, the enzymatic activities that reduce the radicals derived from antioxidants are essential to keep optimal alpha-tocopherol levels in the membrane [135][137][138]. Other types of enzymes, such as glutathione peroxidases, can reduce lipid hydroperoxides to alcohols at the expense of glutathione (GSH). GPX4 is a pharmacological target in cancer [139][140][141], and its inhibition has been found to induce cancer cell death by the accumulation of lipid hydroperoxides [140].
References
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbeck’s Arch. Surg. 2006, 391, 499–510, doi:10.1007/s00423-006-0073-1.
- Klaunig, J.E.; Kamendulis, L.M. The Role of Oxidative Stress in Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267, doi:10.1146/annurev.pharmtox.44.101802.121851.
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324, doi:10.1038/nri.2017.142.
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771, doi:10.1038/nrc3611.
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947, doi:10.1038/nrd4002.
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb Perspect Med 2015, 5, a006098, doi:10.1101/cshperspect.a006098.
- Chaiswing, L.; St Clair, W.H.; St Clair, D.K. Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer. Antioxid. Redox Signal. 2018, 29, 1237–1272, doi:10.1089/ars.2017.7485.
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2016, 8, 15996.
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203, doi:10.1038/s12276-020-0384-2.
- Colombo, S.; Melo, T.; Martínez-López, M.; Carrasco, M.J.; Domingues, M.R.; Pérez-Sala, D.; Domingues, P. Phospholipidome of endothelial cells shows a different adaptation response upon oxidative, glycative and lipoxidative stress. Sci. Rep. 2018, 8, 12365, doi:10.1038/s41598-018-30695-0.
- Hazen, S.L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 2008, 283, 15527–15531, doi:10.1074/jbc.R700054200.
- Dennis, E.A. Liberating Chiral Lipid Mediators, Inflammatory Enzymes, and LIPID MAPS from Biological Grease. J. Biol. Chem. 2016, 291, 24431–24448, doi:10.1074/jbc.X116.723791.
- Freigang, S. The regulation of inflammation by oxidized phospholipids. Eur. J. Immunol. 2016, 46, 1818–1825, doi:10.1002/eji.201545676.
- Miller, Y.I.; Shyy, J.Y.J. Context-Dependent Role of Oxidized Lipids and Lipoproteins in Inflammation. Trends Endocrinol Metab 2017, 28, 143–152, doi:10.1016/j.tem.2016.11.002.
- Dias, I.H.K.; Milic, I.; Heiss, C.; Ademowo, O.S.; Polidori, M.C.; Devitt, A.; Griffiths, H.R. Inflammation, Lipid (Per)oxidation, and Redox Regulation. Antioxid. Redox Signal. 2020, 33, 166–190, doi:10.1089/ars.2020.8022.
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498, doi:10.1039/c5sc02311d.
- Catalá, A. Lipid peroxidation modifies the picture of membranes from the “Fluid Mosaic Model” to the “Lipid Whisker Model”. Biochimie 2012, 94, 101–109, doi:https://doi.org/10.1016/j.biochi.2011.09.025.
- Mouchlis, V.D.; Dennis, E.A. Phospholipase A(2) catalysis and lipid mediator lipidomics. Biochim. Et Biophys. Acta. Mol. Cell Biol. Lipids 2019, 1864, 766–771, doi:10.1016/j.bbalip.2018.08.010.
- Xu, Y.; Qian, S.Y. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed J 2014, 37, 112–119, doi:10.4103/2319-4170.131378.
- Anderson, D.W.; Crowle, A.J. Regression and differentiation of neuroblastoma tumors in mice treated with differentiating agents-prostaglandin E1 and a phosphodiesterase inhibitor, RO 20-1724. Cancer Lett. 1982, 16, 287–295, doi:https://doi.org/10.1016/0304-3835(82)90009-X.
- Hanazaki, K.; Kajikawa, S.; Fujimori, Y.; Nakata, S.; Shimozawa, N.; Koide, N.; Adachi, W.; Amano, J. Effects of prostaglandin E1 administration during hepatectomy for cirrhotic hepatocellular carcinoma. Hepatogastroenterology 2000, 47, 461–464.
- Wang, X.; Lin, H.; Gu, Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 11, 25, doi:10.1186/1476-511x-11-25.
- Davies, P.; Bailey, P.J.; Goldenberg, M.M.; Ford-Hutchinson, A.W. The Role of Arachidonic Acid Oxygenation Products in Pain and Inflammation. Annu. Rev. Immunol. 1984, 2, 335–357, doi:10.1146/annurev.iy.02.040184.002003.
- Castellone, M.D.; Teramoto, H.; Williams, B.O.; Druey, K.M.; Gutkind, J.S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science 2005, 310, 1504–1510, doi:10.1126/science.1116221.
- Pai, R.; Soreghan, B.; Szabo, I.L.; Pavelka, M.; Baatar, D.; Tarnawski, A.S. Prostaglandin E2 transactivates EGF receptor: A novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 2002, 8, 289–293, doi:10.1038/nm0302-289.
- Schrey, M.P.; Patel, K.V. Prostaglandin E2 production and metabolism in human breast cancer cells and breast fibroblasts. Regulation by inflammatory mediators. Br J Cancer 1995, 72, 1412–1419, doi:10.1038/bjc.1995.523.
- Uotila, P. Inhibition of prostaglandin E2 formation and histamine action in cancer immunotherapy. Cancer Immunol Immunother 1993, 37, 251–254, doi:10.1007/bf01518519.
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Reviews. Cancer 2010, 10, 181–193, doi:10.1038/nrc2809.
- Tachtsis, B.; Whitfield, J.; Hawley, J.A.; Hoffman, N.J. Omega-3 Polyunsaturated Fatty Acids Mitigate Palmitate-Induced Impairments in Skeletal Muscle Cell Viability and Differentiation. Front. Physiol. 2020, 11, doi:10.3389/fphys.2020.00563.
- Magtanong, L.; Ko, P.J.; Dixon, S.J. Emerging roles for lipids in non-apoptotic cell death. Cell Death Differ. 2016, 23, 1099–1109, doi:10.1038/cdd.2016.25.
- Khadge, S.; Sharp, J.G.; McGuire, T.R.; Thiele, G.M.; Talmadge, J.E. Lipid Inflammatory Mediators in Cancer Progression and Therapy. In Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy, Kalinski, P., Ed. Springer International Publishing: Cham, 2017; pp. 145-156.
- Zhang, Q.; Zhu, B.; Li, Y. Resolution of Cancer-Promoting Inflammation: A New Approach for Anticancer Therapy. Front. Immunol. 2017, 8, 71–71, doi:10.3389/fimmu.2017.00071.
- Ungaro, F.; D’Alessio, S.; Danese, S. The Role of Pro-Resolving Lipid Mediators in Colorectal Cancer-Associated Inflammation: Implications for Therapeutic Strategies. Cancers 2020, 12, 2060.
- Rigas, B.; Goldman, I.S.; Levine, L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med 1993, 122, 518–523, doi:0022-2143(93)90010-V.
- Wang, D.; DuBois, R.N. Cyclooxygenase-2: A potential target in breast cancer. Semin. Oncol. 2004, 31, 64–73, doi:https://doi.org/10.1053/j.seminoncol.2004.01.008.
- Koppenol, W.H. The centennial of the Fenton reaction. Free Radic Biol Med 1993, 15, 645–651, doi:0891-5849(93)90168-T.
- Koppenol, W.H. The Haber-Weiss cycle – 70 years later. Redox Rep. 2001, 6, 229–234, doi:10.1179/135100001101536373.
- Halliwell, B.; Gutteridge, J.M.C. Free radicals in biology and medicine, 4th ed. ed.; Oxford University Press: Oxford, 2007.
- Johnson, D.R.; Decker, E.A. The Role of Oxygen in Lipid Oxidation Reactions: A Review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190, doi:10.1146/annurev-food-022814-015532.
- Schafer, F.Q.; Qian, S.Y.; Buettner, G.R. Iron and free radical oxidations in cell membranes. Cell Mol Biol (Noisy-Le-Grand) 2000, 46, 657–662.
- Qian, S.Y.; Buettner, G.R. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: An electron paramagnetic resonance spin trapping study. Free Radic. Biol. Med. 1999, 26, 1447–1456, doi:10.1016/S0891-5849(99)00002-7.
- Minotti, G.; Aust, S.D. The role of iron in the initiation of lipid peroxidation. Chem. Phys. Lipids 1987, 44, 191–208, doi:10.1016/0009-3084(87)90050-8.
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. Biochim. Et Biophys. Acta (Bba)-Biomembr. 2012, 1818, 2374–2387, doi:10.1016/j.bbamem.2012.02.002.
- Pratt, D.A.; Tallman, K.A.; Porter, N.A. Free radical oxidation of polyunsaturated lipids: New mechanistic insights and the development of peroxyl radical clocks. Acc Chem Res 2011, 44, 458–467, doi:10.1021/ar200024c.
- Repetto, M.G.; Ferrarotti, N.F.; Boveris, A. The involvement of transition metal ions on iron-dependent lipid peroxidation. Arch Toxicol 2010, 84, 255–262, doi:10.1007/s00204-009-0487-y.
- Repetto, M.; Boveris, A. Transition metals: Bioinorganic and redox reactions in biological systems. Transition Metals: Characteristics, Properties and Uses, New York, USA, 2011, 349-370.
- Pike, L.J.; Han, X.; Chung, K.-N.; Gross, R.W. Lipid Rafts Are Enriched in Arachidonic Acid and Plasmenylethanolamine and Their Composition Is Independent of Caveolin-1 Expression: A Quantitative Electrospray Ionization/Mass Spectrometric Analysis. Biochemistry 2002, 41, 2075–2088, doi:10.1021/bi0156557.
- Gutierrez-Merino, C.; Marques-da-Silva, D.; Fortalezas, S.; Samhan-Arias, A.K. The critical role of lipid rafts nanodomains in the cross-talk between calcium and reactive oxygen and nitrogen species in cerebellar granule neurons apoptosis by extracellular potassium deprivation. Aims Mol. Sci. 2016, 3, 12–29, doi:10.3934/molsci.2016.1.12.
- Yaqoob, P. Fatty acids as gatekeepers of immune cell regulation. Trends Immunol. 2003, 24, 639–645, doi:10.1016/j.it.2003.10.002.
- Tyurina, Y.Y.; Tyurin, V.A.; Kapralova, V.I.; Amoscato, A.A.; Epperly, M.W.; Greenberger, J.S.; Kagan, V.E. Mass-Spectrometric Characterization of Phospholipids and Their Hydroperoxide Derivatives In Vivo: Effects of Total Body Irradiation. In Lipidomics: Volume 2: Methods and Protocols, Armstrong, D., Ed. Humana Press: Totowa, NJ, 2010; pp. 153-183.
- Stark, G. The effect of ionizing radiation on lipid membranes. Biochim. Et Biophys. Acta (Bba)-Rev. Biomembr. 1991, 1071, 103–122, doi:https:10.1016/0304-4157(91)90020-W.
- Corre, I.; Niaudet, C.; Paris, F. Plasma membrane signaling induced by ionizing radiation. Mutat. Res. /Rev. Mutat. Res. 2010, 704, 61–67, doi:10.1016/j.mrrev.2010.01.014.
- Emerit, J.; Klein, J.M.; Coutellier, A.; Congy, F. [Free radicals and lipid peroxidation in cell biology: Physiopathologic prospects]. Pathol Biol (Paris) 1991, 39, 316–327.
- Vlasova, I.I. Peroxidase Activity of Human Hemoproteins: Keeping the Fire under Control. Molecules 2018, 23, 2561.
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V., et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 2005, 1, 223–232, doi:10.1038/nchembio727.
- Ji, J.; Kline, A.E.; Amoscato, A.; Samhan-Arias, A.K.; Sparvero, L.J.; Tyurin, V.A.; Tyurina, Y.Y.; Fink, B.; Manole, M.D.; Puccio, A.M., et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 2012, 15, 1407–1413, doi:10.1038/nn.3195.
- Atkinson, J.; Kapralov, A.A.; Yanamala, N.; Tyurina, Y.Y.; Amoscato, A.A.; Pearce, L.; Peterson, J.; Huang, Z.; Jiang, J.; Samhan-Arias, A.K., et al. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nat Commun 2011, 2, 497, doi:10.1038/ncomms1499.
- Samhan-Arias, A.K.; Tyurina, Y.Y.; Kagan, V.E. Lipid antioxidants: Free radical scavenging versus regulation of enzymatic lipid peroxidation. J. Clin. Biochem. Nutr. 2011, 48, 91–95, doi:10.3164/jcbn.11-009FR.
- Belikova, N.A.; Tyurina, Y.Y.; Borisenko, G.; Tyurin, V.; Samhan Arias, A.K.; Yanamala, N.; Furtmuller, P.G.; Klein-Seetharaman, J.; Obinger, C.; Kagan, V.E. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: Antioxidant function in mitochondria. J Am Chem Soc 2009, 131, 11288–11289, doi:10.1021/ja904343c.
- Samhan-Arias, A.K.; Cordas, C.M.; Carepo, M.S.; Maia, L.B.; Gutierrez-Merino, C.; Moura, I.; Moura, J.J.G. Ligand accessibility to heme cytochrome b5 coordinating sphere and enzymatic activity enhancement upon tyrosine ionization. J. Biol. Inorg. Chem. : Jbic : A Publ. Soc. Biol. Inorg. Chem. 2019, 24, 317–330, doi:10.1007/s00775-019-01649-2.
- Samhan-Arias, A.K.; Maia, L.B.; Cordas, C.M.; Moura, I.; Gutierrez-Merino, C.; Moura, J.J.G. Peroxidase-like activity of cytochrome b5 is triggered upon hemichrome formation in alkaline pH. Biochim Biophys Acta Proteins Proteom 2018, 1866, 373–378, doi:10.1016/j.bbapap.2017.09.010.
- Ascenzi, P.; Coletta, M.; Wilson, M.T.; Fiorucci, L.; Marino, M.; Polticelli, F.; Sinibaldi, F.; Santucci, R. Cardiolipin–cytochrome c complex: Switching cytochrome c from an electron-transfer shuttle to a myoglobin- and a peroxidase-like heme-protein. Iubmb Life 2015, 67, 98–109, doi:10.1002/iub.1350.
- Beckerson, P.; Svistunenko, D.; Reeder, B. Effect of the distal histidine on the peroxidatic activity of monomeric cytoglobin. F1000Res 2015, 4, 87–87, doi:10.12688/f1000research.5971.1.
- Poulos, T.L. Heme enzyme structure and function. Chem Rev 2014, 114, 3919–3962, doi:10.1021/cr400415k.
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438, doi:10.1155/2014/360438.
- Eberhart, C.E.; Coffey, R.J.; Radhika, A.; Giardiello, F.M.; Ferrenbach, S.; Dubois, R.N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994, 107, 1183–1188, doi:10.1016/0016-5085(94)90246-1.
- Ferrandina, G.; Lauriola, L.; Zannoni, G.F.; Distefano, M.G.; Legge, F.; Salutari, V.; Gessi, M.; Maggiano, N.; Scambia, G.; Ranelletti, F.O. Expression of cyclooxygenase-2 (COX-2) in tumour and stroma compartments in cervical cancer: Clinical implications. Br J Cancer 2002, 87, 1145–1152, doi:10.1038/sj.bjc.6600578.
- Tian, F.; Wang, T.Y.; Gong, M.; Lu, X.M.; Hu, J.; Wang, J.; Zhang, C.H. [Overexpression of COX-2 and its clinical significance in non-small cell lung cancer]. Zhonghua Wai Ke Za Zhi 2003, 41, 407–410.
- Brattstrom, D.; Wester, K.; Bergqvist, M.; Hesselius, P.; Malmstrom, P.U.; Nordgren, H.; Wagenius, G.; Brodin, O. HER-2, EGFR, COX-2 expression status correlated to microvessel density and survival in resected non-small cell lung cancer. Acta Oncol 2004, 43, 80–86, doi:10.1080/02841860310017441.
- Liao, Z.; Milas, L. COX-2 and its inhibition as a molecular target in the prevention and treatment of lung cancer. Expert Rev. Anticancer Ther. 2004, 4, 543–560, doi:10.1586/14737140.4.4.543.
- Nix, P.; Lind, M.; Greenman, J.; Stafford, N.; Cawkwell, L. Expression of Cox-2 protein in radioresistant laryngeal cancer. Ann. Oncol. 2004, 15, 797–801, doi:10.1093/annonc/mdh185.
- Panagopoulos, V.; Leach, D.A.; Zinonos, I.; Ponomarev, V.; Licari, G.; Liapis, V.; Ingman, W.V.; Anderson, P.; DeNichilo, M.O.; Evdokiou, A. Inflammatory peroxidases promote breast cancer progression in mice via regulation of the tumour microenvironment. Int J Oncol 2017, 50, 1191–1200, doi:10.3892/ijo.2017.3883.
- Tabariès, S.; Ouellet, V.; Hsu, B.E.; Annis, M.G.; Rose, A.A.N.; Meunier, L.; Carmona, E.; Tam, C.E.; Mes-Masson, A.-M.; Siegel, P.M. Granulocytic immune infiltrates are essential for the efficient formation of breast cancer liver metastases. Breast Cancer Res. 2015, 17, 45, doi:10.1186/s13058-015-0558-3.
- Samoszuk, M.; Lin, F.; Rim, P.; Strathearn, G. New marker for blood vessels in human ovarian and endometrial cancers. Clin. Cancer Res. 1996, 2, 1867–1871.
- Cormier, S.A.; Taranova, A.G.; Bedient, C.; Nguyen, T.; Protheroe, C.; Pero, R.; Dimina, D.; Ochkur, S.I.; O’Neill, K.; Colbert, D., et al. Pivotal Advance: Eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J. Leukoc. Biol. 2006, 79, 1131–1139, doi:10.1189/jlb.0106027.
- Hou, Z.; Falcone, D.J.; Subbaramaiah, K.; Dannenberg, A.J. Macrophages induce COX-2 expression in breast cancer cells: Role of IL-1beta autoamplification. Carcinogenesis 2011, 32, 695–702, doi:10.1093/carcin/bgr027.
- Mustea, A.; Heinze, G.; Sehouli, J.; Koensgen, D.; Wolf, A.; Gutu, L.; Sofroni, D.; Pirvulescu, C.; Braicu, E.I.; Schuster, E., et al. The -463G/A polymorphism in myeloperoxidase gene and cervical cancer. Anticancer Res 2007, 27, 1531–1535.
- Zhu, H.; Yang, L.; Zhou, B.; Yu, R.; Tang, N.; Wang, B. Myeloperoxidase G-463A polymorphism and the risk of gastric cancer: A case-control study. Carcinogenesis 2006, 27, 2491–2496, doi:10.1093/carcin/bgl121.
- London, S.J.; Lehman, T.A.; Taylor, J.A. Myeloperoxidase genetic polymorphism and lung cancer risk. Cancer Res 1997, 57, 5001–5003.
- Limami, Y.; Pinon, A.; Leger, D.Y.; Pinault, E.; Delage, C.; Beneytout, J.-L.; Simon, A.; Liagre, B. The P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic acid-induced apoptosis in colorectal and prostate cancer cells. Biochimie 2012, 94, 1754–1763, doi:https:10.1016/j.biochi.2012.04.006.
- Berger, S.; Weichert, H.; Porzel, A.; Wasternack, C.; Kuhn, H.; Feussner, I. Enzymatic and non-enzymatic lipid peroxidation in leaf development. Biochim Biophys Acta 2001, 1533, 266–276, doi:10.1016/s1388-1981(01)00161-5.
- Stoyanovsky, D.A.; Tyurina, Y.Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.A.; Protchenko, O.; Jadhav, S.; Bolevich, S.B.; Kozlov, A.V.; Vladimirov, Y.A., et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic. Biol. Amp; Med. 2019, 133, 153–161, doi:10.1016/j.freeradbiomed.2018.09.008.
- Noguchi, N.; Yamashita, H.; Hamahara, J.; Nakamura, A.; Kuhn, H.; Niki, E. The specificity of lipoxygenase-catalyzed lipid peroxidation and the effects of radical-scavenging antioxidants. Biol Chem 2002, 383, 619–626, doi:10.1515/BC.2002.064.
- Drew, D.A.; Cao, Y.; Chan, A.T. Aspirin and colorectal cancer: The promise of precision chemoprevention. Nat. Reviews. Cancer 2016, 16, 173–186, doi:10.1038/nrc.2016.4.
- Elwood, P.C.; Morgan, G.; Pickering, J.E.; Galante, J.; Weightman, A.L.; Morris, D.; Kelson, M.; Dolwani, S. Aspirin in the Treatment of Cancer: Reductions in Metastatic Spread and in Mortality: A Systematic Review and Meta-Analyses of Published Studies. Plos One 2016, 11, e0152402–e0152402, doi:10.1371/journal.pone.0152402.
- Dong, L.; Anderson, A.J.; Malkowski, M.G. Arg-513 and Leu-531 Are Key Residues Governing Time-Dependent Inhibition of Cyclooxygenase-2 by Aspirin and Celebrex. Biochemistry 2019, 58, 3990–4002, doi:10.1021/acs.biochem.9b00659.
- Lucido, M.J.; Orlando, B.J.; Vecchio, A.J.; Malkowski, M.G. Crystal Structure of Aspirin-Acetylated Human Cyclooxygenase-2: Insight into the Formation of Products with Reversed Stereochemistry. Biochemistry 2016, 55, 1226–1238, doi:10.1021/acs.biochem.5b01378.
- Giménez-Bastida, J.A.; Boeglin, W.E.; Boutaud, O.; Malkowski, M.G.; Schneider, C. Residual cyclooxygenase activity of aspirin-acetylated COX-2 forms 15 R-prostaglandins that inhibit platelet aggregation. Faseb J. : Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 1033–1041, doi:10.1096/fj.201801018R.
- Jeske, L.; Placzek, S.; Schomburg, I.; Chang, A.; Schomburg, D. BRENDA in 2019: A European ELIXIR core data resource. Nucleic Acids Res 2018, 47, D542-D549, doi:10.1093/nar/gky1048.
- Fan, X.M.; Zheng, F.S.; Liu, H.Y.; Ma, Y.H.; Wong, B.C. [Mechanism of apoptosis induced by specific COX-2 inhibitor SC236 in gastric cancer cells]. Zhonghua Zhong Liu Za Zhi 2005, 27, 145–147.
- Ott, I.; Schmidt, K.; Kircher, B.; Schumacher, P.; Wiglenda, T.; Gust, R. Antitumor-Active Cobalt−Alkyne Complexes Derived from Acetylsalicylic Acid: Studies on the Mode of Drug Action. J. Med. Chem. 2005, 48, 622–629, doi:10.1021/jm049326z.
- Zanellato, I.; Bonarrigo, I.; Ravera, M.; Gabano, E.; Gust, R.; Osella, D. The hexacarbonyldicobalt derivative of aspirin acts as a CO-releasing NSAID on malignant mesothelioma cells. Met. : Integr. Biometal Sci. 2013, 5, 1604–1613, doi:10.1039/c3mt00117b.
- Soumaoro, L.T.; Uetake, H.; Takagi, Y.; Iida, S.; Higuchi, T.; Yasuno, M.; Enomoto, M.; Sugihara, K. Coexpression of VEGF-C and Cox-2 in human colorectal cancer and its association with lymph node metastasis. Dis Colon Rectum 2006, 49, 392–398, doi:10.1007/s10350-005-0247-x.
- Peng, Z.H.; Wan, D.S.; Li, L.R.; Chen, G.; Lu, Z.H.; Wu, X.J.; Kong, L.H.; Pan, Z.Z. Expression of COX-2, MMP-2 and VEGF in stage II and III colorectal cancer and the clinical significance. Hepatogastroenterology 2011, 58, 369–376.
- Ferrandina, G.; Lauriola, L.; Zannoni, G.F.; Fagotti, A.; Fanfani, F.; Legge, F.; Maggiano, N.; Gessi, M.; Mancuso, S.; Ranelletti, F.O., et al. Increased cyclooxygenase-2 (COX-2) expression is associated with chemotherapy resistance and outcome in ovarian cancer patients. Ann Oncol 2002, 13, 1205–1211, doi:10.1093/annonc/mdf207.
- Ferrandina, G.; Ranelletti, F.O.; Lauriola, L.; Fanfani, F.; Legge, F.; Mottolese, M.; Nicotra, M.R.; Natali, P.G.; Zakut, V.H.; Scambia, G. Cyclooxygenase-2 (COX-2), epidermal growth factor receptor (EGFR), and Her-2/neu expression in ovarian cancer. Gynecol Oncol 2002, 85, 305–310, doi:10.1006/gyno.2002.6620.
- Denkert, C.; Köbel, M.; Pest, S.; Koch, I.; Berger, S.; Schwabe, M.; Siegert, A.; Reles, A.; Klosterhalfen, B.; Hauptmann, S. Expression of Cyclooxygenase 2 Is an Independent Prognostic Factor in Human Ovarian Carcinoma. Am. J. Pathol. 2002, 160, 893–903, doi:10.1016/S0002-9440(10)64912-7.
- Ristimäki, A.; Sivula, A.; Lundin, J.; Lundin, M.; Salminen, T.; Haglund, C.; Joensuu, H.; Isola, J. Prognostic Significance of Elevated Cyclooxygenase-2 Expression in Breast Cancer. Cancer Res. 2002, 62, 632–635.
- Mazhar, D.; Ang, R.; Waxman, J. COX inhibitors and breast cancer. Br. J. Cancer 2006, 94, 346–350, doi:10.1038/sj.bjc.6602942.
- Fulton, A.M.; Heppner, G.H. Relationships of Prostaglandin E and Natural Killer Sensitivity to Metastatic Potential in Murine Mammary Adenocarcinomas. Cancer Res. 1985, 45, 4779–4784.
- Singh, B.; Berry, J.A.; Vincent, L.E.; Lucci, A. Involvement of IL-8 in COX-2-mediated bone metastases from breast cancer. J Surg Res 2006, 134, 44–51, doi:10.1016/j.jss.2006.03.018.
- Majumder, M.; Dunn, L.; Liu, L.; Hasan, A.; Vincent, K.; Brackstone, M.; Hess, D.; Lala, P.K. COX-2 induces oncogenic micro RNA miR655 in human breast cancer. Sci. Rep. 2018, 8, 327, doi:10.1038/s41598-017-18612-3.
- Barnes, N.; Haywood, P.; Flint, P.; Knox, W.F.; Bundred, N.J. Survivin expression in in situ and invasive breast cancer relates to COX-2 expression and DCIS recurrence. Br. J. Cancer 2006, 94, 253–258, doi:10.1038/sj.bjc.6602932.
- Nie, F.; Yu, X.L.; Wang, X.G.; Tang, Y.F.; Wang, L.L.; Ma, L. Down-regulation of CacyBP is associated with poor prognosis and the effects on COX-2 expression in breast cancer. Int J Oncol 2010, 37, 1261–1269, doi:10.3892/ijo_00000777.
- Li, E.X.; Wu, Y.Y.; Shi, F.; Wu, Y.; Guo, J.J.; Dong, D.F. [Relationship between serum VEGF level and VEGF, COX-2 and MVD expression in breast cancer tissues]. Zhonghua Zhong Liu Za Zhi 2007, 29, 522–525.
- Siironen, P.; Ristimäki, A.; Nordling, S.; Louhimo, J.; Haapiainen, R.; Haglund, C. Expression of COX-2 is increased with age in papillary thyroid cancer. Histopathology 2004, 44, 490–497, doi:10.1111/j.1365-2559.2004.01880.
- Siironen, P.; Ristimaki, A.; Narko, K.; Nordling, S.; Louhimo, J.; Andersson, S.; Haapiainen, R.; Haglund, C. VEGF-C and COX-2 expression in papillary thyroid cancer. Endocr Relat Cancer 2006, 13, 465–473, doi:10.1677/erc.1.01114.
- Fujiwaki, R.; Iida, K.; Kanasaki, H.; Ozaki, T.; Hata, K.; Miyazaki, K. Cyclooxygenase-2 expression in endometrial cancer: Correlation with microvessel count and expression of vascular endothelial growth factor and thymidine phosphorylase. Hum. Pathol. 2002, 33, 213–219, doi:10.1053/hupa.2002.31292.
- Landen, C.N.; Mathur, S.P.; Richardson, M.S.; Creasman, W.T. Expression of cyclooxygenase-2 in cervical, endometrial, and ovarian malignancies. Am. J. Obstet. Gynecol. 2003, 188, 1174–1176, doi:10.1067/mob.2003.284.
- Ferrandina, G.; Ranelletti, F.O.; Gallotta, V.; Martinelli, E.; Zannoni, G.F.; Gessi, M.; Scambia, G. Expression of cyclooxygenase-2 (COX-2), receptors for estrogen (ER), and progesterone (PR), p53, ki67, and neu protein in endometrial cancer. Gynecol Oncol 2005, 98, 383–389, doi:10.1016/j.ygyno.2005.04.024.
- Fowler, J.M.; Ramirez, N.; Cohn, D.E.; Kelbick, N.; Pavelka, J.; Ben-Shachar, I.; Morrison, C. Correlation of cyclooxygenase-2 (COX-2) and aromatase expression in human endometrial cancer: Tissue microarray analysis. Am. J. Obstet. Gynecol. 2005, 192, 1262–1271, doi:10.1016/j.ajog.2005.01.009.
- Moon, W.S.; Park, H.S.; Lee, H.; Pai, R.; Tarnawski, A.S.; Kim, K.R.; Jang, K.Y. Co-expression of cox-2, C-met and beta-catenin in cells forming invasive front of gallbladder cancer. Cancer Res Treat 2005, 37, 171–176, doi:10.4143/crt.2005.37.3.171.
- Rubio, J.; Ramos, D.; Lopez-Guerrero, J.A.; Iborra, I.; Collado, A.; Solsona, E.; Almenar, S.; Llombart-Bosch, A. Immunohistochemical expression of Ki-67 antigen, cox-2 and Bax/Bcl-2 in prostate cancer; prognostic value in biopsies and radical prostatectomy specimens. Eur Urol 2005, 48, 745–751, doi:10.1016/j.eururo.2005.06.014.
- Cohen, B.L.; Gomez, P.; Omori, Y.; Duncan, R.C.; Civantos, F.; Soloway, M.S.; Lokeshwar, V.B.; Lokeshwar, B.L. Cyclooxygenase-2 (COX-2) expression is an independent predictor of prostate cancer recurrence. Int J Cancer 2006, 119, 1082–1087, doi:10.1002/ijc.21749.
- Rao, D.S.; Gui, D.; Koski, M.E.; Popoviciu, L.M.; Wang, H.; Reiter, R.E.; Said, J.W. An Inverse Relation Between COX-2 and E-cadherin Expression Correlates With Aggressive Histologic Features in Prostate Cancer. Appl. Immunohistochem. Mol. Morphol. 2006, 14, 375–383, doi:10.1097/01.pai.0000210417.61117.6c.
- Richardsen, E.; Uglehus, R.D.; Due, J.; Busch, C.; Busund, L.T. COX-2 is overexpressed in primary prostate cancer with metastatic potential and may predict survival. A comparison study between COX-2, TGF-beta, IL-10 and Ki67. Cancer Epidemiol 2010, 34, 316–322, doi:10.1016/j.canep.2010.03.019.
- Li, M.; Liu, W.; Zhu, Y.F.; Chen, Y.L.; Zhang, B.Z.; Wang, R. Correlation of COX-2 and K-ras expression to clinical outcome in gastric cancer. Acta Oncol 2006, 45, 1115–1119, doi:10.1080/02841860601043066.
- Mrena, J.; Wiksten, J.P.; Nordling, S.; Kokkola, A.; Ristimäki, A.; Haglund, C. MMP-2 but not MMP-9 associated with COX-2 and survival in gastric cancer. J. Clin. Pathol. 2006, 59, 618–623, doi:10.1136/jcp.2005.033761.
- Jo, H.; Kang, S.; Kim, J.W.; Kang, G.H.; Park, N.H.; Song, Y.S.; Park, S.Y.; Kang, S.B.; Lee, H.P. Hypermethylation of the COX-2 gene is a potential prognostic marker for cervical cancer. J Obs. Gynaecol Res 2007, 33, 236–241, doi:10.1111/j.1447-0756.2007.00517.x.
- Matsumoto, G.; Muta, M.; Tsuruta, K.; Horiguchi, S.; Karasawa, K.; Okamoto, A. Tumor size significantly correlates with postoperative liver metastases and COX-2 expression in patients with resectable pancreatic cancer. Pancreatology 2007, 7, 167–173, doi:10.1159/000104241.
- Matsubayashi, H.; Infante, J.R.; Winter, J.; Klein, A.P.; Schulick, R.; Hruban, R.; Visvanathan, K.; Goggins, M. Tumor COX-2 expression and prognosis of patients with resectable pancreatic cancer. Cancer Biol. 2007, 6, 1569–1575, doi:10.4161/cbt.6.10.4711.
- Bergmann, F.; Moldenhauer, G.; Herpel, E.; Gaida, M.M.; Strobel, O.; Werner, J.; Esposito, I.; Muerkoster, S.S.; Schirmacher, P.; Kern, M.A. Expression of L1CAM, COX-2, EGFR, c-KIT and Her2/neu in anaplastic pancreatic cancer: Putative therapeutic targets? Histopathology 2010, 56, 440–448, doi:10.1111/j.1365-2559.2010.03499.x.
- Yin, H.; Porter, N.A. New Insights Regarding the Autoxidation of Polyunsaturated Fatty Acids. Antioxid. Redox Signal. 2004, 7, 170–184, doi:10.1089/ars.2005.7.170.
- Russell, G.A. Deuterium-isotope Effects in the Autoxidation of Aralkyl Hydrocarbons. Mechanism of the Interaction of PEroxy Radicals1. J. Am. Chem. Soc. 1957, 79, 3871–3877, doi:10.1021/ja01571a068.
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F., et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, doi:10.3389/fphys.2013.00242.
- Lee, M.J.; Yaffe, M.B. Protein Regulation in Signal Transduction. Cold Spring Harb Perspect Biol 2016, 8, a005918, doi:10.1101/cshperspect.a005918.
- Rahimi, N.; Costello, C.E. Emerging roles of post-translational modifications in signal transduction and angiogenesis. Proteomics 2015, 15, 300–309, doi:10.1002/pmic.201400183.
- Tomko, N.; Kluever, M.; Wu, C.; Zhu, J.; Wang, Y.; Salomon, R.G. 4-Hydroxy-7-oxo-5-heptenoic acid lactone is a potent inducer of brain cancer cell invasiveness that may contribute to the failure of anti-angiogenic therapies. Free Radic. Biol. Med. 2020, 146, 234–256, doi:10.1016/j.freeradbiomed.2019.11.009.
- Sousa, B.C.; Ahmed, T.; Dann, W.L.; Ashman, J.; Guy, A.; Durand, T.; Pitt, A.R.; Spickett, C.M. Short-chain lipid peroxidation products form covalent adducts with pyruvate kinase and inhibit its activity in vitro and in breast cancer cells. Free Radic. Biol. Med. 2019, 144, 223–233, doi:10.1016/j.freeradbiomed.2019.05.028.
- Sakuma, S.; Sumi, H.; Kohda, T.; Arakawa, Y.; Fujimoto, Y. Effects of Lipid Peroxidation-Derived Products on the Growth of Human Colorectal Cancer Cell Line HT-29. J Clin Biochem Nutr 2009, 45, 171–177, doi:10.3164/jcbn.09-09.
- Gasparovic, A.C.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. Cancer growth regulation by 4-hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 226–234, doi:https://doi.org/10.1016/j.freeradbiomed.2017.01.030.
- Rossin, D.; Calfapietra, S.; Sottero, B.; Poli, G.; Biasi, F. HNE and cholesterol oxidation products in colorectal inflammation and carcinogenesis. Free Radic. Biol. Med. 2017, 111, 186–195, doi:10.1016/j.freeradbiomed.2017.01.017.
- Tappel, A.L. Vitamin E as the Biological Lipid Antioxidant. In Vitamins & Hormones, Harris, R.S., Wool, I.G., Marrian, G.F., Thimann, K.V., Eds. Academic Press: 1962; Vol. 20, pp. 493-510.
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 1993, 300, 535–543, doi:10.1006/abbi.1993.1074.
- Samhan-Arias, A.K.; Duarte, R.O.; Martin-Romero, F.J.; Moura, J.J.; Gutierrez-Merino, C. Reduction of ascorbate free radical by the plasma membrane of synaptic terminals from rat brain. Arch Biochem Biophys 2008, 469, 243–254, doi:10.1016/j.abb.2007.10.004.
- Scarpa, M.; Rigo, A.; Maiorino, M.; Ursini, F.; Gregolin, C. Formation of alpha-tocopherol radical and recycling of alpha-tocopherol by ascorbate during peroxidation of phosphatidylcholine liposomes. An electron paramagnetic resonance study. Biochim. Et Biophys. Acta 1984, 801, 215–219, doi:10.1016/0304-4165(84)90070-9.
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat Res 1996, 145, 532–541.
- Mendiratta, S.; Qu, Z.C.; May, J.M. Enzyme-dependent ascorbate recycling in human erythrocytes: Role of thioredoxin reductase. Free Radic Biol Med 1998, 25, 221–228, doi:10.1016/s0891-5849(98)00060-4.
- Schneider, M.; Wortmann, M.; Mandal, P.K.; Arpornchayanon, W.; Jannasch, K.; Alves, F.; Strieth, S.; Conrad, M.; Beck, H. Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity. Neoplasia (New Yorkn.Y.) 2010, 12, 254–263, doi:10.1593/neo.91782.
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331, doi:10.1016/j.cell.2013.12.010.
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L., et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250, doi:10.1038/nature24297.




