The necessity of finding alternative pathways for power supplementation in a more sustainable way has been promoted because of the current carbon emissions that contribute to climate change
[1]. Thus, the energy-from-waste (EfW) concept has been introduced as an alternative pathway where the use of refuse-derived flues, household waste and non-hazardous industrial by-products are considered as potential sources for energy production
[1]. This productive model fits with the sustainable model of circular economy in which the concept ‘end-of-life’ is replaced by reutilization, recycling and recovering. Hence, by-products or wastes from one industry become raw material for another. In addition, another productive model can be easily combined with bio-economy, which promotes the use renewables based on biomass by-products
[2]. Indeed, in the past years, the production of renewable fuels using lignocellulosic waste from agricultural activities has been considered as an alternative to traditional fuels
[3]. Agricultural residues are defined as unusable and unstable materials derived from agricultural production which are directly linked to the cultivation of crops, and these materials are characterized by their biodegradability and solid and lignocellulosic composition
[3][4]. In this way, the lignocellulosic non-edible biomass discarded by agriculture feedstock can be used as raw material to obtain biofuel, being considered second-generation (2G) biofuel
[1][5]. Lignocellulosic biomass is mainly composed by cellulose, hemicellulose, and lignin, which are low energy-density compounds, so a pretreatment step is necessary so plant-specific enzymes can release sugars for biofuel production
[5][6]. Moreover, 2G biofuels present several advantages compared to first generation biofuels, such as the cheaper non-edible matter used since it is the waste obtained as a result of an industrial activity; no fuel–food competition since non-edible matrices are used; and the reuse of by-products obtained after pretreatment of raw materials to obtain animal fodder to be used, for example, in livestock feed, thus reinforcing the circular economy. In this way, sugarcane bagasse (SCB), wheat, barley, rice and corn straw, and sorghum can be used for bioethanol production
[7]. In the specific case of sugarcane production, it has an associated production of residues that may reach up to 30% of the production
[8], which may represent more than 100 MT of residues. This huge volume of residues has several drawbacks. From an economic point of view, they require being effectively managed, which has an associated cost for the industry. From an environmental point of view, they represent a potential source of CO
2 since they are likely to be burned or, if not, accumulated in landfills, which threatens the quality of environmental (i.e., air and water pollution and noise, among others, and their potential consequences) and public health. Therefore, the reutilization of these by-products as part of a circular economy model is key to reduce management costs of residues to sugarcane industries and their negative environmental impact. In fact, their reutilization as raw material for bioethanol production has double benefits. It would avoid the release of this biomass to the environment, and it would promote the use of bioethanol produced using SCB, which is less carbon intensive compared with fossil fuel, so air pollution can be reduced
[9]. Therefore, these two approaches have the potential of reducing the footprint of sugarcane industries and fuel utilization.
2. Sugarcane Bagasse as a Potential Raw Material for Bioethanol Production
Sugarcane (
Saccharum oficinarum L.) is a tropical grass that belongs to the Gramineae family and the
Saccharum spp. genus
[10] and is characterized for being large and perennial. The sugarcane cultivation requirements are 6–12 months to grow with 60–100 cm
3 of water
[11]. Brazil, China and India are the main producers of SCB, with almost 500 MT generated every year from the sugarcane industry, which provides an important contribution to economic development
[9][10][12]. SCB yielded the highest crop straw production between 2012 and 2022 according to the Food and Agricultural Organization Corporate Statistical Database (FAOSTAT)
[13]. Sucrose is the main product of sugarcane, which accumulates in the internodes of the stalk
[9]. However, the percentage of waste generated during sugarcane production varies between 25 and 30%
[8], which may represent 125–150 MT of residues. The residues produced by the sugarcane industry are mainly two types, and they can be classified as straw, which is the harvest residue, and as bagasse, which is the fibrous fraction after the extraction of the sugarcane stem juice
[10]. These two by-products are characterized by their lignocellulosic composition, with cellulose, lignin and hemicellulose being the major components and having also extractants and ashes in their composition
[14]. SCB is composed of approximately 45–50% cellulose, 25–30% hemicellulose, 25% lignin and 2.4–9% ash
[9]. Nevertheless, this may vary depending on different factors such as chemical composition of the soil, climatic conditions and variety of the crop, among others
[9].
Table 1 shows variations in the composition of SCB, sugarcane fiber and sugarcane straw. The relative abundances of component units are usually calculated on the basis of the volume integration of the raw material and are expressed in percentage of dried weight
[15]. The chemical characterization of vegetal biomass is highly relevant for their consideration as potential sources of carbon to produce bioethanol. Indeed, for bioethanol production, it is important to evaluate the cellulose, hemicellulose and lignin composition of the raw material
[9]. Owing to its high yield of sugar and lignocellulosic biomass, SCB is regarded as an excellent alternative energy source to substitute fossil fuels
[16][17].
Table 1. Composition of the main sugarcane chemical compounds expressed in percentage of dried weight.
As stated above, biomass characterization is a noteworthy step for establishing optimal process conditions. Hence, delineating the specificities of SCB as a lignocellulosic feedstock is key to its close scalability. Concerning these aspects, several authors
[22] conducted a study in which 60 bagasse samples were characterized. The results provided the following average of the structural compounds of SCB: 42.2% of cellulose, 27.6% of hemicelluloses, 21.6% of lignin, 5.63% of extractives and 2.84% of ashes. As can be seen in
Table 1, the composition of the samples did not differ substantially between them. This research supports the vantage of using SBC for obtaining bioethanol since the bioethanol yield is closely related to the biomass composition.
Moreover, the composition of feedstocks tends to vary depending on a large number of factors, so the stability of SCB may be considered an important advantage over other raw materials
[27]. Another work serves as example of the potential of SCB to obtain bioethanol
[28], where the authors considered the biorefinery route to convert SCB into various products, such as nanocrystalline cellulose, lignin and biohydrogen. They further outlined the rationale for selecting SCB as feedstock, arguing the availability of surplus bagasse, elimination of logistics, lower pretreatment costs, and additional revenue for the industry in the off-season. The findings revealed how over 80% of the SCB biomass was biorefined to yield the target products with a zero-liquid discharge strategy
[28]. Another different point from SCB is the fact that it usually provides a high organic content (>90% on a total solids basis) which results in a high theoretical biofuel yield
[29]. It is emphasized that SGB is in a niche as it is being used on a large scale for bioenergy and biorefinery, and therefore provides a training ground for new innovative technologies
[30]. To move further away from the chemical composition of SCB, the world’s annual SCB has a lignocellulose potential of 243 million tons, or 4.3 EJ on an energy basis, equal to 6.8% of the world’s current bioenergy supply. It completes to give prominence to the potential of SCB lignocellulose compared to other feedstocks and its value as a renewable resource
[29][30]. Since cellulose, hemicellulose and lignin have a high association, it is necessary to apply a pretreatment method that disrupts the plant cell wall organization so the polysaccharides can be more accessible to enzymes
[7]. As a suitable application of SE as pretreatment for bioethanol production using lignocellulosic biomass, SCB has been studied as a potential feedstock by different authors
[15][31][32].
As previously explained, SE pretreatment achieves hemicellulose hydrolysis, lignin transformation and cellulose crystallization by applying high temperatures (160–270 °C) and pressurized steam (20–50 bar) for a time that varies between seconds and minutes
[33]. Therefore, when SE is applied, there are different parameters affecting the sugar release off the feedstock. The most relevant ones include particle size, temperature, residence time and the combination of temperature and residence time, also named SF
[33]. In this way, the different applications of SE using SCB have been optimized by several authors. Their achievements and conclusions are presented below. For instance, Espirito Santo et al.
[34] studied SE applied in SCB at different conditions, including the combinatorial use of SE with H
2SO
4 and SE with H
3PO
4. A better cellulose yield was achieved when SE was simply applied since the combined used with H
3PO
4 led to higher lignin yield, whereas the incorporation of H
2SO
4 led to a higher hemicellulose yield, as it is shown in
Table 2. The optimized time, pressure and temperature conditions where the cellulose released was the highest for each pretreatment were 200 °C, 10.5 min, 14.2 atm (SE); 180 °C, 4 min, 10 atm (SE + H
2SO
4); and 195 °C, 7.5 min, 14.2 atm (SE + H
3PO
4)
[34]. Moreover, results obtained in this study showed that SE pretreatments using high temperature and short residence time lead to better yields than the combination of low temperature and long residence time. This phenomenon is explained because of the accumulation of fermentation inhibitory by-products, such as organic acids, furan compounds and phenolic acids, that ultimately lead to yield losses
[34]. Other authors also studied the SCB pretreatment with SE. Results showed how short residence time pretreatments lead to better hemicellulose removal, in agreement with the outcomes of other previously published studies
[35]. The results indicated that the higher removal of hemicellulose and lignin was obtained when operational conditions of SE were 210 °C, 15 min and 1% of H
2O
2, achieving 92.4 and 29.7% removal, respectively
[35]. Researchers also compared SE and acid hydrolysis (AH) applied in SCB to obtain bioethanol
[36]. Their results showed a six-times higher carbohydrate yield when SE was applied when compared to AH. Moreover, the negative impact of long residence time was confirmed since results showed that pretreatment over 30 min leads to lower total amount of carbohydrates
[36]. In fact, the higher yields of carbohydrates obtained in this study were when SE was applied with 160 °C, 30 min and 6.805 atm, as it is shown in
Table 2 [36]. Autohydrolysis (AHS) of SCB was also studied as an environmentally friendly pretreatment to obtain bioethanol. In fact, the authors ran a study where AHS pretreatment was applied in SCB matrix to obtain xylooligosaccharides
[37]. The yields achieved after this pretreatment ranged from 51.88 to 66.67% of bioethanol. The differences obtained were related to the use of a buffer solution that the stabilized pH and led to a maintenance of both cellulose and yeast activity
[37]. A novel pretreatment methodology applied to SCB was run by Duy The Pan and Chung-Sung Tan by using supercritical CO
2 [38]. The authors compared the glucose recovery obtained after 72 h of enzymatic hydrolysis with three pretreatments: single supercritical CO
2, supercritical CO
2 followed by H
2O
2 and supercritical CO
2 followed by ultrasound. The higher glucose recovery, with a yield of 97.8%, was obtained when supercritical CO
2 was combined with H
2O
2. Moreover, this pretreatment was the only capable of increasing the glucose recovery after 48 h of enzymatic hydrolysis
[38]. Considering that one shortcoming of obtaining bioethanol from SCB is the inhibition produced by different compounds, including lignin, the authors ran a study where sequential NaOH and hydroxymethylation pretreatment was applied
[39]. The authors compared this sequential process with the single alkaline pretreatment, achieving an increment of 13% of bioethanol, which was linked to the lower lignin content found in the sequential NaOH pretreatment followed by a hydroxymethylation process
[39].
Table 2. Comparison of bioethanol and other compounds recovery considering different sugarcane bagasse (SCB) pretreatments.
Overall, and as it is shown in Table 2, there are several works that have studied the suitability of SE and other pretreatments in SCB as biomass. In this way, SE has shown successful results that strongly support the application of this pretreatment to produce bioethanol through the reutilization of SCB.
3. Steam Explosion as Lignocellulosic Biomass Pretreatment
SE was pioneered and patented as a biomass pretreatment process in 1926 by Mason
[42]. SE pretreatment is a physicochemical modification technology that couples autohydrolysis and biomass alteration through high temperature and explosive decompression with application in food raw materials
[32]. SE processes can be operated in continuous or batch mode. Batch reactors are usually used for laboratory-scale pretreatment, while continuous systems are typically used for large-scale industrial processes
[32][42]. The lignocellulosic materials that can be treated with SE are extensively diverse
[43]. Indeed, its competence has been successfully demonstrated in the fractionation of a broad range of lignocellulosic raw materials, such as wheat straw, hay, SCB, corn stover, birch wood and numerous other chemical platforms from a large range of lignocellulosic feedstocks
[44][45].
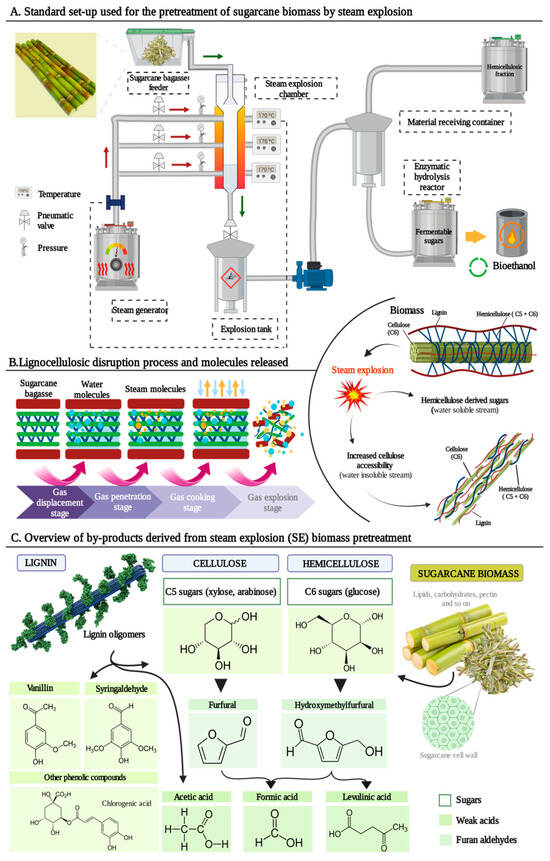
Figure 1A shows a schematic diagram of the continuous operation process of SE using SCB as biomass where the main three parts of the equipment are represented: steam generator, steam explosion chamber, and material receiving container
[46].
Figure 1. An insight into steam explosion (SE) pretreatment for bioethanol production using sugarcane bagasse (SCB) as biomass. (A). Diagram of industrial SE pretreatment of SCB for bioethanol production. (B). Structural changes of SCB during SE pretreatment. (C). By-products obtained after applying the steam explosion pretreatment in sugarcane biomass.
The SE process is usually divided into two independent stages. An initial one where the vapor boiling and explosion phase takes place, hence along this stag thermochemical reactions operate. For this, SE presses steam at high pressure (1–3.5 MPa) and temperature (180–240 °C) into cell walls and plant tissues for an abbreviated period (30 s) to several minutes (20 min). The second phase, in which physical tearing occurs, is a process of adiabatic expansion and conversion of thermal energy into mechanical energy
[47][48]. Thus, its high throughput relies on combination of the thermochemical action of high-temperature boiling coupled with the physical tearing action of instantaneous blasting
[32][46][49]. Temperature and residence time are known as the combined pretreatment severity factor (SF). In this regard, the recalcitrance of the biomass (e.g., lignin content) to the hydrolysis process is one of the conditions that most affects this factor
[44][50]. Generally, SF is utilized in the analysis of reaction kinetics with solid and liquid phases involved. In this way, the aim of the SF calculation is to come up with a pretreatment strategy approach that fulfills the expected requirements for the product and the process
[51]. Specifically, the SF was designed to enable both process monitoring and prediction of cellulose, hemicellulose and lignin after pretreatment. The effects of pretreatment are assumed to follow first-order kinetics and to fit to the Arrhenius equation
[52][53]. Hence, SF is an influential parameter that defines the relationship between hydrothermal severity (operating conditions and physicochemical changes) and lignocellulosic biomass fractionation
[54], and provides an estimation of the intensity of the SE treatment. Indeed, to reach the maximal efficiency of SE pretreatment, it is required to optimize the factors that modulate the toughness of the pretreatment conditions. The inter-dependence of these factors (SF) is calculated through
R0, a parameter that may be considered as a scaling strategy for a batch operation of the SE reactor (Equation (1)).
It is calculated by combining the lignocellulose pretreatment reaction time and temperature related to the boiling point of pure water into one single parameter, where,
Texp refers to the experimental temperature. Therefore, 100 is the reference temperature and 14.75 is the arbitrary constant ω being the activation energy of the first order kinetics
[55].
Other influencing factors are biomass particle size, moisture content, the rate of diffusion of vapor and liquid through the particle, the ratio of solid to liquid loaded in the SE container, the presence of chemical solvents involved in previous steps, or the addition of a chemical catalyst prior to steam pretreatment
[44][50][56]. To determine the optimal combinations of these factors, it would be required to carry out an endless series of experiments which are strongly minimized through the application of statistical designs of the experiments
[57].
To propose the development of a factorial experiment that will cover all possible combinations of the selected levels, considering the impact of all factors and the interactions between them, would serve as a powerful tool that brings the most comprehensive insight into the behavior of the system
[58]. For example, in a work assessing the influence of pretreatment SF on the fractionation of softwood using a protic acidic ionic liquid, the statistical analysis consisted of the design of a three-level business requirement document (BRD)/response surface method (RSM) involving three key pretreatment variables. Thus, the respective levels of each variable were 20, 30 and 40 min (time); 160, 170 and 180 °C (temperature); and 70, 80 and 90 wt%, while the response was the extraction of lignin. The key conditions of the process made it possible to achieve a quick pretreatment, which yielded a pulp rich in highly digestible cellulose (>90% glucose yield)
[52].
In a deeper way, in the above two vapor explosion phases, the following processes are principally involved: acid hydrolysis, thermal degradation, mechanical fracture, hydrogen bond breakdown and structural rearrangement
[46]. Subsequently, these stages will be developed focusing on a specific feedstock.
Figure 1B shows the structural changes of the three main components of lignocellulosic biomass during SE pretreatment. This hydrolysis leads to the decomposition of the lignocellulosic raw material by the alteration of the chemical structure of lignin. Lignin depolymerizes by cleavage of the β-O-4 bonds, and the fragments condense, giving rise to a more stable polymer
[59]. This depolymerization may eventually trigger a partial removal and/or redistribution of lignin
[60]. The alteration of the native lignin structure and its redeposition in the pretreated biomass are complicated interactions. They are dependent on the source of the biomass and the detailed heat and mass transfer reactions occurring inside the specific SE reactor and still require intensive investigation
[61]. Therefore, the removal of biomass components such as hemicelluloses and lignin will lead to a significant increase in glucose yield after enzymatic hydrolysis
[62]. This increase in yield can range from 20% to 85%, depending in many cases on the severity conditions used during SE
[63]. Thus, the benefits of hydrolysis, apart from enhancing the extractability of lignin polymer, result in the enhancement of the biodegradability of the raw material. More specifically it involves the release of mono- and oligosaccharides, the improvement of cellulose accessibility and the reduction in the crystallinity index of the holocellulosic content
[64][65]. In the case of cellulose, it suffers nearly no structural changes, it is mostly retained in its original form, and only mild depolymerization occurs under soft reaction conditions. However, apart from the solubilization of carbohydrate polymers into soluble sugars (mainly glucose, xylose and arabinose), the pretreatment also results in the formation of lignocellulosic by-products, as illustrated by
Figure 1C
[66].
Nowadays, SE is considered to be the only physical pretreatment method that can be applied alone or in combination with other chemical pretreatments to efficiently delignify biomass
[67]. This has often been combined, for example, with wet oxidation intended to treat larger particle sizes and to operate with higher substrate loadings
[68]. Further outstanding benefits of SE pretreatment are the extensive hydrolysis of hemicellulose polymers and the reduction in biomass particle size
[13][61]. Smaller particles have more available surface area and the lignin droplets act as a binder, which improves particle-to-particle contact and binding capacity
[32]. Also, SE has a high potential for energy efficiency, low capital investment, and lower environmental impact compared to other pretreatment technologies.
[69]. Nonetheless, a significant contribution of studies is still needed to propose an economically valuable utilization of biomasses such as SCB and to explore the different limitations that may arise when selecting a pretreatment as SE.