Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ferdinando Nicoletti | + 1860 word(s) | 1860 | 2021-08-24 10:11:24 | | | |
| 2 | Camila Xu | -212 word(s) | 1648 | 2021-08-30 07:41:39 | | | | |
| 3 | Camila Xu | -212 word(s) | 1648 | 2021-08-30 07:42:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nicoletti, F.; Dragomanova, S.; Staykov, H. Alpha-Lipoic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/13626 (accessed on 08 February 2026).
Nicoletti F, Dragomanova S, Staykov H. Alpha-Lipoic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/13626. Accessed February 08, 2026.
Nicoletti, Ferdinando, Stela Dragomanova, Hristian Staykov. "Alpha-Lipoic Acid" Encyclopedia, https://encyclopedia.pub/entry/13626 (accessed February 08, 2026).
Nicoletti, F., Dragomanova, S., & Staykov, H. (2021, August 27). Alpha-Lipoic Acid. In Encyclopedia. https://encyclopedia.pub/entry/13626
Nicoletti, Ferdinando, et al. "Alpha-Lipoic Acid." Encyclopedia. Web. 27 August, 2021.
Copy Citation
Alpha-lipoic acid (LA) is a powerful endogenous and exogenous antioxidant. It is a disulfide compound soluble in both water and oil.
alfa-lipoic acid
antioxidants
natural products
viral infections
COVID-19
1. Beneficial Effects of LA
LA is a powerful endogenous and exogenous antioxidant. It is a disulfide compound soluble in both water and oil. The reduced form of LA, dihydrolipoic acid, represents the active metabolite (Figure 1).
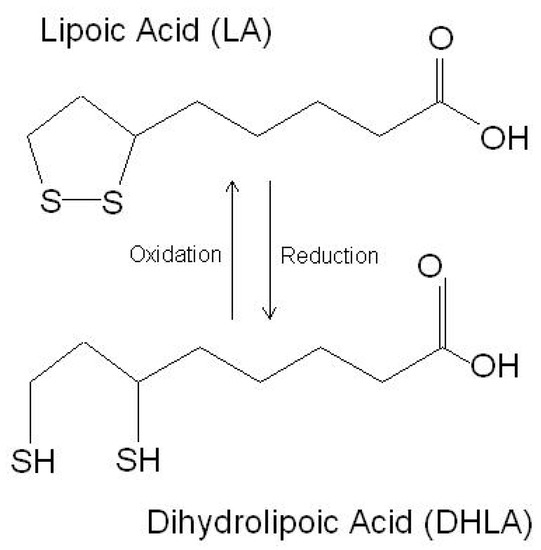
Figure 1. The chemical structure of lipoic acid and its active metabolite dihydrolipoic acid (https://www.robertbarrington.net/alpha-lipoic-acid-antioxidant/; posted on 3 January 2014 by Robert Barrington (accessed on 5 February 2021)).
In the cell, LA increases the utilization of glucose in the mitochondria via supplying acetyl-CoA for the production of acetylcholine [1]. LA induces the activity of the enzymes responsible for the synthesis of GSH and other antioxidant enzymes and is a cofactor of several mitochondrial enzymes [2]. Hence, LA regulates several processes, such as nucleic acid synthesis and energy production, via the citric acid cycle [2].
LA is present in foods and supplements and possesses pleiotropic biological activities. As a free radical scavenger, LA increases the level of reduced GSH. Moreover, it regenerates vitamins C and E as part of the antioxidant defense system.
LA stimulates insulin production and improves glucose utilization, thus ameliorating the oxidative damage caused by hyperglycemia [3]. In addition, it reduces the insulin resistance and improves the insulin sensitivity and blood sugar control [4]. In patients with type 1 diabetes mellitus, LA leads to the downregulation of nuclear factor-kB in monocytes [5].
Additionally, LA has been shown to improve the symptoms of diabetic neuropathy, to reduce the risk of diabetic retinopathy [6][7][8], and to ameliorate diabetes-associated autonomic neuropathy [9].
Furthermore, LA improves endothelial dysfunction [10] and decreases the risks of heart attack and stroke [11].
LA can cross the blood–brain barrier (BBB) [1][12] and may alleviate the neuroinflammatory damage, preventing neuronal death, in models of Alzheimer’s disease and Parkinson’s disease [13][14][15][16][17]. Additionally, it significantly attenuates the inflammatory response in microglial cells, downregulating the production of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-6, and other cytotoxic molecules, such as nitric oxide and ROS [18].
Maldonado-Rojas and colleagues (2011) performed an in silico screening of the molecular targets for LA, and, among several targets, leukotriene A4 hydrolase, voltage-gated potassium channel, alpha hydroxysteroid dehydrogenase, and epoxide hydrolase were found to be the most likely bound by LA. As these genes are involved in the pathogenesis of a number of diseases, this finding supports the potential use of LA in the treatment of cancer, diabetes, and neurological and cardiovascular disorders [19].
The proposed mechanisms of action for LA are summarized in Table 1.
Table 1. Proposed mechanisms of action of LA.
| Mechanism of Action | References |
|---|---|
| Scavenging reactive oxygen species | [20][21][22] |
| Regeneration other endogenous antioxidants (e.g., vitamins C and E) | [23] |
| Chelation of redox-active metals | [24][25][26][27] |
| Induction of endogenous antioxidants (e.g., ascorbate, GSH) | [28][29][30][31][32][33] |
| Induction of the Nrf2/ARE pathway | [34][35][36][37] |
| Inhibition of NF-κB activation | [38] |
| Activation of: | -- |
| - PKC | [39] |
| - Erk1/2 | [40][41] |
| - p38 MAPK | [42] |
| - PI3K | [42] |
| - Akt | [42][43][44][45] |
| - Inhibition of: | -- |
| - PTEN | [43] |
| - PP2A | [43] |
| - PTP1B | [46] |
| Activation of insulin receptor by direct binding | [47] |
| Activation of AMP-activated protein kinase (AMPK) | [48][49][50] |
2. Effects of LA in Viral Infection
2.1. Effects of LA on Influenza Virus Infection
Influenza viruses are RNA viruses of the Orthomyxoviridae family. Seasonal epidemics are associated with 3–5 million cases of severe illness each year and from 290- to 650-thousand deaths worldwide (https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal); accessed on 3 August 2021). Although safe and effective vaccines are available, their specificity is limited because of the diversity of the circulating influenza strains and the ability of the virus to acquire mutations. The treatment options for influenza infections are limited to inhibitors of neuraminidase and blockers of the M2 proton channel. In patients infected with IV, marked increases of oxidation byproducts are found, including 8-hydroxydeoxyguanosine, malondialdehyde, F2-isoprostane, 7-ketocholesterol, and 7β-hydroxycholesterol (reviewed in reference [51]). Additionally, elevated levels of sterol oxidation products are observed up to three months after IV clearance (reviewed in reference [51]). Finally, increased levels of ROS, as well as of nitric oxide synthase 2 (iNOS) and nitrotyrosine, are found in the lung tissues of patients that died from an IV infection (reviewed in reference [51]). Several reports have shown that NAC, ascorbic acid, and vitamin E have positive effects on IV infections by inhibiting viral replication and inflammation both in the cells and in the mice (reviewed in reference [51]). In an in vitro study, LA inhibited IV replication in MDCK cells and reduced the nuclear translocation of NF-κB. Additionally, the caspase-3 activity was remarkably inhibited and type I interferons (IFNs) were upregulated upon LA treatment [52]. Overall, the available data indicated that LA might be a potential anti-influenza agent, deserving further investigation.
2.2. Effects of LA in Herpes Infections
Herpes virus infection is widely spread, as estimates show that 80% of people worldwide are carriers of at least one strain. Nine herpesvirus types are known to primarily infect humans: herpes simplex viruses 1 and 2 (HSV-1 and HSV-2), varicella zoster virus, Epstein–Barr virus, human cytomegalovirus human herpesvirus 6A and 6B (HHV-6A and HHV-6B), human herpesvirus 7 (HHV-7), and Kaposi’s sarcoma-associated herpesvirus (KSHV) [53]
LA has been recognized for its potential to control herpes virus infections in a patented invention—a formulation for external use containing different naturally occurring substances, together with LA. The expected beneficial effect is to narrow the period of inflammation, allowing the affected tissues to be restored (US patent application No.: US 2011/0229584A1).
The neuroprotective properties of LA could potentially find their role in post-herpetic neuralgia, following shingles or Herpes zoster infection [12][17]. The chronic damage caused by the virus results in so-called post-herpetic neuralgia, could potentially be alleviated thanks to the neuroprotective properties of LA, that have been demonstrated in diabetic polyneuropathy—another condition related to peripheral nerve damage. In addition, by acting on the endothelial cells, LA improves the endoneural blood flow and favors the transport of nutrients to the compression site responsible for neuronal damage [10].
2.3. Effects of LA in Viral Hepatitis
Smallpox was declared eradicated in 1980; however, in recent years, due to the potential use of this virus as a biological weapon, concerns have been raised for an improved method for the prevention and treatment of this disease. The currently available vaccines, which have not been updated since the eradication of smallpox, could lead to severe adverse reactions, and interest in drugs with good safety profiles is emerging. In addition to the prevention and treatment of vaccinia-related infections and variola infections, worth mentioning is the so-called monkey pox virus, which is endemic for Africa, although cases are also sporadically reported in the United States.
An in vitro study of LA and ethacrynic acid has shown that both substances have inhibitory effect towards virus growth, while they are relatively nontoxic to fibroblasts and epithelial cells [54]. The effect of LA and ethacrynic acid was dose-dependent and seemed to be related to the inhibition of the expression of VACV late genes, resulting in decreased levels of infectious virus progeny. The two molecules, however, did not seem to affect either VACV entry into the cell or viral DNA synthesis [54].
2.4. Effects of LA in HIV Infection
Chronic viral hepatitis is the leading cause of liver fibrosis and cirrhosis. Replication of the virus in liver cells results in decreased levels of glutathione in hepatocytes. The viral load in hepatitis C is directly related to the glutathione levels [55]. Eventually, the oxidative stress leads to necrotic inflammation and the necrosis of liver cells. Increased lipid peroxidation leads to hepatic cytotoxicity, causing immune-mediated inflammation and fibrosis [56][57][58]. Supportive therapeutic effects of LA have been demonstrated in the clinical setting in patients with a Hepatitis C infection [59][60]. The LA antioxidant properties have been recognized as potential therapeutic effects based on its ability to exert anti-inflammatory, antifibrotic, and anti-TNF-α effects that produce restoration of the damaged areas of the liver, as well as its function, as demonstrated in HCV patients [59][60]. According to a clinical study with a small number of patients conducted by Berkson in 1999, therapy with the antioxidant LA, the hepatoprotector silymarin, and the element selenium, completely restores the liver function of patients, and the proposed combination allows avoiding liver transplantation [59]. The beneficial effect of the application of various antioxidants, including LA, was later confirmed in a clinical trial enrolling 50 patients with chronic hepatitis C infection [60]. Among them, 48% responded with improvement in at least one of the observed liver parameters, and a decrease in the viral load was observed in 25% of the patients.
2.5. Effects of LA in COVID-19
A major pathogenetic factor in HIV infection is the induced oxidative stress, which causes apoptosis and a decrease in the number of CD4 + T cells, which may eventually lead to marked immunosuppression [61][62]. Increased oxidative reactions [61] and higher concentrations of hydrogen peroxide [63] and MDA [64], as well as suppression of the antioxidant protection response, with decreased activity of the antioxidant enzymes SOD, GPx, CAT, and thioredoxin, have been found in the plasma, lung mucosa, erythrocytes, and lymphocytes from patients with HIV infection [62][63][65].
The potential antiviral effects of LA were first proposed by Baur and colleagues in 1991 [66]. They observed that the addition of LA to the T-cell lines Jurkat, SupT1, and Molt-4 incubated with a wild-type HIV-1 isolate was associated with a dose-dependent inhibition of HIV-1-replication, a reduced cytopathic effect, and the inhibition of reverse transcriptase activity and plaque formation [66]. Moreover, LA synergized with zidovudine in inhibiting viral replication by affecting the reverse transcriptase enzyme [66].
In HIV patients, the administration of LA was associated with positive effects characterized by the stable restoration of the total blood GSH levels and lymphocyte function in comparison to the placebo group, where a progressive decline was observed [67].
In another clinical study, upon the oral administration of LA to 11 AIDS patients, an increase in the blood concentrations of ascorbic acid and total GSH and a decrease in the levels of lipid peroxidation, accompanied by a significant increase in the CD4/CD8 ratio and the number of CD4 + T cells in the plasma, was observed [68].
References
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2011, 48, 26–32.
- Bustamante, J.; Lodge, J.K.; Marcocci, L.; Tritschler, H.J.; Packer, L.; Rihn, B.H. α-lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998, 24, 1023–1039.
- Clavreul, N.; Bachschmid, M.M.; Hou, X.; Shi, C.; Idrizovic, A.; Ido, Y.; Pimentel, D.; Cohen, R.A. S-glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2454–2461.
- Ibrahimpasic, K. Alpha lipoic acid and glycaemic control in diabetic neuropathies at type 2 diabetes treatment. Med. Arch. 2013, 67, 7–9.
- Hofmann, M.A.; Schiekofer, S.; Kanitz, M.; Klevesath, M.S.; Joswig, M.; Lee, V.; Morcos, M.; Tritschler, H.; Ziegler, R.; Wahl, P.; et al. Insufficient glycemic control increases nuclear factor-κB binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetes. Diabetes Care 1998, 21, 1310–1316.
- Ziegler, D.; Reljanovic, M.; Mehnert, H.; Gries, F.A. α-lipoic acid in the treatment of diabetic polyneuropathy in Germany: Current evidence from clinical trials. Exp. Clin. Endocrinol. Diabetes 1999, 107, 421–430.
- Nagamatsu, M.; Nickander, K.K.; Schmelzer, J.D.; Raya, A.; Wittrock, D.A.; Tritschler, H.; Low, P.A. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care 1995, 18, 1160–1167.
- Androne, L.; Gavan, N.A.; Veresiu, I.A.; Orasan, R. In vivo effect of lipoic acid on lipid peroxidation in patients with diabetic neuropathy. In Vivo 2000, 14, 327–330.
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895.
- Jones, W.; Li, X.; Qu, Z.; Perriott, L.; Whitesell, R.R.; May, J.M. Uptake, recycling, and antioxidant actions of α-lipoic acid in endothelial cells. Free Radic. Biol. Med. 2002, 33, 83–93.
- Ziegler, D.; Gries, F.A. α-lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy. Diabetes 1997, 46, S62–S66.
- Lynch, M.A. Lipoic acid confers protection against oxidative injury in non-neuronal and neuronal tissue. Nutr. Neurosci. 2001, 4, 419–438.
- Tancheva, L.P.; Lazarova, M.I.; Alexandrova, A.V.; Dragomanova, S.T.; Nicoletti, F.; Tzvetanova, E.R.; Hodzhev, Y.K.; Kalfin, R.E.; Miteva, S.A.; Mazzon, E.; et al. Neuroprotective mechanisms of three natural antioxidants on a rat model of parkinson’s disease: A comparative study. Antioxidants 2020, 9, 49.
- Tzvetanova, E.R.; Georgieva, A.P.; Alexandrova, A.V.; Tancheva, L.P.; Lazarova, M.I.; Dragomanova, S.T.; Alova, L.G.; Stefanova, M.O.; Kalfin, R.E. Antioxidant mechanisms in neuroprotective action of lipoic acid on learning and memory of rats with experimental dementia. Bulg. Chem. Commun. 2018, 50, 52–57.
- Hager, K.; Marahrens, A.; Kenklies, M.; Riederer, P.; Münch, G. Alpha-lipoic acid as a new treatment option for Azheimer type dementia. Arch. Gerontol. Geriatr. 2001, 32, 275–282.
- Holmquist, L.; Stuchbury, G.; Berbaum, K.; Muscat, S.; Young, S.; Hager, K.; Engel, J.; Münch, G. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol. Ther. 2007, 113, 154–164.
- Lovell, M.A.; Xie, C.; Xiong, S.; Markesbery, W.R. Protection against amyloid beta peptide and iron/hydrogen peroxide toxicity by alpha lipoic acid. J. Alzheimer’s Dis. 2003, 5, 229–239.
- Kim, S.M.; Ha, J.S.; Han, A.R.; Cho, S.W.; Yang, S.J. Effects of α-lipoic acid on LPS-induced neuroinflammation and NLRP3 inflammasome activation through the regulation of BV-2 microglial cells activation. BMB Rep. 2019, 52, 613–618.
- Maldonado-Rojas, W.; Olivero-Verbel, J.; Ortega-Zuñiga, C. Searching of protein targets for alpha lipoic acid. J. Braz. Chem. Soc. 2011, 22, 2250–2259.
- Suzuki, Y.J.; Tsuchiya, M.; Packer, L. Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Radic. Res. 1991, 15, 255–263.
- Scott, B.C.; Aruoma, O.I.; Evans, P.J.; O’Neill, C.; Van Der Vliet, A.; Cross, C.E.; Tritschler, H.; Halliwell, B. Lipoic and dihydrolipoic acids as antioxidants. A critical evaluation. Free Radic. Res. 1994, 20, 119–133.
- Trujillo, M.; Radi, R. Peroxynitrite reaction with the reduced and the oxidized forms of lipoic acid: New insights into the reaction of peroxynitrite with thiols. Arch. Biochem. Biophys. 2002, 397, 91–98.
- Biewenga, G.P.; Haenen, G.R.M.M.; Bast, A. The pharmacology of the antioxidant: Lipoic acid. Gen. Pharmacol. 1997, 29, 315–331.
- Ou, P.; Tritschler, H.J.; Wolff, S.P. Thioctic (lipoic) acid: A therapeutic metal-chelating antioxidant? Biochem. Pharmacol. 1995, 50, 123–126.
- Lodge, J.K.; Traber, M.G.; Packer, L. Thiol chelation of Cu2+ by dihydrolipoic acid prevents human low density lipoprotein peroxidation. Free Radic. Biol. Med. 1998, 25, 287–297.
- Goralska, M.; Dackor, R.; Holley, B.; McGahan, M.C. Alpha lipoic acid changes iron uptake and storage in lens epithelial cells. Exp. Eye Res. 2003, 76, 241–248.
- Suh, J.H.; Moreau, R.; Heath, S.H.D.; Hagen, T.M. Dietary supplementation with (R)-α-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep. 2005, 10, 52–60.
- Lykkesfeldt, J.; Hagen, T.M.; Vinarsky, V.; Ames, B.N. Age-associated decline in ascorbic acid concentration, recycling, and biosynthesis in rat hepatocytes—Reversal with (R)-α-lipoic acid supplementation. FASEB J. 1998, 12, 1183–1189.
- Xu DP, W.W. Alpha-Lipoic acid dependent regeneration of ascorbic acid from dehydroascorbic acid in rat liver mitochondria. J Bioenerg. Biomembr. 1996, 28, 77–85.
- Bast, A.; Haenen, G.R.M.M. Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim. Biophys. Acta BBA Lipids Lipid Metab. 1988, 963, 558–561.
- Busse E Zimmer G Schopohl B Kornhuber B Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneim. Forsch. 1992, 42, 829–831.
- Suh, J.H.; Wang, H.; Liu, R.M.; Liu, J.; Hagen, T.M. (R)-α-Lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: Evidence for increased cysteine requirement for zGSH synthesis. Arch. Biochem. Biophys. 2004, 423, 126–135.
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386.
- Lii, C.-K.; Liu, K.-L.; Cheng, Y.-P.; Lin, A.-H.; Chen, H.-W.; Tsai, C.-W. Sulforaphane and α-Lipoic Acid Upregulate the Expression of the π Class of Glutathione S-Transferase through c-Jun and Nrf2 Activation. J. Nutr. 2010, 140, 885–892.
- Lee, J.; Jung, S.-Y.; Yang, K.-J.; Kim, Y.; Lee, D.; Lee, M.H.; Kim, D.-K. α-Lipoic acid prevents against cisplatin cytotoxicity via activation of the NRF2/HO-1 antioxidant pathway. PLoS ONE 2019, 14, e0226769.
- Koriyama, Y.; Nakayama, Y.; Matsugo, S.; Kato, S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Res. 2013, 1499, 145–157.
- Pilar Valdecantos, M.; Prieto-Hontoria, P.L.; Pardo, V.; Módol, T.; Santamaría, B.; Weber, M.; Herrero, L.; Serra, D.; Muntané, J.; Cuadrado, A.; et al. Essential role of Nrf2 in the protective effect of lipoic acid against lipoapoptosis in hepatocytes. Free Radic. Biol. Med. 2015, 84, 263–278.
- Ying, Z.; Kampfrath, T.; Sun, Q.; Parthasarathy, S.; Rajagopalan, S. Evidence that α-lipoic acid inhibits NF-κB activation independent of its antioxidant function. Inflamm. Res. 2011, 60, 219–225.
- Sen, C.K.; Roy, S.; Packer, L. Fas mediated apoptosis of human Jurkat T-cells: Intracellular events and potentiation by redox-active α-lipoic acid. Cell Death Differ. 1999, 6, 481–491.
- Suzuki, Y.J.; Shi, S.S.; Day, R.M.; Blumberg, J.B. Differential regulation of MAP kinase signaling by pro- and antioxidant biothiols. Ann. N. Y. Acad. Sci. 2000, 899, 159–167.
- Shi, S.S.; Day, R.M.; Halpner, A.D.; Blumberg, J.B.; Suzuki, Y.J. Homocysteine and α-Lipoic Acid Regulate p44/42 MAP Kinase Phosphorylation in NIH/3T3 Cells. Antioxid. Redox Signal. 1999, 1, 123–128.
- Konrad, D.; Somwar, R.; Sweeney, G.; Yaworsky, K.; Hayashi, M.; Ramlal, T.; Klip, A. The antihyperglycemic drug α-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: Potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001, 50, 1464–1471.
- Petersen Shay, K.; Hagen, T.M. Age-associated impairment of Akt phosphorylation in primary rat hepatocytes is remediated by alpha-lipoic acid through PI3 kinase, PTEN, and PP2A. Biogerontology 2009, 10, 443–456.
- Zhang, W.J.; Wei, H.; Hagen, T.; Frei, B. α-Lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 4077–4082.
- Smith, A.R.; Hagen, T.M. Vascular endothelial dysfunction in aging: Loss of Akt-dependent endothelial nitric oxide synthase phosphorylation and partial restoration by (R)-α-lipoic acid. Biochem. Soc. Trans. 2003, 31, 1447–1449.
- Cho, K.J.; Moini, H.; Shon, H.K.; Chung, A.S.; Packer, L. α-Lipoic acid decreases thiol reactivity of the insulin receptor and protein tyrosine phosphatase 1B in 3T3-L1 adipocytes. Biochem. Pharmacol. 2003, 66, 849–858.
- Diesel, B.; Kulhanek-Heinze, S.; Höltje, M.; Brandt, B.; Höltje, H.D.; Vollmar, A.M.; Kiemer, A.K. α-lipoic acid as a directly binding activator of the insulin receptor: Protection from hepatocyte apoptosis. Biochemistry 2007, 46, 2146–2155.
- Kim, M.S.; Park, J.Y.; Namkoong, C.; Jang, P.G.; Ryu, J.W.; Song, H.S.; Yun, J.Y.; Namgoong, I.S.; Ha, J.; Park, I.S.; et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 2004, 10, 727–733.
- Woo, J.L.; Song, K.H.; Eun, H.K.; Jong, C.W.; Hyoun, S.K.; Park, H.S.; Kim, M.S.; Kim, S.W.; Lee, K.U.; Park, J.Y. α-Lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 885–891.
- Lee, W.J.; In, K.L.; Hyoun, S.K.; Yun, M.K.; Eun, H.K.; Jong, C.W.; Sung, M.H.; Kim, M.S.; Jo, I.; Goo, T.O.; et al. α-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2488–2494.
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox biology of respiratory viral infections. Viruses 2018, 10, 392.
- Bai, S.W.; Chen, C.Y.; Ji, J.; Xie, Q.M.; Ma, Y.; Sun, B.L.; Xue, C.Y.; Cao, Y.C.; Ma, J.Y.; Bi, Y.Z. Inhibition Effect of Alpha-Lipoic Acid on the Propagation of Influenza A Virus in MDCK Cells. Pak. Vet. J. 2012, 32, 101–106.
- McGeoch, D.J.; Cook, S.; Dolan, A.; Jamieson, F.E.; Telford, E.A.R. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J. Mol. Biol. 1995, 247, 443–458.
- Spisakova, M.; Cizek, Z.; Melkova, Z. Ethacrynic and α-lipoic acids inhibit vaccinia virus late gene expression. Antiviral Res. 2009, 81, 156–165.
- Hepatitis viral load correlates to glutathione levels. Posit. Health News 1998, 17, 14–15.
- Vendemiale, G.; Grattagliano, I.; Portincasa, P.; Serviddio, G.; Palasciamo, G.; Altomare, E. Oxidative stress in symptom-free HCV carriers: Relation with ALT flare-up. Eur. J. Clin. Investig. 2001, 31, 54–63.
- Tanyalcin, T.; Taskiran, D.; Topalak, O.; Batur, Y.; Kutay, F. The effects of chronic hepatitis C and B virus infections on liver reduced and oxidized glutathione concentrations. Hepatol. Res. 2000, 18, 104–109.
- Von Herbay, A.; Stahl, W.; Niederau, C.; Von Laar, J.; Strohmeyer, G.; Sies, H. Diminished plasma levels of vitamin E in patients with severe viral hepatitis. Free Radic. Res. 1996, 25, 461–466.
- Berkson, B.M. A conservative triple antioxidant approach to the treatment of hepatitis C. Combination of alpha lipoic acid (Thioctic acid), silymarin, and selenium: Three case histories. Med. Klin. 1999, 94, 84–89.
- Melhem, A.; Stern, M.; Shibolet, O.; Israeli, E.; Ackerman, Z.; Pappo, O.; Hemed, N.; Rowe, M.; Ohana, H.; Zabrecky, G.; et al. Treatment of chronic hepatitis C virus infection via antioxidants: Results of a phase I clinical trial. J. Clin. Gastroenterol. 2005, 39, 737–742.
- Greenspan, H.C.; Aruoma, O.I. Oxidative stress and apoptosis in HIV infection: A role for plant-derived metabolites with synergistic antioxidant activity. Immunol. Today 1994, 15, 209–213.
- Pace, G.W.; Leaf, C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995, 19, 523–528.
- Sandstrom, P.A.; Tebbey, R.W.; Van Cleave, S.; Buttke, T.M. Lipid hydroperoxides induce apoptosis in T cells displaying a HIV- associated glutathione peroxidase deficiency. J. Biol. Chem. 1994, 269, 798–801.
- Jarstrand, C.; Åkerlund, B. Oxygen radical release by neutrophils of HIV-infected patients. Chem. Biol. Interact. 1994, 91, 141–146.
- Polyakov, V.M.; Shepelev, A.P.; Kokovkina, O.E.; Vtornikova, I.V. B15—Superoxide anion (O2−) production and enzymatic disbalance in peripheral blood cells isolated from HIV-infected children. Free Radic. Biol. Med. 1994, 16, 15.
- Baur, A.; Harrer, T.; Peukert, M.; Jahn, G.; Kalden, J.R.; Fleckenstein, B. Alpha-lipoic acid is an effective inhibitor of human immuno-deficiency virus (HIV-1) replication. Klin. Wochenschr. 1991, 69, 722–724.
- Jariwalla, R.J.; Lalezari, J.; Cenko, D.; Mansour, S.E.; Kumar, A.; Gangapurkar, B.; Nakamura, D. Restoration of blood total glutathione status and lymphocyte function following α-lipoic acid supplementation in patients with HIV infection. J. Altern. Complement. Med. 2008, 14, 139–146.
- Studies on Lipoate Effects on Blood Redox State in Human Immunodeficiency Virus Infected Patients—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8141828/ (accessed on 9 February 2021).
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
3 times
(View History)
Update Date:
30 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




