Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agnieszka Maruszewska | -- | 1268 | 2023-02-03 09:55:31 | | | |
| 2 | Sirius Huang | Meta information modification | 1268 | 2023-02-03 11:43:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Gutowska, I. Fluoride as an Environmental Toxin. Encyclopedia. Available online: https://encyclopedia.pub/entry/40804 (accessed on 07 February 2026).
Żwierełło W, Maruszewska A, Skórka-Majewicz M, Gutowska I. Fluoride as an Environmental Toxin. Encyclopedia. Available at: https://encyclopedia.pub/entry/40804. Accessed February 07, 2026.
Żwierełło, Wojciech, Agnieszka Maruszewska, Marta Skórka-Majewicz, Izabela Gutowska. "Fluoride as an Environmental Toxin" Encyclopedia, https://encyclopedia.pub/entry/40804 (accessed February 07, 2026).
Żwierełło, W., Maruszewska, A., Skórka-Majewicz, M., & Gutowska, I. (2023, February 03). Fluoride as an Environmental Toxin. In Encyclopedia. https://encyclopedia.pub/entry/40804
Żwierełło, Wojciech, et al. "Fluoride as an Environmental Toxin." Encyclopedia. Web. 03 February, 2023.
Copy Citation
Fluorine in its elemental form is practically not found on Earth, but it is present in the ecosphere in the form of fluorine compounds. A growing body of literature suggests that labelling fluorides as an environmental toxin appears to be correct.
fluoride
environmental pollution
neurotoxicity
1. Fluoride as an Environmental Toxin
Fluoride is regarded as an environmental pollutant associated with serious effects on the functioning of organisms and ecosystems [1]. Fluorine in its elemental form is practically not found on Earth, but it is present in the ecosphere in the form of fluorine compounds. They occur naturally in a wide variety of minerals in the Earth’s crust, from where fluorides are released into the soil and water through the Earth’s volcanic activity and rock erosion [2][3]. Fluoride may pose a threat to human health, which has been specifically documented for populations inhabiting industrialised areas. In these areas, soil and water fluoride levels are elevated due to release from anthropogenic sources. These include fertilizers, pesticides, and deposits of industrial air pollution. Sources of industrial fluoride emissions include combustion of fluoride-rich coal, petroleum refining, production of steel, clay, glass, enamels, bricks and ceramics, manufacture of chemicals, and nuclear fuels [1][2].
The fluorides distributed in soil, air, and water are accumulated by plants and animals [4]. Consequently, drinking water, which may also be artificially fluoridated as a public health measure, and food are major sources of fluoride uptake in humans. The degree of fluoride exposure is affected by the quality of food and water, the amount consumed, as well as individual variability [5][6][7][8].
The harmfulness of fluoride has been the topic of intense debate in the last twenty years. There are different opinions as to the role of fluorine as an essential element and the magnitude of its toxic effects on humans (especially through water fluoridation) [9], but a growing body of literature suggests that labelling fluorides as an environmental toxin appears to be correct.
The effects of fluoride on the human body can be considered in two ways. Low supply of fluoride interferes with dental enamel formation and promotes growth of cariogenic oral bacteria, leading to dental caries. Fluoride deficiency also causes bone demineralization [1][3][10]. On the other hand, through complex molecular mechanisms of fluoride action on the cellular level, acute and chronic exposure to elevated doses may trigger a broad spectrum of disorders, both physiological and developmental.
Fluoride has been shown to inhibit or activate numerous enzymes crucial for cell metabolism and signalling (Figure 1). It suppresses the activity of Mg-dependent enzymes, including those that catalyse glycolytic reactions. It has also been shown to inhibit pyrophosphatases, ATPases, acetylcholinesterase, and cytochrome c oxidase. On the other hand, stimulatory effects of fluoride have been observed in, for example, glycogen phosphorylase, aspartate transaminase, and tyrosine kinase [9]. Furthermore, fluoride influences intracellular signal transduction pathways by affecting signalling cascades involving, e.g., G proteins, adenylate cyclase, Hedgehog proteins, and transcription factors such as NF-κB and Nrf2 [11][12]. It has also been demonstrated to induce abnormal methylation in some regions of the genome [13]. Consequently, with increased exposure, fluorine compounds can exert toxic effects, including organelle damage, oxidative stress on the cellular level, cell cycle disruption, inflammatory cytokine secretion, induction of apoptosis, and disruption of synaptic neurotransmission [9][14][15]. Fluoride is also regarded as a potential endocrine disruptor leading to the development of thyroid dysfunction [16].
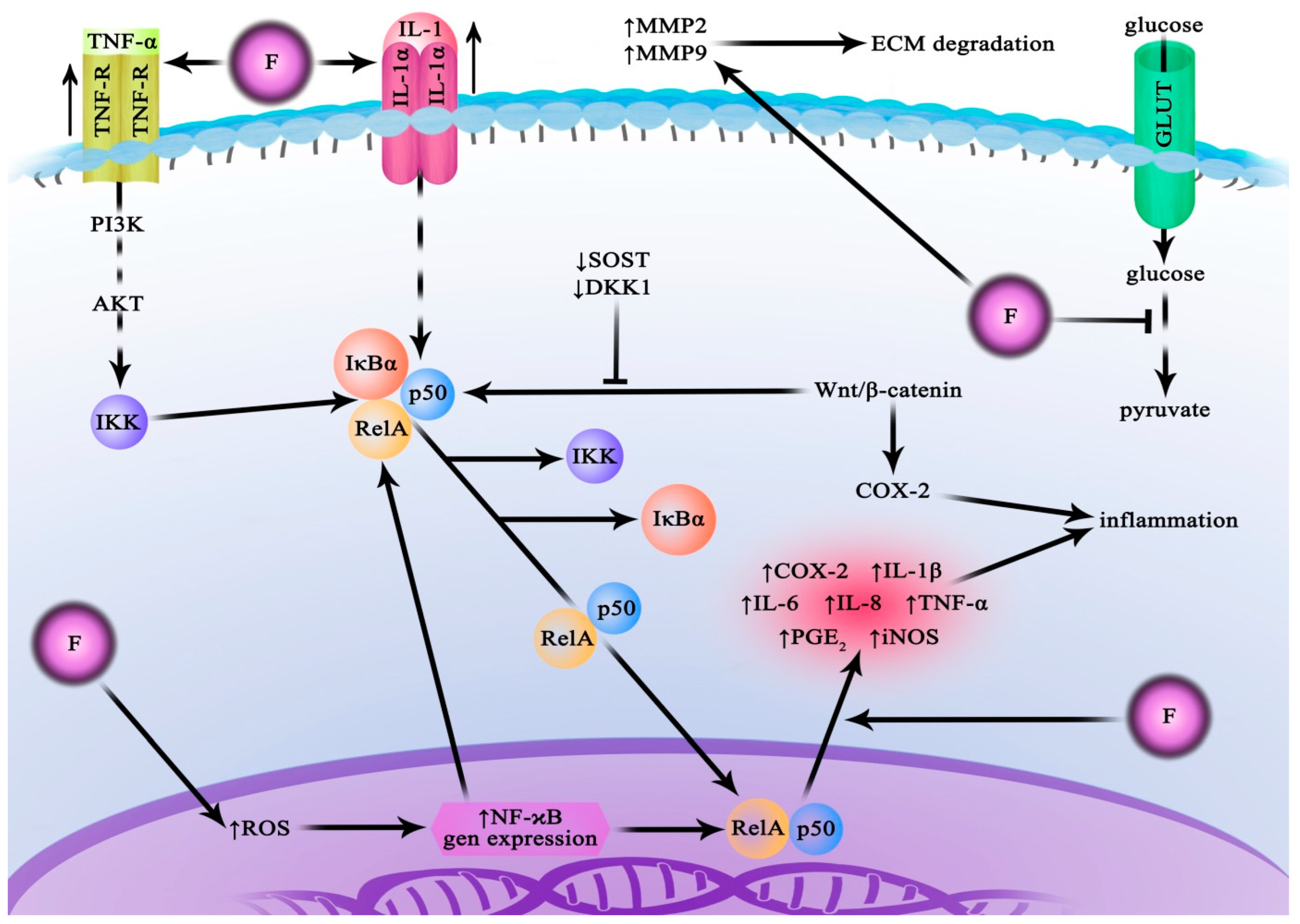
Figure 1. Fluoride action in normal cells. Fluoride affects normal cells in a pro-inflammatory manner, which is associated with the activation of the NF-kB pathway. As an inhibitor of glycolysis enzymes, it leads to disturbances in energy metabolism. AKT—protein kinase B; COX-2—cyclooxygenase-2; DKK1—Dickkopf-related protein 1; GLUT—glucose transporter; IKK—IκB kinase; Il—interleukin; iNOS—inducible nitric oxide synthases; IκBα—nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; MMP—matrix metalloproteinase; NaF—sodium fluoride; NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells; p50—NF-kappa-B p105 subunit; PGE2—prostaglandin E2; PI3K—phosphoinositide 3-kinase; RelA—NF-kappa-B p65 subunit; ROS—reactive oxygen species; SOST—clerostin protein; TNF—tumour necrosis factor; TNFR—tumour necrosis factor receptor; Wnt/β-catenin—Wnt signalling pathway.
2. Fluoride Neurotoxicity
One of the better-known toxic effects of chronic fluoride exposure is dental and bone fluorosis, manifested by structural abnormalities in dental enamel as well as bones, ligaments, and tendons [3]. Besides teeth and bones, fluoride accumulates in soft tissues, hence chronic exposure can cause damage to the liver, kidneys, cardiovascular system, and reproductive system [17][18][19][20][21][22][23]. Still, perhaps the most concerning data point to the significant role of fluoride as a neurotoxin, fluoride penetrates the BBB and alters the structure and function of nervous tissue [24][25][26][27]. Furthermore, in a study with a rat model, chronic fluoride exposure was shown to increase the levels of metalloproteinase 9 (MMP-9) and p53 protein, leading to cell apoptosis and damage of the blood–spinal cord barrier [28]. The neurodegenerative effects of fluoride are particularly critical in the early stages of biological development, which many authors attribute to its ability to cross the blood–placenta barrier [29]. Fluoride causes degenerative changes in all parts of the brain and in the spinal cord, including axon deterioration, myelin sheath degeneration, mitochondrial damage, and alterations in synaptic ultrastructure [26][30]. It also affects neurotransmitter metabolism and causes changes in the expression of neurotransmitter receptors [31][32][33]. Fluorine compounds impair energy metabolism of the brain, dependent primarily on the burning of glucose. Fluoride exposure may be associated with changes in the profile of proteins involved in energy metabolism [34], and researchers have suggested that impaired glucose metabolism in neurons is correlated with decreased expression of the GLUT-1 transporter [35]. On the other hand, increased glucose transport into brain cells has also been documented, although without changes in transporter expression, suggesting a compensatory mechanism in response to damage [36]. Chronic fluoride exposure also affects amino acid and lipid metabolism [31][37]. Neuronal damage as a result of exposure to high doses of fluoride is associated with the induction of cellular oxidative stress and inflammation. In vitro and in vivo studies have shown that fluoride increases ROS levels through lipid peroxidation, decreasing GSH levels, and suppressing antioxidant enzyme activity [38][39][40]. Fluoride exposure results in increased secretion of pro-inflammatory interleukins and decreased production of anti-inflammatory interleukins [11][38][41]. Fluoride-induced neuronal degeneration is associated with the activation of apoptotic signalling cascades [42], increased expression or higher levels of death receptors [43] and pro-apoptotic proteins [28][41][44], as well as caspase activation [38][43] and downregulation of anti-apoptotic protein expression [41]. Neuronal degeneration can also occur via autophagy (Figure 2) [45].
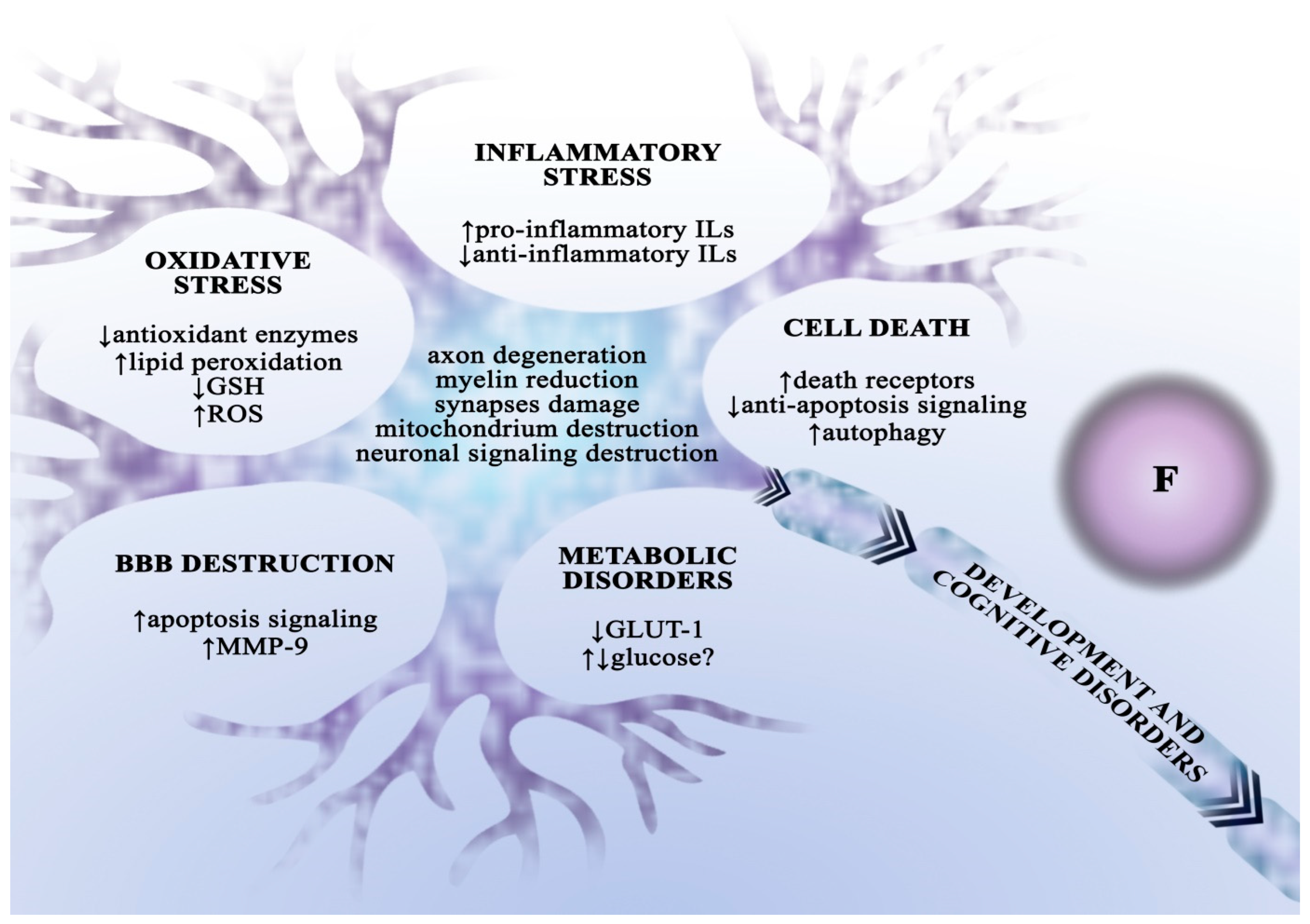
Figure 2. The neurodegenerative effect of fluoride. The toxic effect of fluoride on the central nervous system is multidimensional—it results from disturbances in metabolism regulation, synaptic functioning, the blood–brain barrier integrity, as well as oxidative stress and inflammation induction in neurons and microglia cells. These multidimensional interactions are believed to cause developmental and cognitive impairments in the body. ADHD—attention deficit hyperactivity disorder; BBB—blood–brain barrier; GLUT—glucose transporter; GSH—reduced glutathione; Ils—interleukins; ROS—reactive oxygen species.
The described molecular-level changes leading to neuronal degeneration manifest themselves in developmental and cognitive disorders that have been observed both in animal models and in population studies. It has been observed that chronic fluoride exposure during the prenatal period and early life may manifest as deficits in learning and memory, reduced non-verbal intelligence (PIQ), and lower intelligence quotient (IQ) [24][46][47][48][49]. Some authors have also suggested that elevated fluoride levels are correlated with the risk of dementia [50] and ADHD prevalence [51]. It should be noted that many authors disagree with these conclusions, arguing that population-based studies are incomplete. They also note that many of the behavioural studies were conducted in animal models utilizing acute doses [52][53][54][55]. Nevertheless, as Till and Green [56] point out, the evidence is relatively new and should rather be regarded as a potential early warning.
References
- Jha, S.; Mishra, V.; Sharma, D.; Damodaran, T. Fluoride in the Environment and Its Metabolism in Humans. In Reviews of Environmental Contamination and Toxicology Volume 211; Whitacre, D., Ed.; Springer New York: New York, NY, USA, 2011; pp. 121–142.
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; Lennon, M.A.; E Petersen, P.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and Oral Health. Community Dent Health 2016, 33, 69–99.
- World Health Organization. Preventing Disease through Healthy Environments: Inadequate or Excess Fluoride: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019.
- Vithanage, M.; Bhattacharya, P. Fluoride in the environment: Sources, distribution and defluoridation. Environ. Chem. Lett. 2015, 13, 131–147.
- Bombik, E.; Bombik, A.; Rymuza, K. The influence of environmental pollution with fluorine compounds on the level of fluoride in soil, feed and eggs of laying hens in Central Pomerania, Poland. Environ. Monit. Assess. 2020, 192, 1–11.
- Ghanbarian, M.; Ghanbarian, M.; Tabatabaie, T.; Ghanbarian, M.; Ghadiri, S.-K. Distributing and assessing fluoride health risk in urban drinking water resources in Fars Province, Iran, using the geographical information system. Environ. Geochem. Health 2021, 44, 771–781.
- Jaudenes, J.R.; Gutiérrez, J.; Paz, S.; Rubio, C.; Hardisson, A. Fluoride Risk Assessment from Consumption of Different Foods Commercialized in a European Region. Appl. Sci. 2020, 10, 6582.
- Riddell, J.; Malin, A.; McCague, H.; Flora, D.; Till, C. Urinary Fluoride Levels among Canadians with and without Community Water Fluoridation. Int. J. Environ. Res. Public Health 2021, 18, 6203.
- Strunecka, A.; Strunecky, O. Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks. Appl. Sci. 2020, 10, 7100.
- Medjedovic, E.; Medjedovic, S.; Deljo, D.; Sukalo, A. Impact of Fluoride on Dental Health Quality. Mater. Socio Medica 2015, 27, 395–398.
- Chen, L.; Kuang, P.; Liu, H.; Wei, Q.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; et al. Sodium Fluoride (NaF) Induces Inflammatory Responses Via Activating MAPKs/NF-κB Signaling Pathway and Reducing Anti-inflammatory Cytokine Expression in the Mouse Liver. Biol. Trace Elem. Res. 2019, 189, 157–171.
- Refsnes, M.; Skuland, T.; Schwarze, P.; Lag, M.; Ovrevik, J. Differential NF-κB and MAPK activation underlies fluoride- and TPA-mediated CXCL8 (IL-8) induction in lung epithelial cells. JIR 2014, 7, 169.
- Pan, X.; Yan, W.; Qiu, B.; Liao, Y.; Liao, Y.; Wu, S.; Ming, J.; Zhang, A. Aberrant DNA methylation of Cyclind-CDK4-p21 is associated with chronic fluoride poisoning. Chem. Interact. 2019, 315, 108875.
- Aulestia, F.J.; Groeling, J.; Bomfim, G.H.S.; Costiniti, V.; Manikandan, V.; Chaloemtoem, A.; Concepcion, A.R.; Li, Y.; Ii, L.E.W.; Idaghdour, Y.; et al. Fluoride exposure alters Ca2+ signaling and mitochondrial function in enamel cells. Sci. Signal. 2020, 13, eaay0086.
- Nagendra, A.H.; Bose, B.; Shenoy, P.S. Recent advances in cellular effects of fluoride: An update on its signalling pathway and targeted therapeutic approaches. Mol. Biol. Rep. 2021, 48, 5661–5673.
- Jianjie, C.; Wenjuan, X.; Jinling, C.; Jie, S.; Ruhui, J.; Meiyan, L. Fluoride caused thyroid endocrine disruption in male zebrafish (Danio rerio). Aquat. Toxicol. 2016, 171, 48–58.
- Cao, J.; Chen, Y.; Chen, J.; Yan, H.; Li, M.; Wang, J. Fluoride exposure changed the structure and the expressions of Y chromosome related genes in testes of mice. Chemosphere 2016, 161, 292–299.
- Han, H.; Sun, Z.; Luo, G.; Wang, C.; Wei, R.; Wang, J. Fluoride exposure changed the structure and the expressions of reproductive related genes in the hypothalamus–pituitary–testicular axis of male mice. Chemosphere 2015, 135, 297–303.
- Iano, F.G.; Ferreira, M.C.; Quaggio, G.B.; Fernandes, M.S.; Oliveira, R.C.; Ximenes, V.F.; Buzalaf, M.A.R. Effects of chronic fluoride intake on the antioxidant systems of the liver and kidney in rats. J. Fluor. Chem. 2014, 168, 212–217.
- Liu, H.; Gao, Y.; Sun, L.; Li, M.; Li, B.; Sun, D. Assessment of relationship on excess fluoride intake from drinking water and carotid atherosclerosis development in adults in fluoride endemic areas, China. Int. J. Hyg. Environ. Health 2014, 217, 413–420.
- Liu, P.; Li, R.; Tian, X.; Zhao, Y.; Li, M.; Wang, M.; Ying, X.; Yuan, J.; Xie, J.; Yan, X.; et al. Co-exposure to fluoride and arsenic disrupts intestinal flora balance and induces testicular autophagy in offspring rats. Ecotoxicol. Environ. Saf. 2021, 222, 112506.
- Raina, R.; Baba, N.A.; Verma, P.K.; Sultana, M.; Singh, M. Hepatotoxicity Induced by Subchronic Exposure of Fluoride and Chlorpyrifos in Wistar Rats: Mitigating Effect of Ascorbic Acid. Biol. Trace Elem. Res. 2015, 166, 157–162.
- Song, C.; Fu, B.; Zhang, J.; Zhao, J.; Yuan, M.; Peng, W.; Zhang, Y.; Wu, H. Sodium fluoride induces nephrotoxicity via oxidative stress-regulated mitochondrial SIRT3 signaling pathway. Sci. Rep. 2017, 7, 1–15.
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 1–17.
- Liu, Y.-J.; Gao, Q.; Wu, C.-X.; Guan, Z.-Z. Alterations of nAChRs and ERK1/2 in the brains of rats with chronic fluorosis and their connections with the decreased capacity of learning and memory. Toxicol. Lett. 2010, 192, 324–329.
- Reddy, P.; Reddy, K.; Kumar, K. Neurodegenerative Changes in Different Regions of Brain, Spinal Cord and Sciatic Nerve of Rats Treated with Sodium Fluoride. J. Med. Allied Sci. 2011, 1, 30–35.
- Wu, C.; Gu, X.; Ge, Y.; Jianhai, Z.; Wang, J. Effects of high fluoride and arsenic on brain biochemical indexes and learning-memory in rats. Fluoride 2006, 39, 274–279.
- Qing-Feng, S.; Ying-Peng, X.; Tian-Tong, X. Matrix metalloproteinase-9 and p53 involved in chronic fluorosis induced blood-brain barrier damage and neurocyte changes. Arch. Med. Sci. 2019, 15, 457–466.
- Opydo, J.; Borysewicz-Lewickaa, M. Transplacental passage of fluoride in pregnant Polish women assessed on the basis of fluoride concentrations in maternal and cord blood plasma. Fluoride 2007, 40, 46–50.
- Niu, Q.; Chen, J.; Xia, T.; Li, P.; Zhou, G.; Xu, C.; Zhao, Q.; Dong, L.; Zhang, S.; Wang, A. Excessive ER stress and the resulting autophagic flux dysfunction contribute to fluoride-induced neurotoxicity. Environ. Pollut. 2018, 233, 889–899.
- Bartos, M.; Gumilar, F.; Gallegos, C.E.; Bras, C.; Dominguez, S.; Cancela, L.M.; Minetti, A. Effects of Perinatal Fluoride Exposure on Short- and Long-Term Memory, Brain Antioxidant Status, and Glutamate Metabolism of Young Rat Pups. Int. J. Toxicol. 2019, 38, 405–414.
- Kupnicka, P.; Listos, J.; Tarnowski, M.; Kolasa-Wołosiuk, A.; Wąsik, A.; Łukomska, A.; Barczak, K.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Fluoride Affects Dopamine Metabolism and Causes Changes in the Expression of Dopamine Receptors (D1R and D2R) in Chosen Brain Structures of Morphine-Dependent Rats. Int. J. Mol. Sci. 2020, 21, 2361.
- Sun, Z.; Zhang, Y.; Xue, X.; Niu, R.; Wang, J. Maternal fluoride exposure during gestation and lactation decreased learning and memory ability, and glutamate receptor mRNA expressions of mouse pups. Hum. Exp. Toxicol. 2018, 37, 87–93.
- Lopes, G.O.; Ferreira, M.K.M.; Davis, L.; Bittencourt, L.O.; Aragão, W.A.B.; Dionizio, A.; Buzalaf, M.A.R.; Crespo-Lopez, M.E.; Maia, C.S.F.; Lima, R.R. Effects of Fluoride Long-Term Exposure over the Cerebellum: Global Proteomic Profile, Oxidative Biochemistry, Cell Density, and Motor Behavior Evaluation. Int. J. Mol. Sci. 2020, 21, 7297.
- Jiang, C.; Zhang, S.; Liu, H.; Guan, Z.; Zeng, Q.; Zhang, C.; Lei, R.; Xia, T.; Wang, Z.; Yang, L.; et al. Low Glucose Utilization and Neurodegenerative Changes Caused by Sodium Fluoride Exposure in Rat’s Developmental Brain. Neuromol. Med. 2014, 16, 94–105.
- Rogalska, A.; Kuter, K.; Żelazko, A.; Głogowska-Gruszka, A.; Świętochowska, E.; Nowak, P. Fluoride Alteration of Glucose Uptake in Wistar Rat Brain and Peripheral Tissues. Neurotox. Res. 2017, 31, 436–443.
- Guan, Z.-Z.; Wang, Y.-N.; Xiao, K.-Q.; Dai, D.-Y.; Chen, Y.-H.; Liu, J.-L.; Sindelar, P.; Dallner, G. Influence of Chronic Fluorosis on Membrane Lipids in Rat Brain. Neurotoxicol. Teratol. 1998, 20, 537–542.
- Adedara, I.; Olabiyi, B.; Ojuade, T.; Idris, U.; Onibiyo, E.; Farombi, E. Taurine reverses sodium fluoride-mediated increase in inflammation, caspase-3 activity, and oxidative damage along the brain-pituitary-gonadal axis in male rats. Can. J. Physiol. Pharmacol. 2017, 95, 1019–1029.
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Jakubczyk, K.; Tarnowski, M.; Lubkowska, A.; Baranowska-Bosiacka, I.; Styburski, D.; Skórka-Majewicz, M.; Maciejewska, D.; et al. Chronic Exposure to Fluoride Affects GSH Level and NOX4 Expression in Rat Model of This Element of Neurotoxicity. Biomolecules 2020, 10, 422.
- Shuhua, X.; Ziyou, L.; Ling, Y.; Fei, W.; Sun, G. A Role of Fluoride on Free Radical Generation and Oxidative Stress in BV-2 Microglia Cells. Mediat. Inflamm. 2012, 2012, 1–8.
- Yan, N.; Liu, Y.; Liu, S.; Cao, S.; Wang, F.; Wang, Z.; Xi, S. Fluoride-Induced Neuron Apoptosis and Expressions of Inflammatory Factors by Activating Microglia in Rat Brain. Mol. Neurobiol. 2016, 53, 4449–4460.
- Liu, X.-L.; Li, C.-C.; Liu, K.-J.; Cui, C.-Y.; Zhang, Y.-Z.; Liu, Y. The Influence of Fluoride on the Expression of Inhibitors of Wnt/β-Catenin Signaling Pathway in Rat Skin Fibroblast Cells. Biol. Trace Elem. Res. 2012, 148, 117–121.
- Xu, B.; Xu, Z.; Xia, T.; He, P.; Gao, P.; He, W.; Zhang, M.; Guo, L.; Niu, Q.; Wang, A. Effects of the Fas/Fas-L pathway on fluoride-induced apoptosis in SH-SY5Y cells. Environ. Toxicol. 2011, 26, 86–92.
- Tu, W.; Zhang, Q.; Liu, Y.; Han, L.; Wang, Q.; Chen, P.; Zhang, S.; Wang, A.; Zhou, X. Fluoride induces apoptosis via inhibiting SIRT1 activity to activate mitochondrial p53 pathway in human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 2018, 347, 60–69.
- Zhou, G.; Tang, S.; Yang, L.; Niu, Q.; Chen, J.; Xia, T.; Wang, S.; Wang, M.; Zhao, Q.; Liu, L.; et al. Effects of long-term fluoride exposure on cognitive ability and the underlying mechanisms: Role of autophagy and its association with apoptosis. Toxicol. Appl. Pharmacol. 2019, 378, 114608.
- Bashash, M.; Thomas, D.; Hu, H.; Martinez-Mier, E.A.; Sanchez, B.; Basu, N.; Peterson, K.; Ettinger, A.; Wright, R.; Zhang, Z.; et al. Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6–12 Years of Age in Mexico. Environ. Health Perspect. 2017, 125, 097017.
- Cao, K.; Xiang, J.; Dong, Y.-T.; Xu, Y.; Li, Y.; Song, H.; Zeng, X.-X.; Ran, L.-Y.; Hong, W.; Guan, Z.-Z. Exposure to fluoride aggravates the impairment in learning and memory and neuropathological lesions in mice carrying the APP/PS1 double-transgenic mutation. Alzheimer’s Res. Ther. 2019, 11, 35.
- Farmus, L.; Till, C.; Green, R.; Hornung, R.; Mier, E.A.M.; Ayotte, P.; Muckle, G.; Lanphear, B.P.; Flora, D.B. Critical windows of fluoride neurotoxicity in Canadian children. Environ. Res. 2021, 200, 111315.
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019, 173, 940–948.
- Russ, T.C.; Killin, L.O.J.; Hannah, J.; Batty, G.; Deary, I.J.; Starr, J.M. Aluminium and fluoride in drinking water in relation to later dementia risk. Br. J. Psychiatry 2020, 216, 29–34.
- Malin, A.J.; Till, C. Exposure to fluoridated water and attention deficit hyperactivity disorder prevalence among children and adolescents in the United States: An ecological association. Environ. Health 2015, 14, 17.
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415.
- Agalakova, N.I.; Nadei, O. Inorganic fluoride and functions of brain. Crit. Rev. Toxicol. 2020, 50, 28–46.
- Broadbent, J.M.; Thomson, W.M.; Ramrakha, S.; Moffitt, T.E.; Zeng, J.; Page, L.A.F.; Poulton, R. Community Water Fluoridation and Intelligence: Prospective Study in New Zealand. Am. J. Public Health 2015, 105, 72–76.
- Saeed, M.; Malik, R.N.; Kamal, A. Fluorosis and cognitive development among children (6–14 years of age) in the endemic areas of the world: A review and critical analysis. Environ. Sci. Pollut. Res. 2020, 27, 2566–2579.
- Till, C.; Green, R. Controversy: The evolving science of fluoride: When new evidence doesn’t conform with existing beliefs. Pediatr. Res. 2020, 90, 1093–1095.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
03 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




