The necessity of finding alternative pathways for power supplementation in a more sustainable way has been promoted because of the current carbon emissions that contribute to climate change
[1]. Thus, the energy-from-waste (EfW) concept has been introduced as an alternative pathway where the use of refuse-derived flues, household waste and non-hazardous industrial by-products are considered as potential sources for energy production
[1]. This productive model fits with the sustainable model of circular economy in which the concept ‘end-of-life’ is replaced by reutilization, recycling and recovering. Hence, by-products or wastes from one industry become raw material for another. In addition, another productive model can be easily combined with bio-economy, which promotes the use renewables based on biomass by-products
[2]. Indeed, in the past years, the production of renewable fuels using lignocellulosic waste from agricultural activities has been considered as an alternative to traditional fuels
[3]. Agricultural residues are defined as unusable and unstable materials derived from agricultural production which are directly linked to the cultivation of crops, and these materials are characterized by their biodegradability and solid and lignocellulosic composition
[3][4][3,4]. In this way, the lignocellulosic non-edible biomass discarded by agriculture feedstock can be used as raw material to obtain biofuel, being considered second-generation (2G) biofuel
[1][5][1,5]. Lignocellulosic biomass is mainly composed by cellulose, hemicellulose, and lignin, which are low energy-density compounds, so a pretreatment step is necessary so plant-specific enzymes can release sugars for biofuel production
[5][6][5,6]. Moreover, 2G biofuels present several advantages compared to first generation biofuels, such as the cheaper non-edible matter used since it is the waste obtained as a result of an industrial activity; no fuel–food competition since non-edible matrices are used; and the reuse of by-products obtained after pretreatment of raw materials to obtain animal fodder to be used, for example, in livestock feed, thus reinforcing the circular economy. In this way, sugarcane bagasse (SCB), wheat, barley, rice and corn straw, and sorghum can be used for bioethanol production
[7]. In the specific case of sugarcane production, it has an associated production of residues that may reach up to 30% of the production
[8], which may represent more than 100 MT of residues. This huge volume of residues has several drawbacks. From an economic point of view, they require being effectively managed, which has an associated cost for the industry. From an environmental point of view, they represent a potential source of CO
2 since they are likely to be burned or, if not, accumulated in landfills, which threatens the quality of environmental (i.e., air and water pollution and noise, among others, and their potential consequences) and public health. Therefore, the reutilization of these by-products as part of a circular economy model is key to reduce management costs of residues to sugarcane industries and their negative environmental impact. In fact, their reutilization as raw material for bioethanol production has double benefits. It would avoid the release of this biomass to the environment, and it would promote the use of bioethanol produced using SCB, which is less carbon intensive compared with fossil fuel, so air pollution can be reduced
[9]. Therefore, these two approaches have the potential of reducing the footprint of sugarcane industries and fuel utilization.
2. Sugarcane Bagasse as a Potential Raw Material for Bioethanol Production
Sugarcane (
Saccharum oficinarum L.) is a tropical grass that belongs to the Gramineae family and the
Saccharum spp. genus
[10][22] and is characterized for being large and perennial. The sugarcane cultivation requirements are 6–12 months to grow with 60–100 cm
3 of water
[11][23]. Brazil, China and India are the main producers of SCB, with almost 500 MT generated every year from the sugarcane industry, which provides an important contribution to economic development
[9][10][12][9,22,24]. SCB yielded the highest crop straw production between 2012 and 2022 according to the Food and Agricultural Organization Corporate Statistical Database (FAOSTAT)
[13][25]. Sucrose is the main product of sugarcane, which accumulates in the internodes of the stalk
[9]. However, the percentage of waste generated during sugarcane production varies between 25 and 30%
[8], which may represent 125–150 MT of residues. The residues produced by the sugarcane industry are mainly two types, and they can be classified as straw, which is the harvest residue, and as bagasse, which is the fibrous fraction after the extraction of the sugarcane stem juice
[10][22]. These two by-products are characterized by their lignocellulosic composition, with cellulose, lignin and hemicellulose being the major components and having also extractants and ashes in their composition
[14][26]. SCB is composed of approximately 45–50% cellulose, 25–30% hemicellulose, 25% lignin and 2.4–9% ash
[9]. Nevertheless, this may vary depending on different factors such as chemical composition of the soil, climatic conditions and variety of the crop, among others
[9].
Table 1 shows variations in the composition of SCB, sugarcane fiber and sugarcane straw. The relative abundances of component units are usually calculated on the basis of the volume integration of the raw material and are expressed in percentage of dried weight
[15][27]. The chemical characterization of vegetal biomass is highly relevant for their consideration as potential sources of carbon to produce bioethanol. Indeed, for bioethanol production, it is important to evaluate the cellulose, hemicellulose and lignin composition of the raw material
[9]. Owing to its high yield of sugar and lignocellulosic biomass, SCB is regarded as an excellent alternative energy source to substitute fossil fuels
[16][17][28,29].
Table 1.
Composition of the main sugarcane chemical compounds expressed in percentage of dried weight.
As stated above, biomass characterization is a noteworthy step for establishing optimal process conditions. Hence, delineating the specificities of SCB as a lignocellulosic feedstock is key to its close scalability. Concerning these aspects, several authors
[22][34] conducted a study in which 60 bagasse samples were characterized. The results provided the following average of the structural compounds of SCB: 42.2% of cellulose, 27.6% of hemicelluloses, 21.6% of lignin, 5.63% of extractives and 2.84% of ashes. As can be seen in
Table 1, the composition of the samples did not differ substantially between them. This research supports the vantage of using SBC for obtaining bioethanol since the bioethanol yield is closely related to the biomass composition.
Moreover, the composition of feedstocks tends to vary depending on a large number of factors, so the stability of SCB may be considered an important advantage over other raw materials
[27][38]. Another work serves as example of the potential of SCB to obtain bioethanol
[28][39], where the authors considered the biorefinery route to convert SCB into various products, such as nanocrystalline cellulose, lignin and biohydrogen. They further outlined the rationale for selecting SCB as feedstock, arguing the availability of surplus bagasse, elimination of logistics, lower pretreatment costs, and additional revenue for the industry in the off-season. The findings revealed how over 80% of the SCB biomass was biorefined to yield the target products with a zero-liquid discharge strategy
[28][39]. Another different point from SCB is the fact that it usually provides a high organic content (>90% on a total solids basis) which results in a high theoretical biofuel yield
[29][40]. It is emphasized that SGB is in a niche as it is being used on a large scale for bioenergy and biorefinery, and therefore provides a training ground for new innovative technologies
[30][41]. To move further away from the chemical composition of SCB, the world’s annual SCB has a lignocellulose potential of 243 million tons, or 4.3 EJ on an energy basis, equal to 6.8% of the world’s current bioenergy supply. It completes to give prominence to the potential of SCB lignocellulose compared to other feedstocks and its value as a renewable resource
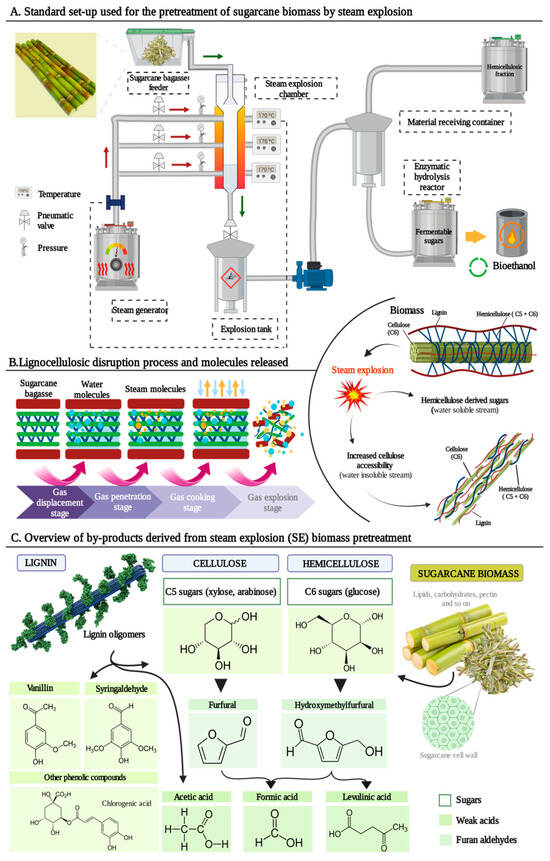
[29][30][40,41]. Since cellulose, hemicellulose and lignin have a high association, it is necessary to apply a pretreatment method that disrupts the plant cell wall organization so the polysaccharides can be more accessible to enzymes
[7]. As a suitable application of SE as pretreatment for bioethanol production using lignocellulosic biomass, SCB has been studied as a potential feedstock by different authors
[15][31][32][27,42,43].
As previously explained, SE pretreatment achieves hemicellulose hydrolysis, lignin transformation and cellulose crystallization by applying high temperatures (160–270 °C) and pressurized steam (20–50 bar) for a time that varies between seconds and minutes
[33][44]. Therefore, when SE is applied, there are different parameters affecting the sugar release off the feedstock. The most relevant ones include particle size, temperature, residence time and the combination of temperature and residence time, also named SF
[33][44]. In this way, the different applications of SE using SCB have been optimized by several authors. Their achievements and conclusions are presented below. For instance, Espirito Santo et al.
[34][45] studied SE applied in SCB at different conditions, including the combinatorial use of SE with H
2SO
4 and SE with H
3PO
4. A better cellulose yield was achieved when SE was simply applied since the combined used with H
3PO
4 led to higher lignin yield, whereas the incorporation of H
2SO
4 led to a higher hemicellulose yield, as it is shown in
Table 2. The optimized time, pressure and temperature conditions where the cellulose released was the highest for each pretreatment were 200 °C, 10.5 min, 14.2 atm (SE); 180 °C, 4 min, 10 atm (SE + H
2SO
4); and 195 °C, 7.5 min, 14.2 atm (SE + H
3PO
4)
[34][45]. Moreover, results obtained in this study showed that SE pretreatments using high temperature and short residence time lead to better yields than the combination of low temperature and long residence time. This phenomenon is explained because of the accumulation of fermentation inhibitory by-products, such as organic acids, furan compounds and phenolic acids, that ultimately lead to yield losses
[34][45]. Other authors also studied the SCB pretreatment with SE. Results showed how short residence time pretreatments lead to better hemicellulose removal, in agreement with the outcomes of other previously published studies
[35][46]. The results indicated that the higher removal of hemicellulose and lignin was obtained when operational conditions of SE were 210 °C, 15 min and 1% of H
2O
2, achieving 92.4 and 29.7% removal, respectively
[35][46]. Researchers also compared SE and acid hydrolysis (AH) applied in SCB to obtain bioethanol
[36][47]. Their results showed a six-times higher carbohydrate yield when SE was applied when compared to AH. Moreover, the negative impact of long residence time was confirmed since results showed that pretreatment over 30 min leads to lower total amount of carbohydrates
[36][47]. In fact, the higher yields of carbohydrates obtained in this study were when SE was applied with 160 °C, 30 min and 6.805 atm, as it is shown in
Table 2 [36][47]. Autohydrolysis (AHS) of SCB was also studied as an environmentally friendly pretreatment to obtain bioethanol. In fact, the authors ran a study where AHS pretreatment was applied in SCB matrix to obtain xylooligosaccharides
[37][48]. The yields achieved after this pretreatment ranged from 51.88 to 66.67% of bioethanol. The differences obtained were related to the use of a buffer solution that the stabilized pH and led to a maintenance of both cellulose and yeast activity
[37][48]. A novel pretreatment methodology applied to SCB was run by Duy The Pan and Chung-Sung Tan by using supercritical CO
2 [38][49]. The authors compared the glucose recovery obtained after 72 h of enzymatic hydrolysis with three pretreatments: single supercritical CO
2, supercritical CO
2 followed by H
2O
2 and supercritical CO
2 followed by ultrasound. The higher glucose recovery, with a yield of 97.8%, was obtained when supercritical CO
2 was combined with H
2O
2. Moreover, this pretreatment was the only capable of increasing the glucose recovery after 48 h of enzymatic hydrolysis
[38][49]. Considering that one shortcoming of obtaining bioethanol from SCB is the inhibition produced by different compounds, including lignin, the authors ran a study where sequential NaOH and hydroxymethylation pretreatment was applied
[39][50]. The authors compared this sequential process with the single alkaline pretreatment, achieving an increment of 13% of bioethanol, which was linked to the lower lignin content found in the sequential NaOH pretreatment followed by a hydroxymethylation process
[39][50].
Table 2.
Comparison of bioethanol and other compounds recovery considering different sugarcane bagasse (SCB) pretreatments.