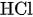| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | João Paulo Sousa | + 13848 word(s) | 13848 | 2021-09-13 05:10:07 | | | |
| 2 | Peter Tang | Meta information modification | 13848 | 2021-10-14 04:43:42 | | |
Video Upload Options
Redox Flow Batteries (RFB) are electrochemical energy storage devices that converts chemical energy into electrical energy through reversible oxidation and reduction of the working fluids. Redox flow batteries are considered by many to be a promising technology for the storage of energy for days or even weeks. Other advantages of RFBs are modularly and the ability to change the output power and energy capacity independently, by changing the size and number of cells in a stack and by adjusting the volume of electrolyte. Also, RFBs show a long lifecycle compared to lithium-ion batteries.
1. Introduction
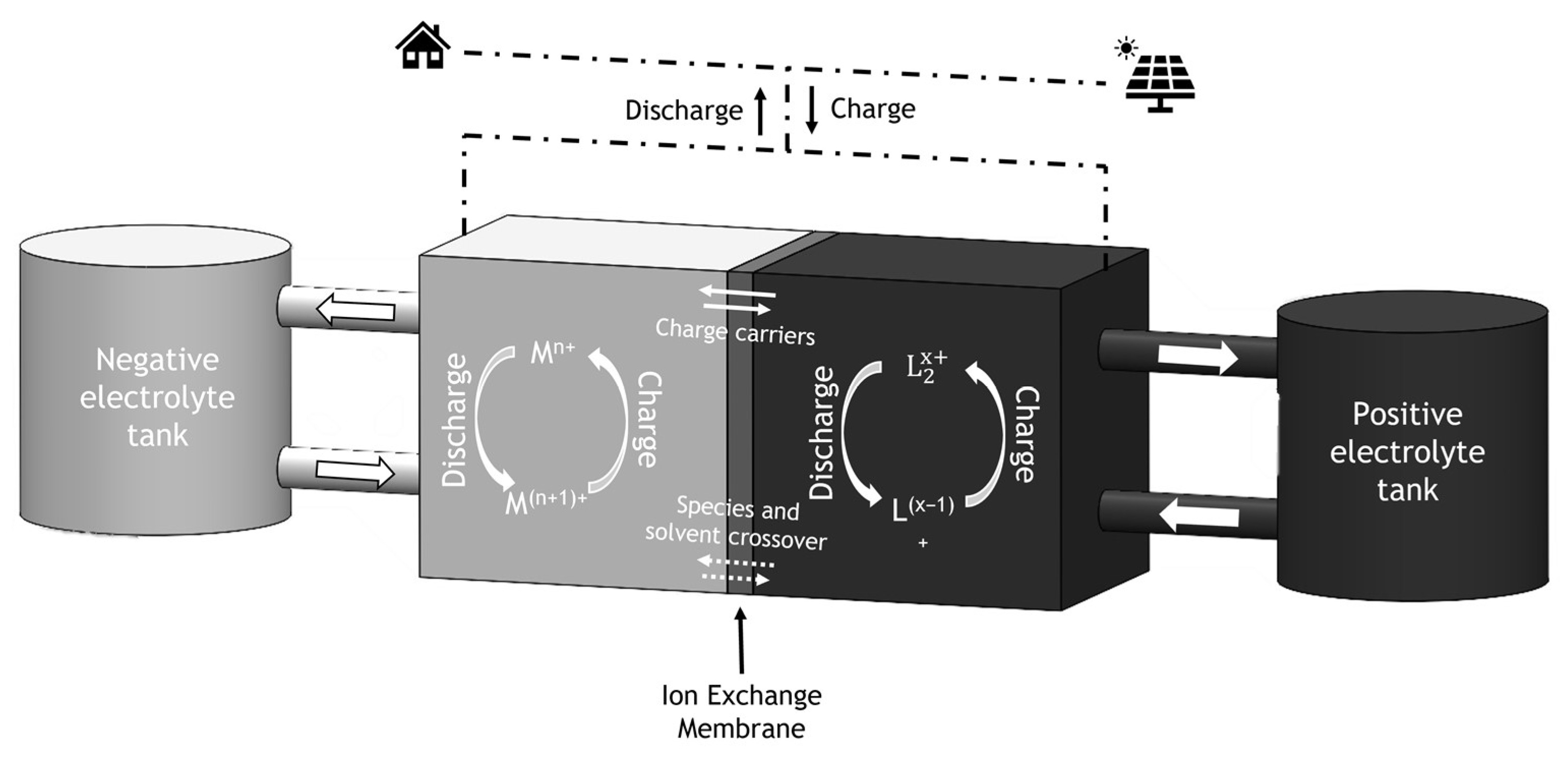
2. Redox Flow Batteries (RFB)
|
Year |
Ref. |
Authors |
Discussed Subject Matter |
|---|---|---|---|
|
2006 |
[9] |
Ponce de Léon et al. |
The different RFB systems were compared considering the OCP, power density, EE and charge–discharge behaviour. |
|
2011 |
[10] |
Weber et al. |
RFB chemistries, kinetics and transport of RFBs were discussed. The electrode/cell modeling and designs were reviewed as well as future research needs. |
|
[11] |
Li et al. |
The requirements for ion exchange membranes for VRFBs were reviewed, as well as the development prospects for next-generation materials. |
|
|
[12] |
Skyllas-Kazacos et al. |
Discussion focused on the technology in general. An historical review was also considered as well as the latest commercial developments and large-scale field testing. |
|
|
2012 |
[13] |
Kear et al. |
Development, commercialisation history, and current performance properties of intermediate- and large-scale VRFBs were reviewed. The potential for VRFB systems to meet the economic requirements was compared to the economic performance of thermal-based generators. |
|
[14] |
Leung et al. |
The development of RFB systems was reviewed. It was concluded that fundamental studies on chemistry and kinetics are necessary for many RFB technologies. |
|
|
[15] |
Wang et al. |
The chemistries and progresses of Li-ion and RFB were reviewed and compared. The authors discussed the research status of a Li–RFB hybrid system and concluded that it was still in its infancy. |
|
|
2013 |
[16] |
Wang et al. |
Review of the main developments, particullarly new chemistries reported since 2010. The field of NA-RFBs was also included (i.e., redox chemistries, new RFB configurations) and was limited to R&D on cell-level components, excluding stack system, e.g., flow-field simulations, shunt-current analysis, and bipolar plate development. |
|
[17] |
Shin et al. |
Non-aqueous RFB (NA-RFB) systems were compared to aqueous RFBs in terms of the current and power density through membranes. |
|
|
2014 |
[18] |
Chakrabarti et al. |
The application of ionic liquids (ILs) and deep eutetic solvents (DESs) in different RFB configurations was reviewed. The prospect of applying DESs in RFBs was discussed using the results reported in the literature considering the electrochemical engineering aspects of these solvents. |
|
[19] |
Alotto et al. |
The state-of-the-art of the most important plants in service and programs development were discussed. The most relevant research issues were debated. |
|
|
2015 |
[20] |
Pan and Wang |
The redox species of RFB were discussed. It was concluded that most of the non-aqueous electrolytes were focused on the catholyte, that the anodic species were limited, and that to fabricate a NA-RFB with high energy density, the development of anodic species was necessary. |
|
[21] |
Soloveichik |
A discussion on the different types of flow batteries was conducted. Technical and economical issues were also approached. |
|
|
[22] |
Kim et al. |
The technical trends in the selection, characterization, evaluation, and modification of electrodes for VRFBs were reviewed between 1985 and 2015. |
|
|
[23] |
Xu and Zhao |
The various issues associated with flow batteries were summarized and a critical review on the numerical investigations of each issue was performed. |
|
|
[24] |
Huang et al. |
NA-RFBs were compared with aqueous systems. The parameters included wider voltage windows, intrinsically faster electron-transfer kinetics, and more extended working temperature ranges. |
|
|
2016 |
[25] |
Winsber et al. |
Overview focused on different flow-battery systems ranging from the classical inorganic to organic/inorganic to RFBs with organic redox-active cathode and anode materials in terms of technical, economic, and environmental aspects. |
|
[26] |
Kowalski et al. |
Review focused on describing the main advances in the developments of redox active organic molecules for all-organic flow batteries. |
|
|
[27] |
Park et al. |
Review on the development of flow batteries focused on materials and chemistries, i.e., conventional aqueous RFBs and the next-generation flow batteries. Despite progress, next-generation battery systems based on organic, iodine, polysulfide or semi-solid materials are still uncertain. |
|
|
2017 |
[28] |
Arenas et al. |
Review focused on the engineering aspects of RFBs. An approach to RFB design and scale-up was performed in order to reduce the gap in technological and research awareness between the academic literature and the industry. |
|
[29] |
Leung et al. |
Review of organic based RFB. Emphasis was given to electrode reactions in both aqueous and non-aqueous electrolytes. It was concluded that organic RFB containing materials of high solubilities and multi-electron-transfers meet the cost target for practical applications at the grid scale and in the automotive industry. |
|
|
[30] |
Ye et al. |
The impact on the voltage efficiency, CE, and EE of the types and properties of membranes on the VRFBs were reviewed. Material modification of carbon-based electrodes, catalyst application, and electrolytes using solid redox-active compounds in semi-solid RFB systems were also discussed. |
|
|
[31] |
Choi et al. |
Vanadium electrolyte technologies from the viewpoint of VRFB design was reviewed providing a logical understanding of how the electrolyte design influences battery performance. |
|
|
[32] |
Li and Liu |
Review comparing the future of RFB technology with Li-ion batteries. The questions regarding breakthroughs needed to enable large-scale deployment of RFBs remain. It was concluded that finding a low-cost, highly soluble aqueous system was the most attractive approach. |
|
|
[33] |
Musbaudeen et al. |
Membraneless cell designs for RFBs were reviewed considering the evolutionary trend of membraneless flow cell design concepts. |
|
|
[34] |
Zhou et al. |
The progress in research on the transport phenomena of RFBs, as well as the critical transport issues, were reviewed. |
|
|
2018 |
[35] |
Chen et al. |
The review focused on the advantages of organic materials for RFBs compared with inorganic-based RFBs and on the recent progress in organic RFBs in redox active materials. The properties of the electrolyte and the design of the membrane, including polymeric and ceramic membranes, were also debated. |
|
[36] |
Zhang et al. |
The performance metrics of RFBs and the progress on the key components of RFBs, including the membranes and new redox-active electrolytes, were reviewed. |
|
|
[37] |
Liu et al. |
The state-of-the-art of several modification methods on the electrode materials for VRFB were reviewed. |
|
|
[38] |
Cao et al. |
Review focused on vanadium electrolyte additives studied for VRFB regarding its function, including precipitation inhibitors, immobilizing agents, kinetic enhancers, electrolyte impurities, and chemical reductants. |
|
|
[39] |
Xu et al. |
Review focus on understanding the evaluation criteria of energy efficiency for RFBs. |
|
|
[40] |
Ke et al. |
The first review focused on the influence on RFB cell performance linked with flow field designs, including their implementation in stacks. Several aspects were considered, e.g., flow field architecture types, flow distribution, cell performance, large-scale stack designs, stack performance, optimization of non-uniform flow distributions, shunt currents, and localized current distributions. |
|
|
[41] |
Arenas et al. |
Review focused on the four main types of RFB employing zinc electrodes, i.e., zinc–bromine, zinc–cerium, zinc–air and zinc–nickel. The main drawbacks linked with zinc deposition and dissolution, particularly in acid media, were also reviewed. |
|
|
[42] |
Minke and Turek |
The literature focused on the techno-economic assessment of VFB was reviewed. The data regarding materials, system designs, and modelling approaches were considred and critically analyzed. |
|
|
2019 |
[43] |
Lourenssen et al. |
The current state of the art of VRFB technology was discussed, including the design and working principles. The critical research areas were highlighted along with future developments. |
|
[44] |
Narayan et al. |
The authors discussed the basic requirements to be satisfied by next-generation aqueous RFBs and concluded that a safe, affordable, sustainable, and robust long-duration energy storage system was promising with next-generation RFBs. |
|
|
[45] |
Hogue and Toghill |
A review of the metal coordination complexes studied as electrolytes for NA-RFBs that were reported in the previous decade. |
|
|
[46] |
Gubler |
The review focused on the key requirements and current development trends for membranes and separators for the VRFB. |
|
|
[47] |
Arenas et al. |
The research needs were reviewed. It was concluded that most academic studies focus on the development of catalysts tested in small electrochemical cells was not realistic for advancing RFB technology; they are limited to short-term laboratory experiments. |
|
|
2020 |
[48] |
Rhodes et al. |
A review on NA-RFBs was conducted. It was concluded that these are predicted to be sustainable systems as the components are mostly organic materials and that NA-RFBs represent the next generation of RFB for green energy storage. |
|
[49] |
Clemente and Costa-Castelló |
A review focused on the RFB models and the main control strategies of RFB systems, as well as the main techniques to estimate the state of charge. |
|
|
[50] |
Gencten and Sahin |
The electrode materials for VRFBs were reviewed. It was concluded that graphene coatings, heteroatom doping, and metal oxide modified carbon-based electrodes were mainly used. Most of the work regarding VRFBs is focused on novel stack design, electrode, membrane, and electrolyte components. |
|
|
[51] |
Kwabi et al. |
The review focused on the electrolyte lifetime in aqueous organic RFB. It was concluded that RFBs are promising alternatives for surpassing lithium ion batteries and aqueous organic RFBs have potentially lower cost than their vanadium-based counterparts. |
|
|
[52] |
Zhong et al. |
The state of the art of organic electroactive molecules for aqueous and non-aqueous RFBs were reviewed. It was concluded that this field was still in its initial stage since no RFB has been deemed suitable to replace VRFBs. |
|
|
[53] |
Gentil et al. |
The challenges in the past five years for the development of next-generation RFBs were discussed. NA-RFBs were not included. The review addressed aqueous organic RFBs (AO-RFBs) and the technologies developed to increase the energy density of RFBs. |
|
|
[54] |
Ortiz-Martínez |
This work reviewed the advances in the application of ILs in RFBs. The authors showed that most of the studies focused on the use of Ils as supporting electrolytes and the latest studies showed their potential as electroactive species and electrolyte membranes. |
|
|
[55] |
Ambrosi and Webster |
The review focused on the most commonly used 3D printing fabrication methods as well as on the additive manufacturing technologies for the fabrication of RFB components that were classified according to the electrolyte nature used (i.e., aqueous and non-aqueous solvents). |
|
|
[56] |
Aberoumand et al. |
The review focused on VRFB technology methods developed to enhance the performance of the electrode and electrolyte as the main components. |
|
|
[57] |
Esan et al. |
The review focused on the modeling and simulation of RFB beyond the all-vanadium, including soluble lead–acid, semisolid, organic, zinc–nickel, zinc–bromine, hydrogen–bromine, sodium–air, and vanadium–cerium flow batteries. |
|
|
[58] |
Tempelman et al. |
A review on the most recent advancements in the structure design and optimization to improve the selectivity and conductivity of membranes. |
|
|
2021 |
[5] |
Wang et al. |
The research progress of insoluble flow batteries was reviewed. The key challenges from the fundamental research point of view and practical application perspectives were compared. |
|
[3] |
Sánchez-Díez et al. |
A review of the aqueous system technologies that potentially fulfill cost requirements and enable large scale storage. |
|
|
[59] |
Zhang and Sun |
The review focused on iron-based aqueous RFBs. The main achievements were highlightned and it was concluded that there is no “perfect chemistry”. |
|
|
[60] |
Emmet and Roberts |
The review focused on the advances in aqueous RFBs with lesser known chemistries than vanadium. The authors expect that these chemistries will become more viable than vanadium due to their lower material costs and less caustic nature. |
|
|
[61] |
Symons |
The review focused on the use of quinones for RFBs. It was concluded that most of the development on quinone-based RFBs is far from being commercially viable. The stability of quinones in high potential electrolytes is not enough and the attempts have led to very low overall cell voltages. |
|
|
[62] |
Aramendia et al. |
The studies and numerical models carried out by means of computational fluid dynamics (CFD) techniques were reviewed. Studies with stacks and approaches for VRFB optimization with CFD based models and different flow field designs to improve the electrochemical performance were discussed. |
|
|
[63] |
Yuan et al. |
The development of the membranes used in the three types of NA-RFBs were summarized and a comprehensive overview of the fundamentals, classification, and performance of the membranes applied in NA-RFBs was provided. |
2.1. Inorganic Aqueous
2.1.1. Vanadium Redox Flow Batteries (VRFB)
|
G1 |
G2 |
G3 |
|
|---|---|---|---|
|
Name |
All-Vanadium |
Vanadium-Polyhalide |
Mixed Acid Vanadium |
|
Positive Couple |
V(III)/V(II) |
V(III)/V(II) |
V(III)/V(II) |
|
Negative Couple |
V(IV)/V(V) |
Cl−/ClBr2− |
V(IV)/V(V) |
|
Supporting Electrolyte |
|
|
|
|
Vanadium Concentration (M) |
1.5–2 [76] |
2.0–3.0 [77] |
2.5–3.0 [78] |
|
Temperature Range (°C) |
10–40 [76] |
0–50 [79] |
|
|
Specific Energy (Wh/L) |
20–33 [77] |
35–70 [77] |
All-Vanadium Redox Flow Battery (G1 RFB)
Vanadium-Polyhalide Redox Flow Battery (G2 RFB)
Positive Side: \( 2\text{ Br}^-+ \text{Cl}^−⇌ \text{ClBr}^-_2 + 2\text{ e}^- \) (2)
Mixed Acid Vanadium Redox Flow Battery (G3 RFB)
2.1.2. Polyoxometalates
2.2. Organic Aqueous
2.3. Non-Aqueous Solvents
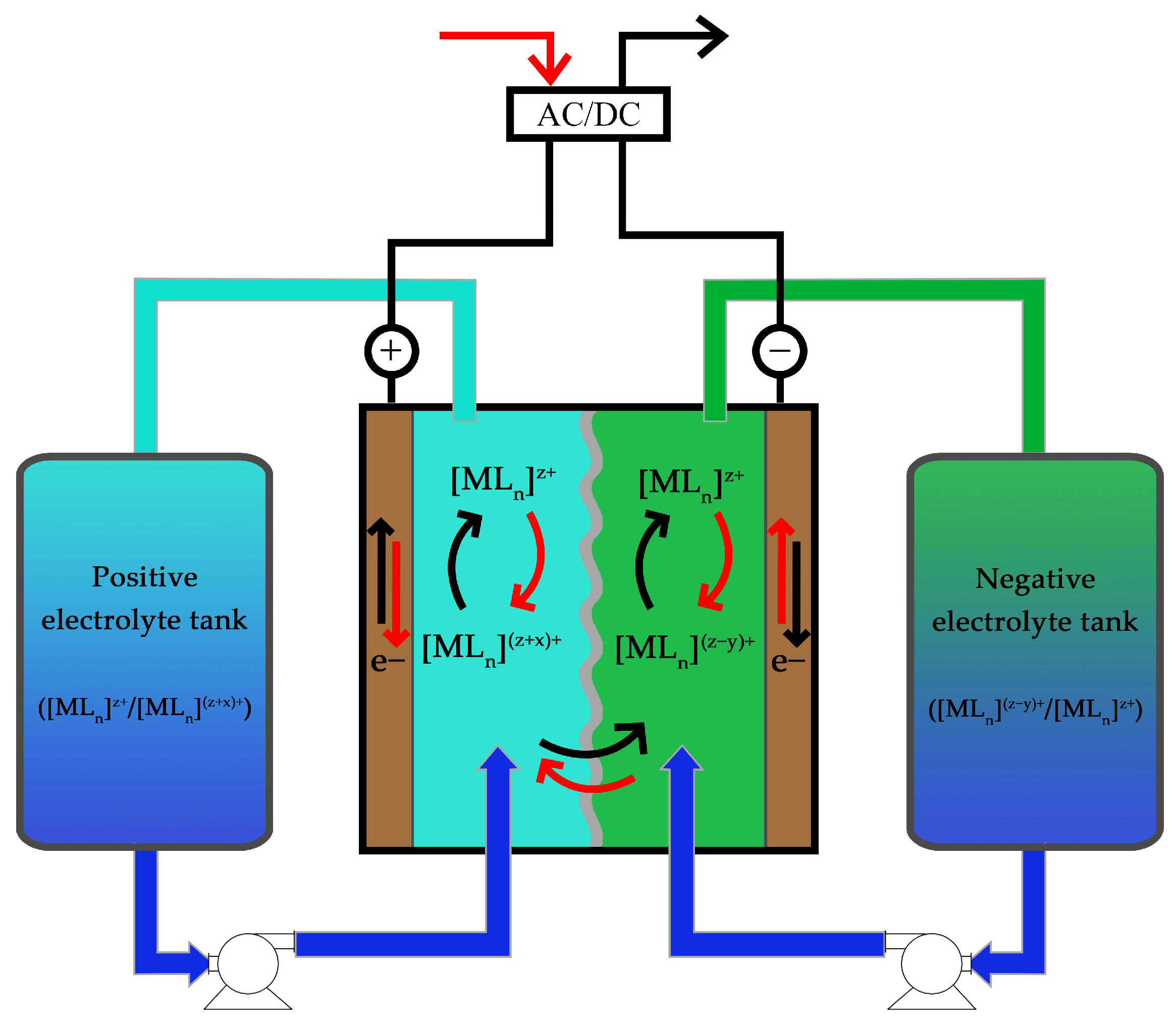
3. Other RFB Configurations
3.1. Membraneless
3.2. Metal–Air Flow Batteries (MAFB) and Metal–Air Fuel Cells (MAFC)
3.3. Zinc–Bromine Flow Batteries
3.4. Semi-Solid (Slurry Flow Batteries)
3.4.1. Without Redox Mediator
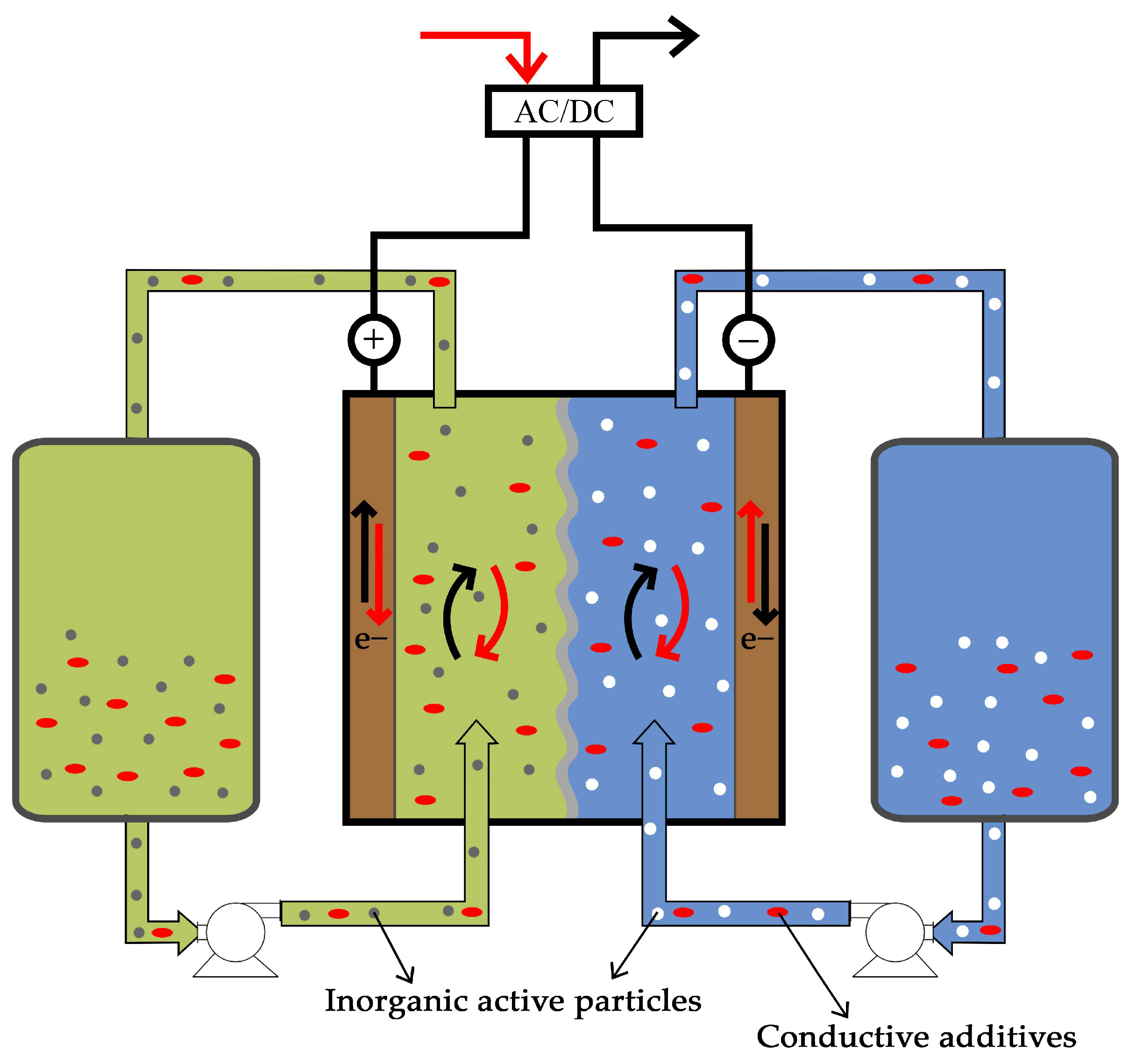
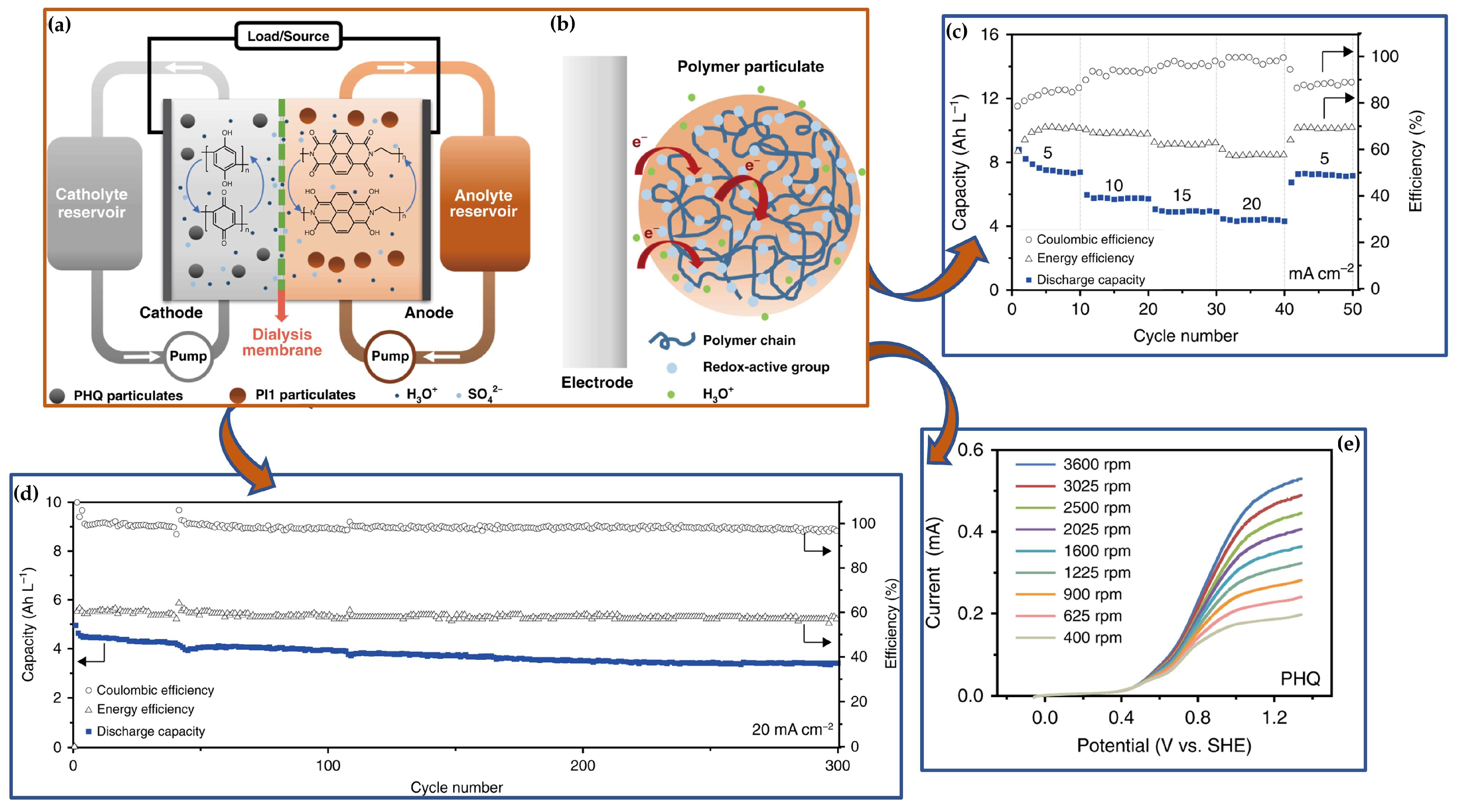
3.4.2. Redox Mediator
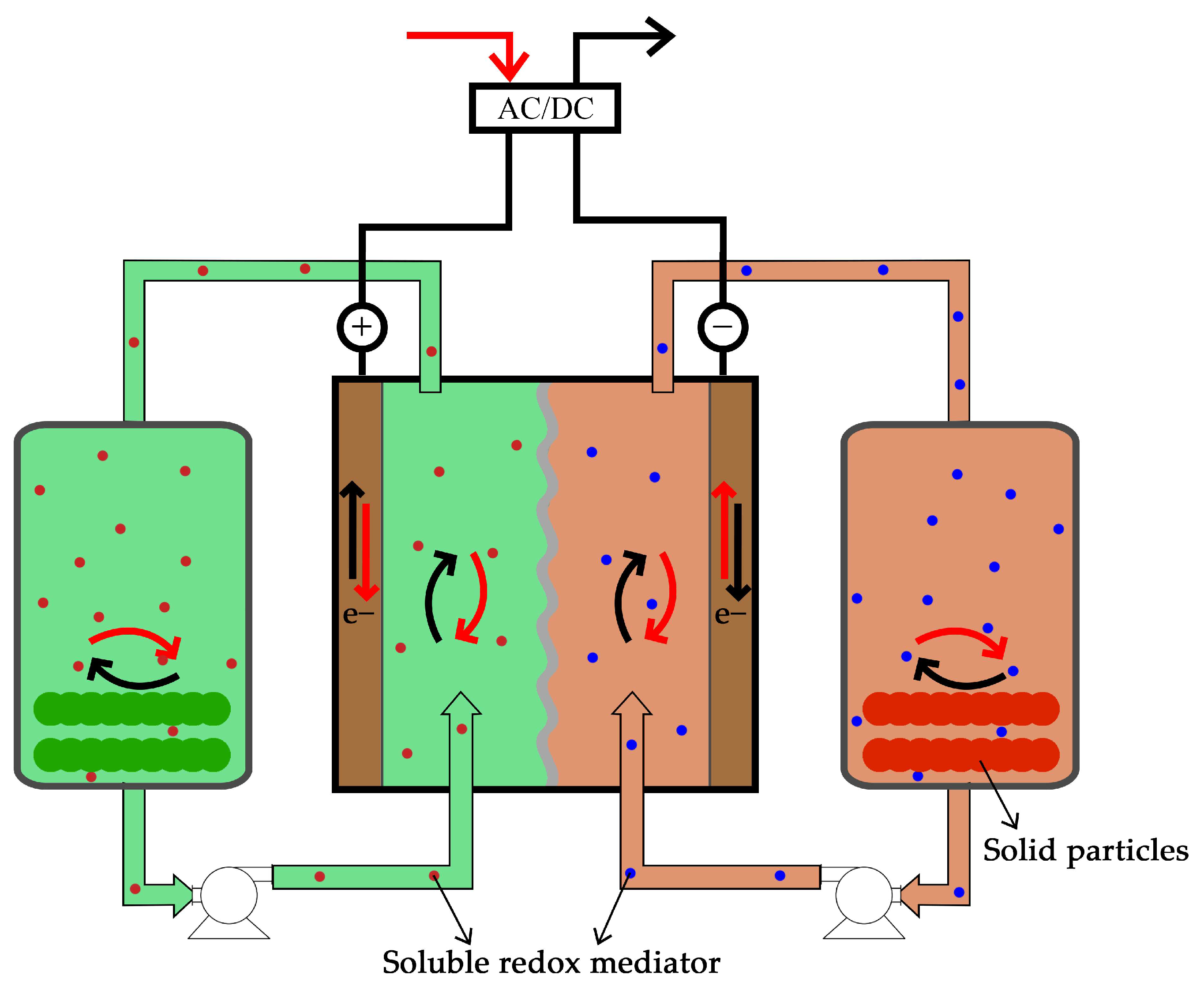
4. Challenges and Future Perspectives
References
- International Renewable Energy Agency (IRENA). Global Energy Transformation: A Roadmap to 2050 (2019 Edition); IRENA: Abu Dhabi, United Arab Emirates, 2019.
- International Renewable Energy Agency (IRENA). Electricity Storage and Renewables: Costs and Markets to 2030; IRENA: Abu Dhabi, United Arab Emirates, 2017.
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox Flow Batteries: Status and Perspective towards Sustainable Stationary Energy Storage. J. Power Sources 2021, 481, 228804.
- Zhang, H.; Li, X.; Zhang, J. (Eds.) Redox Flow Batteries: Fundamentals and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017.
- Wang, X.; Chai, J.; Jiang, J. “Jimmy” Redox Flow Batteries Based on Insoluble Redox-Active Materials. A Review. Nano Mater. Sci. 2021, 3, 17–24.
- Barelli, L.; Bidini, G.; Ottaviano, P.A.; Pelosi, D. Vanadium Redox Flow Batteries Application to Electric Buses Propulsion: Performance Analysis of Hybrid Energy Storage System. J. Energy Storage 2019, 24, 100770.
- Gouveia, J.R.; Silva, E.; Mata, T.M.; Mendes, A.; Caetano, N.S.; Martins, A.A. Life Cycle Assessment of a Renewable Energy Generation System with a Vanadium Redox Flow Battery in a NZEB Household. Energy Rep. 2020, 6, 87–94.
- Elio, J.; Phelan, P.; Villalobos, R.; Milcarek, R.J. A Review of Energy Storage Technologies for Demand-Side Management in Industrial Facilities. J. Clean. Prod. 2021, 307, 127322.
- Ponce de León, C.; Frías-Ferrer, A.; González-García, J.; Szánto, D.A.; Walsh, F.C. Redox Flow Cells for Energy Conversion. J. Power Sources 2006, 160, 716–732.
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox Flow Batteries: A Review. J. Appl. Electrochem. 2011, 41, 1137.
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion Exchange Membranes for Vanadium Redox Flow Battery (VRB) Applications. Energy Environ. Sci. 2011, 4, 1147–1160.
- Skyllas-Kazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in Flow Battery Research and Development. J. Electrochem. Soc. 2011, 158, R55.
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the All-Vanadium Redox Flow Battery for Energy Storage: A Review of Technological, Financial and Policy Aspects. Int. J. Energy Res. 2012, 36, 1105–1120.
- Leung, P.; Li, X.; de León, C.P.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in Redox Flow Batteries, Remaining Challenges and Their Applications in Energy Storage. RSC Adv. 2012, 2, 10125–10156.
- Wang, Y.; He, P.; Zhou, H. Li-Redox Flow Batteries Based on Hybrid Electrolytes: At the Cross Road between Li-Ion and Redox Flow Batteries. Adv. Energy Mater. 2012, 2, 770–779.
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986.
- Shin, S.-H.; Yun, S.-H.; Moon, S.-H. A Review of Current Developments in Non-Aqueous Redox Flow Batteries: Characterization of Their Membranes for Design Perspective. RSC Adv. 2013, 3, 9095–9116.
- Chakrabarti, M.H.; Mjalli, F.S.; AlNashef, I.M.; Hashim, M.A.; Hussain, M.A.; Bahadori, L.; Low, C.T.J. Prospects of Applying Ionic Liquids and Deep Eutectic Solvents for Renewable Energy Storage by Means of Redox Flow Batteries. Renew. Sustain. Energy Rev. 2014, 30, 254–270.
- Alotto, P.; Guarnieri, M.; Moro, F. Redox Flow Batteries for the Storage of Renewable Energy: A Review. Renew. Sustain. Energy Rev. 2014, 29, 325–335.
- Pan, F.; Wang, Q. Redox Species of Redox Flow Batteries: A Review. Molecules 2015, 20, 20499–20517.
- Soloveichik, G.L. Flow Batteries: Current Status and Trends. Available online: https://pubs.acs.org/doi/abs/10.1021/cr500720t (accessed on 19 August 2021).
- Kim, K.J.; Park, M.-S.; Kim, Y.-J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A Technology Review of Electrodes and Reaction Mechanisms in Vanadium Redox Flow Batteries. J. Mater. Chem. A 2015, 3, 16913–16933.
- Xu, Q.; Zhao, T.S. Fundamental Models for Flow Batteries. Prog. Energy Combust. Sci. 2015, 49, 40–58.
- Huang, Y.; Gu, S.; Yan, Y.; Li, S.F.Y. Nonaqueous Redox-Flow Batteries: Features, Challenges, and Prospects. Curr. Opin. Chem. Eng. 2015, 8, 105–113.
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem. Int. Ed. 2017, 56, 686–711.
- Kowalski, J.A.; Su, L.; Milshtein, J.D.; Brushett, F.R. Recent Advances in Molecular Engineering of Redox Active Organic Molecules for Nonaqueous Flow Batteries. Curr. Opin. Chem. Eng. 2016, 13, 45–52.
- Park, M.; Ryu, J.; Wang, W.; Cho, J. Material Design and Engineering of Next-Generation Flow-Battery Technologies. Nat. Rev. Mater. 2016, 2, 1–18.
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Engineering Aspects of the Design, Construction and Performance of Modular Redox Flow Batteries for Energy Storage. J. Energy Storage 2017, 11, 119–153.
- Leung, P.; Shah, A.A.; Sanz, L.; Flox, C.; Morante, J.R.; Xu, Q.; Mohamed, M.R.; Ponce de León, C.; Walsh, F.C. Recent Developments in Organic Redox Flow Batteries: A Critical Review. J. Power Sources 2017, 360, 243–283.
- Ye, R.; Henkensmeier, D.; Yoon, S.J.; Huang, Z.; Kim, D.K.; Chang, Z.; Kim, S.; Chen, R. Redox Flow Batteries for Energy Storage: A Technology Review. J. Electrochem. Energy Convers. Storage 2017, 15, 010801.
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.; Yang, J.H.; Kim, H.-T. A Review of Vanadium Electrolytes for Vanadium Redox Flow Batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274.
- Li, B.; Liu, J. Progress and Directions in Low-Cost Redox-Flow Batteries for Large-Scale Energy Storage. Natl. Sci. Rev. 2017, 4, 91–105.
- Bamgbopa, M.O.; Almheiri, S.; Sun, H. Prospects of Recently Developed Membraneless Cell Designs for Redox Flow Batteries. Renew. Sustain. Energy Rev. 2017, 70, 506–518.
- Zhou, X.L.; Zhao, T.S.; An, L.; Zeng, Y.K.; Wei, L. Critical Transport Issues for Improving the Performance of Aqueous Redox Flow Batteries. J. Power Sources 2017, 339, 1–12.
- Chen, H.; Cong, G.; Lu, Y.-C. Recent Progress in Organic Redox Flow Batteries: Active Materials, Electrolytes and Membranes. J. Energy Chem. 2018, 27, 1304–1325.
- Zhang, C.; Zhang, L.; Ding, Y.; Peng, S.; Guo, X.; Zhao, Y.; He, G.; Yu, G. Progress and Prospects of Next-Generation Redox Flow Batteries. Energy Storage Mater. 2018, 15, 324–350.
- Liu, T.; Li, X.; Zhang, H.; Chen, J. Progress on the Electrode Materials towards Vanadium Flow Batteries (VFBs) with Improved Power Density. J. Energy Chem. 2018, 27, 1292–1303.
- Cao, L.; Skyllas-Kazacos, M.; Menictas, C.; Noack, J. A Review of Electrolyte Additives and Impurities in Vanadium Redox Flow Batteries. J. Energy Chem. 2018, 27, 1269–1291.
- Xu, Q.; Ji, Y.N.; Qin, L.Y.; Leung, P.K.; Qiao, F.; Li, Y.S.; Su, H.N. Evaluation of Redox Flow Batteries Goes beyond Round-Trip Efficiency: A Technical Review. J. Energy Storage 2018, 16, 108–115.
- Ke, X.; Prahl, J.M.; Alexander, J.I.D.; Wainright, J.S.; Zawodzinski, T.A.; Savinell, R.F. Rechargeable Redox Flow Batteries: Flow Fields, Stacks and Design Considerations. Chem. Soc. Rev. 2018, 47, 8721–8743.
- Arenas, L.F.; Loh, A.; Trudgeon, D.P.; Li, X.; Ponce de León, C.; Walsh, F.C. The Characteristics and Performance of Hybrid Redox Flow Batteries with Zinc Negative Electrodes for Energy Storage. Renew. Sustain. Energy Rev. 2018, 90, 992–1016.
- Minke, C.; Turek, T. Materials, System Designs and Modelling Approaches in Techno-Economic Assessment of All-Vanadium Redox Flow Batteries—A Review. J. Power Sources 2018, 376, 66–81.
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium Redox Flow Batteries: A Comprehensive Review. J. Energy Storage 2019, 25, 100844.
- Narayan, S.R.; Nirmalchandar, A.; Murali, A.; Yang, B.; Hoober-Burkhardt, L.; Krishnamoorthy, S.; Prakash, G.K.S. Next-Generation Aqueous Flow Battery Chemistries. Curr. Opin. Electrochem. 2019, 18, 72–80.
- Hogue, R.W.; Toghill, K.E. Metal Coordination Complexes in Nonaqueous Redox Flow Batteries. Curr. Opin. Electrochem. 2019, 18, 37–45.
- Gubler, L. Membranes and Separators for Redox Flow Batteries. Curr. Opin. Electrochem. 2019, 18, 31–36.
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Redox Flow Batteries for Energy Storage: Their Promise, Achievements and Challenges. Curr. Opin. Electrochem. 2019, 16, 117–126.
- Rhodes, Z.; Cabrera-Pardo, J.R.; Li, M.; Minteer, S.D. Electrochemical Advances in Non-Aqueous Redox Flow Batteries. Isr. J. Chem. 2021, 61, 101–112.
- Clemente, A.; Costa-Castelló, R. Redox Flow Batteries: A Literature Review Oriented to Automatic Control. Energies 2020, 13, 4514.
- Gencten, M.; Sahin, Y. A Critical Review on Progress of the Electrode Materials of Vanadium Redox Flow Battery. Int. J. Energy Res. 2020, 44, 7903–7923.
- Kwabi, D.G.; Ji, Y.; Aziz, M.J. Electrolyte Lifetime in Aqueous Organic Redox Flow Batteries: A Critical Review. Chem. Rev. 2020, 120, 6467–6489.
- Zhong, F.; Yang, M.; Ding, M.; Jia, C. Organic Electroactive Molecule-Based Electrolytes for Redox Flow Batteries: Status and Challenges of Molecular Design. Front. Chem. 2020, 8, 451.
- Gentil, S.; Reynard, D.; Girault, H.H. Aqueous Organic and Redox-Mediated Redox Flow Batteries: A Review. Curr. Opin. Electrochem. 2020, 21, 7–13.
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Pérez, G.; Ortiz, A.; Ortiz, I. The Roles of Ionic Liquids as New Electrolytes in Redox Flow Batteries. Sep. Purif. Technol. 2020, 252, 117436.
- Ambrosi, A.; Webster, R.D. 3D Printing for Aqueous and Non-Aqueous Redox Flow Batteries. Curr. Opin. Electrochem. 2020, 20, 28–35.
- Aberoumand, S.; Woodfield, P.; Shabani, B.; Dao, D.V. Advances in Electrode and Electrolyte Improvements in Vanadium Redox Flow Batteries with a Focus on the Nanofluidic Electrolyte Approach. Phys. Rep. 2020, 881, 1–49.
- Esan, O.C.; Shi, X.; Pan, Z.; Huo, X.; An, L.; Zhao, T.S. Modeling and Simulation of Flow Batteries. Adv. Energy Mater. 2020, 10, 2000758.
- Tempelman, C.H.L.; Jacobs, J.F.; Balzer, R.M.; Degirmenci, V. Membranes for All Vanadium Redox Flow Batteries. J. Energy Storage 2020, 32, 101754.
- Zhang, H.; Sun, C. Cost-Effective Iron-Based Aqueous Redox Flow Batteries for Large-Scale Energy Storage Application: A Review. J. Power Sources 2021, 493, 229445.
- Emmett, R.K.; Roberts, M.E. Recent Developments in Alternative Aqueous Redox Flow Batteries for Grid-Scale Energy Storage. J. Power Sources 2021, 506, 230087.
- Symons, P. Quinones for Redox Flow Batteries. Curr. Opin. Electrochem. 2021, 29, 100759.
- Aramendia, I.; Fernandez-Gamiz, U.; Martinez-San-Vicente, A.; Zulueta, E.; Lopez-Guede, J.M. Vanadium Redox Flow Batteries: A Review Oriented to Fluid-Dynamic Optimization. Energies 2021, 14, 176.
- Yuan, J.; Pan, Z.-Z.; Jin, Y.; Qiu, Q.; Zhang, C.; Zhao, Y.; Li, Y. Membranes in Non-Aqueous Redox Flow Battery: A Review. J. Power Sources 2021, 500, 229983.
- Rychcik, M.; Skyllas-Kazacos, M. Evaluation of Electrode Materials for Vanadium Redox Cell. J. Power Sources 1987, 19, 45–54.
- Roznyatovskaya, N.; Noack, J.; Mild, H.; Fühl, M.; Fischer, P.; Pinkwart, K.; Tübke, J.; Skyllas-Kazacos, M. Vanadium Electrolyte for All-Vanadium Redox-Flow Batteries: The Effect of the Counter Ion. Batteries 2019, 5, 13.
- Heo, J.; Han, J.-Y.; Kim, S.; Yuk, S.; Choi, C.; Kim, R.; Lee, J.-H.; Klassen, A.; Ryi, S.-K.; Kim, H.-T. Catalytic Production of Impurity-Free V 3.5+ Electrolyte for Vanadium Redox Flow Batteries. Nat. Commun. 2019, 10, 4412.
- Martin, J.; Schafner, K.; Turek, T. Preparation of Electrolyte for Vanadium Redox-Flow Batteries Based on Vanadium Pentoxide. Energy Technol. 2020, 8, 2000522.
- Zhang, Z.H.; Wei, L.; Wu, M.C.; Bai, B.F.; Zhao, T.S. Chloride Ions as an Electrolyte Additive for High Performance Vanadium Redox Flow Batteries. Appl. Energy 2021, 289, 116690.
- Endres, F. Ionic Liquids: Promising Solvents for Electrochemistry. Z. Für Phys. Chem. 2004, 218, 255–284.
- Viswanathan, V.; Crawford, A.; Stephenson, D.; Kim, S.; Wang, W.; Li, B.; Coffey, G.; Thomsen, E.; Graff, G.; Balducci, P.; et al. Cost and Performance Model for Redox Flow Batteries. J. Power Sources 2014, 247, 1040–1051.
- Lim, H.S.; Lackner, A.M.; Knechtli, R.C. Zinc-Bromine Secondary Battery. J. Electrochem. Soc. 1977, 124, 1154–1157.
- Rychcik, M.; Skyllas-Kazacos, M. Characteristics of a New All-Vanadium Redox Flow Battery. J. Power Sources 1988, 22, 59–67.
- Kazacos, M.; Skyllas-Kazacos, M. High Energy Density Vanadium Electrolyte Solutions, Methods of Preparation Thereof and All-Vanadium Redox Cells and Batteries Containing High Energy Vanadium Electrolyte Solutions. CA2220075C, 7 September 1996.
- Skyllas-Kazacos, M. Vanadium/Polyhalide Redox Flow Battery. U.S. Patent US7320844B2, 22 January 2008.
- Skyllas-Kazacos, M. Novel Vanadium Chloride/Polyhalide Redox Flow Battery. J. Power Sources 2003, 124, 299–302.
- Wu, X.; Liu, J.; Xiang, X.; Zhang, J.; Hu, J.; Wu, Y. Electrolytes for Vanadium Redox Flow Batteries. Pure Appl. Chem. 2014, 86, 661–669.
- Skyllas-Kazacos, M.; Kazacos, G.; Poon, G.; Verseema, H. Recent Advances with UNSW Vanadium-Based Redox Flow Batteries. Int. J. Energy Res. 2010, 34, 182–189.
- Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G.; et al. A Stable Vanadium Redox-Flow Battery with High Energy Density for Large-Scale Energy Storage. Adv. Energy Mater. 2011, 1, 394–400.
- Menictas, C.; Skyllas-Kazacos, M.; Lim, T.M. Advances in Batteries for Medium and Large-Scale Energy Storage; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-1-78242-013-2.
- Rahman, F.; Skyllas-Kazacos, M. Vanadium Redox Battery: Positive Half-Cell Electrolyte Studies. J. Power Sources 2009, 189, 1212–1219.
- Bryans, D.; Amstutz, V.; Girault, H.H.; Berlouis, L.E.A. Characterisation of a 200 KW/400 KWh Vanadium Redox Flow Battery. Batteries 2018, 4, 54.
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The Chemistry of Redox-Flow Batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809.
- Cunha, Á.; Martins, J.; Rodrigues, N.; Brito, F.P. Vanadium Redox Flow Batteries: A Technology Review. Int. J. Energy Res. 2015, 39, 889–918.
- Aaron, D.; Tang, Z.; Papandrew, A.B.; Zawodzinski, T.A. Polarization Curve Analysis of All-Vanadium Redox Flow Batteries. J. Appl. Electrochem. 2011, 41, 1175.
- Zhou, X.L.; Zeng, Y.K.; Zhu, X.B.; Wei, L.; Zhao, T.S. A High-Performance Dual-Scale Porous Electrode for Vanadium Redox Flow Batteries. J. Power Sources 2016, 325, 329–336.
- Wei, L.; Zhao, T.S.; Zeng, L.; Zhou, X.L.; Zeng, Y.K. Copper Nanoparticle-Deposited Graphite Felt Electrodes for All Vanadium Redox Flow Batteries. Appl. Energy 2016, 180, 386–391.
- Sun, J.; Zeng, L.; Jiang, H.; Chao, C.; Zhao, T. Formation of Electrodes by Self-Assembling Porous Carbon Fibers into Bundles for Vanadium Redox Flow Batteries. J. Power Sources 2018, 405, 106–113.
- Busacca, C.; Blasi, O.D.; Giacoppo, G.; Briguglio, N.; Antonucci, V.; Blasi, A.D. High Performance Electrospun Nickel Manganite on Carbon Nanofibers Electrode for Vanadium Redox Flow Battery. Electrochim. Acta 2020, 355, 136755.
- Jiang, H.R.; Sun, J.; Wei, L.; Wu, M.C.; Shyy, W.; Zhao, T.S. A High Power Density and Long Cycle Life Vanadium Redox Flow Battery. Energy Storage Mater. 2020, 24, 529–540.
- Xu, Z.; Zhu, M.; Zhang, K.; Zhang, X.; Xu, L.; Liu, J.; Liu, T.; Yan, C. Inspired by “Quenching-Cracking” Strategy: Structure-Based Design of Sulfur-Doped Graphite Felts for Ultrahigh-Rate Vanadium Redox Flow Batteries. Energy Storage Mater. 2021, 39, 166–175.
- Mustafa, I.; Susantyoko, R.; Wu, C.-H.; Ahmed, F.; Hashaikeh, R.; Almarzooqi, F.; Almheiri, S. Nanoscopic and Macro-Porous Carbon Nano-Foam Electrodes with Improved Mass Transport for Vanadium Redox Flow Batteries. Sci. Rep. 2019, 9, 17655.
- Han, J.; Yoo, H.; Kim, M.; Lee, G.; Choi, J. High-Performance Bipolar Plate of Thin IrOx-Coated TiO2 Nanotubes in Vanadium Redox Flow Batteries. Catal. Today 2017, 295, 132–139.
- Liao, W.; Zhang, Y.; Zhou, X.; Zhuang, M.; Guo, D.; Jiang, F.; Yu, Q. Low-Carbon-Content Composite Bipolar Plates: A Novel Design and Its Performance in Vanadium Redox Flow Batteries. ChemistrySelect 2019, 4, 2421–2427.
- Liao, W.; Jiang, F.; Zhang, Y.; Zhou, X.; He, Z. Highly-Conductive Composite Bipolar Plate Based on Ternary Carbon Materials and Its Performance in Redox Flow Batteries. Renew. Energy 2020, 152, 1310–1316.
- Jiang, F.; Liao, W.; Ayukawa, T.; Yoon, S.-H.; Nakabayashi, K.; Miyawaki, J. Enhanced Performance and Durability of Composite Bipolar Plate with Surface Modification of Cactus-like Carbon Nanofibers. J. Power Sources 2021, 482, 228903.
- Zhang, D.; Xin, L.; Xia, Y.; Dai, L.; Qu, K.; Huang, K.; Fan, Y.; Xu, Z. Advanced Nafion Hybrid Membranes with Fast Proton Transport Channels toward High-Performance Vanadium Redox Flow Battery. J. Membr. Sci. 2021, 624, 119047.
- Zhang, B.; Zhao, M.; Liu, Q.; Zhang, X.; Fu, Y.; Zhang, E.; Wang, G.; Zhang, Z.; Yuan, X.; Zhang, S. High Performance Membranes Based on Pyridine Containing Poly (Aryl Ether Ketone Ketone) for Vanadium Redox Flow Battery Applications. J. Power Sources 2021, 506, 230128.
- Wan, Y.H.; Sun, J.; Jiang, H.R.; Fan, X.Z.; Zhao, T.S. A Highly-Efficient Composite Polybenzimidazole Membrane for Vanadium Redox Flow Battery. J. Power Sources 2021, 489, 229502.
- Kushner, D.I.; Crothers, A.R.; Kusoglu, A.; Weber, A.Z. Transport Phenomena in Flow Battery Ion-Conducting Membranes. Curr. Opin. Electrochem. 2020, 21, 132–139.
- Shin, J.; Jeong, B.; Chinannai, M.F.; Ju, H. Mitigation of Water and Electrolyte Imbalance in All-Vanadium Redox Flow Batteries. Electrochim. Acta 2021, 390, 138858.
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium Electrolyte Studies for the Vanadium Redox Battery-A Review. ChemSusChem 2016, 9, 1521–1543.
- Kazacos, M.; Skyllas-Kazacos, M.; Kazacos, N. Vanadium Halide Redox Flow Battery. U.S. Patent US7976974B2, 12 July 2011.
- Kim, D.; Kim, Y.; Lee, Y.; Jeon, J. 1,2-Dimethylimidazole Based Bromine Complexing Agents for Vanadium Bromine Redox Flow Batteries. Int. J. Hydrog. Energy 2019, 44, 12024–12032.
- Vafiadis, H.; Skyllas-Kazacos, M. Evaluation of Membranes for the Novel Vanadium Bromine Redox Flow Cell. J. Membr. Sci. 2006, 279, 394–402.
- Vijayakumar, M.; Li, L.; Graff, G.; Liu, J.; Zhang, H.; Yang, Z.; Hu, J.Z. Towards Understanding the Poor Thermal Stability of V5+ Electrolyte Solution in Vanadium Redox Flow Batteries. J. Power Sources 2011, 196, 3669–3672.
- Roe, S.; Menictas, C.; Skyllas-Kazacos, M. A High Energy Density Vanadium Redox Flow Battery with 3 M Vanadium Electrolyte. J. Electrochem. Soc. 2015, 163, A5023.
- Kim, S.; Thomsen, E.; Xia, G.; Nie, Z.; Bao, J.; Recknagle, K.; Wang, W.; Viswanathan, V.; Luo, Q.; Wei, X.; et al. 1 KW/1 KWh Advanced Vanadium Redox Flow Battery Utilizing Mixed Acid Electrolytes. J. Power Sources 2013, 237, 300–309.
- Reed, D.; Thomsen, E.; Li, B.; Wang, W.; Nie, Z.; Koeppel, B.; Kizewski, J.; Sprenkle, V. Stack Developments in a KW Class All Vanadium Mixed Acid Redox Flow Battery at the Pacific Northwest National Laboratory. J. Electrochem. Soc. 2016, 163, A5211–A5219.
- Yang, Y.; Zhang, Y.; Tang, L.; Liu, T.; Huang, J.; Peng, S.; Yang, X. Investigations on Physicochemical Properties and Electrochemical Performance of Sulfate-Chloride Mixed Acid Electrolyte for Vanadium Redox Flow Battery. J. Power Sources 2019, 434, 226719.
- Kim, S.; Vijayakumar, M.; Wang, W.; Zhang, J.; Chen, B.; Nie, Z.; Chen, F.; Hu, J.; Li, L.; Yang, Z. Chloride Supporting Electrolytes for All-Vanadium Redox Flow Batteries. Phys. Chem. Chem. Phys. 2011, 13, 18186–18193.
- Li, B.; Gu, M.; Nie, Z.; Shao, Y.; Luo, Q.; Wei, X.; Li, X.; Xiao, J.; Wang, C.; Sprenkle, V.; et al. Bismuth Nanoparticle Decorating Graphite Felt as a High-Performance Electrode for an All-Vanadium Redox Flow Battery. Nano Lett. 2013, 13, 1330–1335.
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane Development for Vanadium Redox Flow Batteries. ChemSusChem 2011, 4, 1388–1406.
- Vijayakumar, M.; Bhuvaneswari, M.S.; Nachimuthu, P.; Schwenzer, B.; Kim, S.; Yang, Z.; Liu, J.; Graff, G.L.; Thevuthasan, S.; Hu, J. Spectroscopic Investigations of the Fouling Process on Nafion Membranes in Vanadium Redox Flow Batteries. J. Membr. Sci. 2011, 366, 325–334.
- Reed, D.; Thomsen, E.; Wang, W.; Nie, Z.; Li, B.; Wei, X.; Koeppel, B.; Sprenkle, V. Performance of Nafion® N115, Nafion® NR-212, and Nafion® NR-211 in a 1 KW Class All Vanadium Mixed Acid Redox Flow Battery. J. Power Sources 2015, 285, 425–430.
- Yang, Y.; Zhang, Y.; Liu, T.; Huang, J. Improved Broad Temperature Adaptability and Energy Density of Vanadium Redox Flow Battery Based on Sulfate-Chloride Mixed Acid by Optimizing the Concentration of Electrolyte. J. Power Sources 2019, 415, 62–68.
- Ueda, T. Electrochemistry of Polyoxometalates: From Fundamental Aspects to Applications. ChemElectroChem 2018, 5, 823–838.
- Yang, L.; Lei, J.; Fan, J.; Yuan, R.; Zheng, M.; Chen, J.; Dong, Q. The Intrinsic Charge Carrier Behaviors and Applications of Polyoxometalate Clusters Based Materials. Adv. Mater. 2021, 2005019.
- Friedl, J.; Holland-Cunz, M.V.; Cording, F.; Pfanschilling, F.L.; Wills, C.; McFarlane, W.; Schricker, B.; Fleck, R.; Wolfschmidt, H.; Stimming, U. Asymmetric Polyoxometalate Electrolytes for Advanced Redox Flow Batteries. Energy Environ. Sci. 2018, 11, 3010–3018.
- Ji, Y.; Huang, L.; Hu, J.; Streb, C.; Song, Y.-F. Polyoxometalate-Functionalized Nanocarbon Materials for Energy Conversion, Energy Storage and Sensor Systems. Energy Environ. Sci. 2015, 8, 776–789.
- Friedl, J.; Al-Oweini, R.; Herpich, M.; Keita, B.; Kortz, U.; Stimming, U. Electrochemical Studies of Tri-Manganese Substituted Keggin Polyoxoanions. Electrochim. Acta 2014, 141, 357–366.
- Chen, H.-Y.; Friedl, J.; Pan, C.-J.; Haider, A.; Al-Oweini, R.; Cheah, Y.L.; Lin, M.-H.; Kortz, U.; Hwang, B.-J.; Srinivasan, M.; et al. In Situ X-ray Absorption near Edge Structure Studies and Charge Transfer Kinetics of Na6 Electrodes. Phys. Chem. Chem. Phys. 2017, 19, 3358–3365.
- Laborda, E.; Henstridge, M.C.; Batchelor-McAuley, C.; Compton, R.G. Asymmetric Marcus–Hush Theory for Voltammetry. Chem. Soc. Rev. 2013, 12.
- Cao, Y.; Chen, J.-J.J.; Barteau, M.A. Systematic Approaches to Improving the Performance of Polyoxometalates in Non-Aqueous Redox Flow Batteries. J. Energy Chem. 2020, 50, 115–124.
- Friedl, J.; Pfanschilling, F.L.; Holland-Cunz, M.V.; Fleck, R.; Schricker, B.; Wolfschmidt, H.; Stimming, U. A Polyoxometalate Redox Flow Battery: Functionality and Upscale. Clean Energy 2019, 3, 278–287.
- VanGelder, L.E.; Pratt, H.D.; Anderson, T.M.; Matson, E.M. Surface Functionalization of Polyoxovanadium Clusters: Generation of Highly Soluble Charge Carriers for Nonaqueous Energy Storage. Chem. Commun. 2019, 55, 12247–12250.
- Cameron, J.M.; Holc, C.; Kibler, A.J.; Peake, C.L.; Walsh, D.A.; Newton, G.N.; Johnson, L.R. Molecular Redox Species for Next-Generation Batteries. Chem. Soc. Rev. 2021, 50, 5863–5883.
- Yang, D.; Liang, Y.; Ma, P.; Li, S.; Wang, J.; Niu, J. Self Assembly of Carboxylate/Alcoholate Functionalized Ring-Shape Phosphomolybdates. CrystEngComm 2014, 16, 8041–8046.
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and Post-Functionalization: A Step towards Polyoxometalate-Based Materials. Chem. Soc. Rev. 2012, 41, 7605.
- VanGelder, L.E.; Kosswattaarachchi, A.M.; Forrestel, P.L.; Cook, T.R.; Matson, E.M. Polyoxovanadate-Alkoxide Clusters as Multi-Electron Charge Carriers for Symmetric Non-Aqueous Redox Flow Batteries. Chem. Sci. 2018, 9, 1692–1699.
- VanGelder, L.E.; Schreiber, E.; Matson, E.M. Physicochemical Implications of Alkoxide “Mixing” in Polyoxovanadium Clusters for Nonaqueous Energy Storage. J. Mater. Chem. A 2019, 7, 4893–4902.
- Chakrabarti, M.H.; Dryfe, R.A.W.; Roberts, E.P.L. Evaluation of Electrolytes for Redox Flow Battery Applications. Electrochim. Acta 2007, 52, 2189–2195.
- Bai, P.; Bazant, M.Z. Charge Transfer Kinetics at the Solid–Solid Interface in Porous Electrodes. Nat. Commun. 2014, 5, 3585.
- Liu, Y.; Lu, S.; Wang, H.; Yang, C.; Su, X.; Xiang, Y. An Aqueous Redox Flow Battery with a Tungsten–Cobalt Heteropolyacid as the Electrolyte for Both the Anode and Cathode. Adv. Energy Mater. 2017, 7, 1601224.
- Chen, J.-J.J.; Barteau, M.A. Molybdenum Polyoxometalates as Active Species for Energy Storage in Non-Aqueous Media. J. Energy Storage 2017, 13, 255–261.
- Laramie, S.M.; Milshtein, J.D.; Breault, T.M.; Brushett, F.R.; Thompson, L.T. Performance and Cost Characteristics of Multi-Electron Transfer, Common Ion Exchange Non-Aqueous Redox Flow Batteries. J. Power Sources 2016, 327, 681–692.
- Li, Q.; Zhang, L.; Dai, J.; Tang, H.; Li, Q.; Xue, H.; Pang, H. Polyoxometalate-Based Materials for Advanced Electrochemical Energy Conversion and Storage. Chem. Eng. J. 2018, 351, 441–461.
- Wang, D.; Liu, L.; Jiang, J.; Chen, L.; Zhao, J. Polyoxometalate-Based Composite Materials in Electrochemistry: State-of-the-Art Progress and Future Outlook. Nanoscale 2020, 12, 5705–5718.
- Chen, H.-Y.; Wee, G.; Al-Oweini, R.; Friedl, J.; Tan, K.S.; Wang, Y.; Wong, C.L.; Kortz, U.; Stimming, U.; Srinivasan, M. A Polyoxovanadate as an Advanced Electrode Material for Supercapacitors. ChemPhysChem 2014, 15, 2162–2169.
- Luo, J.; Hu, B.; Hu, M.; Zhao, Y.; Liu, T.L. Status and Prospects of Organic Redox Flow Batteries toward Sustainable Energy Storage. ACS Energy Lett. 2019, 4, 2220–2240.
- Singh, V.; Kim, S.; Kang, J.; Byon, H.R. Aqueous Organic Redox Flow Batteries. Nano Res. 2019, 12, 1988–2001.
- Hollas, A.; Wei, X.; Murugesan, V.; Nie, Z.; Li, B.; Reed, D.; Liu, J.; Sprenkle, V.; Wang, W. A Biomimetic High-Capacity Phenazine-Based Anolyte for Aqueous Organic Redox Flow Batteries. Nat. Energy 2018, 3, 508–514.
- Lee, W.; Park, G.; Kwon, Y. Alkaline Aqueous Organic Redox Flow Batteries of High Energy and Power Densities Using Mixed Naphthoquinone Derivatives. Chem. Eng. J. 2020, 386, 123985.
- Liu, L.; Yao, Y.; Wang, Z.; Lu, Y.-C. Viologen Radical Stabilization by Molecular Spectators for Aqueous Organic Redox Flow Batteries. Nano Energy 2021, 84, 105897.
- Korshunov, A.; Gibalova, A.; Grünebaum, M.; Ravoo, B.J.; Winter, M.; Cekic-Laskovic, I. Host-Guest Interactions Enhance the Performance of Viologen Electrolytes for Aqueous Organic Redox Flow Batteries. Batter. Supercaps 2021, 4, 923–928.
- Xia, L.; Huo, W.; Gao, H.; Zhang, H.; Chu, F.; Liu, H.; Tan, Z. Intramolecular Hydrogen Bonds Induced High Solubility for Efficient and Stable Anthraquinone Based Neutral Aqueous Organic Redox Flow Batteries. J. Power Sources 2021, 498, 229896.
- Hoober-Burkhardt, L.; Krishnamoorthy, S.; Yang, B.; Murali, A.; Nirmalchandar, A.; Prakash, G.K.S.; Narayanan, S.R. A New Michael-Reaction-Resistant Benzoquinone for Aqueous Organic Redox Flow Batteries. J. Electrochem. Soc. 2017, 164, A600.
- Murali, A.; Nirmalchandar, A.; Krishnamoorthy, S.; Hoober-Burkhardt, L.; Yang, B.; Soloveichik, G.; Prakash, G.K.S.; Narayanan, S.R. Understanding and Mitigating Capacity Fade in Aqueous Organic Redox Flow Batteries. J. Electrochem. Soc. 2018, 165, A1193.
- Hu, B.; Fan, H.; Li, H.; Ravivarma, M.; Song, J. Five-Membered Ring Nitroxide Radical: A New Class of High-Potential, Stable Catholyte for Neutral Aqueous Organic Redox Flow Batteries. Adv. Funct. Mater. 2021, 31, 2102734.
- Yang, X.; Garcia, S.N.; Janoschka, T.; Kónya, D.; Hager, M.D.; Schubert, U.S. Novel, Stable Catholyte for Aqueous Organic Redox Flow Batteries: Symmetric Cell Study of Hydroquinones with High Accessible Capacity. Molecules 2021, 26, 3823.
- Liu, T.; Wei, X.; Nie, Z.; Sprenkle, V.; Wang, W. A Total Organic Aqueous Redox Flow Battery Employing a Low Cost and Sustainable Methyl Viologen Anolyte and 4-HO-TEMPO Catholyte. Adv. Energy Mater. 2016, 6, 1501449.
- Hu, B.; Seefeldt, C.; DeBruler, C.; Liu, T.L. Boosting the Energy Efficiency and Power Performance of Neutral Aqueous Organic Redox Flow Batteries. J. Mater. Chem. A 2017, 5, 22137–22145.
- Hu, B.; Tang, Y.; Luo, J.; Grove, G.; Guo, Y.; Liu, T.L. Improved Radical Stability of Viologen Anolytes in Aqueous Organic Redox Flow Batteries. Chem. Commun. 2018, 54, 6871–6874.
- Feng, R.; Zhang, X.; Murugesan, V.; Hollas, A.; Chen, Y.; Shao, Y.; Walter, E.; Wellala, N.P.N.; Yan, L.; Rosso, K.M.; et al. Reversible Ketone Hydrogenation and Dehydrogenation for Aqueous Organic Redox Flow Batteries. Science 2021, 372, 836–840.
- Noh, C.; Chung, Y.; Kwon, Y. Organometallic Redox Flow Batteries Using Iron Triethanolamine and Cobalt Triethanolamine Complexes. J. Power Sources 2020, 466, 228333.
- Noh, C.; Chung, Y.; Kwon, Y. Highly Stable Aqueous Organometallic Redox Flow Batteries Using Cobalt Triisopropanolamine and Iron Triisopropanolamine Complexes. Chem. Eng. J. 2021, 405, 126966.
- Ruan, W.; Mao, J.; Yang, S.; Shi, C.; Jia, G.; Chen, Q. Designing Cr Complexes for a Neutral Fe–Cr Redox Flow Battery. Chem. Commun. 2020, 56, 3171–3174.
- Shin, M.; Noh, C.; Chung, Y.; Kwon, Y. All Iron Aqueous Redox Flow Batteries Using Organometallic Complexes Consisting of Iron and 3--2-Hydroxypropanesulfonic Acid Ligand and Ferrocyanide as Redox Couple. Chem. Eng. J. 2020, 398, 125631.
- Shinkle, A.A.; Sleightholme, A.E.S.; Thompson, L.T.; Monroe, C.W. Electrode Kinetics in Non-Aqueous Vanadium Acetylacetonate Redox Flow Batteries. J. Appl. Electrochem. 2011, 41, 1191–1199.
- Ding, Y.; Zhang, C.; Zhang, L.; Zhou, Y.; Yu, G. Molecular Engineering of Organic Electroactive Materials for Redox Flow Batteries. Chem. Soc. Rev. 2018, 47, 69–103.
- Sun, C.-N.; Mench, M.M.; Zawodzinski, T.A. High Performance Redox Flow Batteries: An Analysis of the Upper Performance Limits of Flow Batteries Using Non-Aqueous Solvents. Electrochim. Acta 2017, 237, 199–206.
- Sevov, C.S.; Fisher, S.L.; Thompson, L.T.; Sanford, M.S. Mechanism-Based Development of a Low-Potential, Soluble, and Cyclable Multielectron Anolyte for Nonaqueous Redox Flow Batteries. J. Am. Chem. Soc. 2016, 138, 15378–15384.
- Chalamala, B.R.; Soundappan, T.; Fisher, G.R.; Anstey, M.R.; Viswanathan, V.V.; Perry, M.L. Redox Flow Batteries: An Engineering Perspective. Proc. IEEE 2014, 102, 976–999.
- Li, M.; Rhodes, Z.; Cabrera-Pardo, J.R.; Minteer, S.D. Recent Advancements in Rational Design of Non-Aqueous Organic Redox Flow Batteries. Sustain. Energy Fuels 2020, 4, 4370–4389.
- Matsuda, Y.; Tanaka, K.; Okada, M.; Takasu, Y.; Morita, M. A Rechargeable Redox Battery Utilizing Ruthenium Complexes with Non-Aqueous Organic Electrolyte. J. Appl. Electrochem. 1988, 18, 909–914.
- Milshtein, J.D.; Kaur, A.P.; Casselman, M.D.; Kowalski, J.A.; Modekrutti, S.; Zhang, P.L.; Harsha Attanayake, N.; Elliott, C.F.; Parkin, S.R.; Risko, C.; et al. High Current Density, Long Duration Cycling of Soluble Organic Active Species for Non-Aqueous Redox Flow Batteries. Energy Environ. Sci. 2016, 9, 3531–3543.
- Yuan, J.; Zhang, C.; Liu, T.; Zhen, Y.; Pan, Z.-Z.; Li, Y. Two-Dimensional Metal-Organic Framework Nanosheets-Modified Porous Separator for Non-Aqueous Redox Flow Batteries. J. Membr. Sci. 2020, 612, 118463.
- Armstrong, C.G.; Hogue, R.W.; Toghill, K.E. Characterisation of the Ferrocene/Ferrocenium Ion Redox Couple as a Model Chemistry for Non-Aqueous Redox Flow Battery Research. J. Electroanal. Chem. 2020, 872, 114241.
- Li, Y.; Geysens, P.; Zhang, X.; Sniekers, J.; Fransaer, J.; Binnemans, K.; Vankelecom, I.F.J. Cerium-Containing Complexes for Low-Cost, Non-Aqueous Redox Flow Batteries (RFBs). J. Power Sources 2020, 450, 227634.
- Kosswattaarachchi, A.M.; Cook, T.R. Concentration-Dependent Charge-Discharge Characteristics of Non-Aqueous Redox Flow Battery Electrolyte Combinations. Electrochim. Acta 2018, 261, 296–306.
- Sleightholme, A.E.S.; Shinkle, A.A.; Liu, Q.; Li, Y.; Monroe, C.W.; Thompson, L.T. Non-Aqueous Manganese Acetylacetonate Electrolyte for Redox Flow Batteries. J. Power Sources 2011, 196, 5742–5745.
- Liu, Q.; Shinkle, A.A.; Li, Y.; Monroe, C.W.; Thompson, L.T.; Sleightholme, A.E.S. Non-Aqueous Chromium Acetylacetonate Electrolyte for Redox Flow Batteries. Electrochem. Commun. 2010, 12, 1634–1637.
- Liu, Q.; Sleightholme, A.E.S.; Shinkle, A.A.; Li, Y.; Thompson, L.T. Non-Aqueous Vanadium Acetylacetonate Electrolyte for Redox Flow Batteries. Electrochem. Commun. 2009, 11, 2312–2315.
- Kaur, A.P.; Holubowitch, N.E.; Ergun, S.; Elliott, C.F.; Odom, S.A. A Highly Soluble Organic Catholyte for Non-Aqueous Redox Flow Batteries. Energy Technol. 2015, 3, 476–480.
- Su, L.; Ferrandon, M.; Kowalski, J.A.; Vaughey, J.T.; Brushett, F.R. Electrolyte Development for Non-Aqueous Redox Flow Batteries Using a High-Throughput Screening Platform. J. Electrochem. Soc. 2014, 161, A1905–A1914.
- Mun, J.; Lee, M.-J.; Park, J.-W.; Oh, D.-J.; Lee, D.-Y.; Doo, S.-G. Non-Aqueous Redox Flow Batteries with Nickel and Iron Tris(2,2ʹ-Bipyridine) Complex Electrolyte. Electrochem. Solid-State Lett. 2012, 15, A80.
- Hamelet, S.; Tzedakis, T.; Leriche, J.-B.; Sailler, S.; Larcher, D.; Taberna, P.-L.; Simon, P.; Tarascon, J.-M. Non-Aqueous Li-Based Redox Flow Batteries. J. Electrochem. Soc. 2012, 159, A1360–A1367.
- Li, Z.; Li, S.; Liu, S.; Huang, K.; Fang, D.; Wang, F.; Peng, S. Electrochemical Properties of an All-Organic Redox Flow Battery Using 2,2,6,6-Tetramethyl-1-Piperidinyloxy and N-Methylphthalimide. Electrochem. Solid-State Lett. 2011, 14, A171.
- Kwon, G.; Lee, K.; Lee, M.H.; Lee, B.; Lee, S.; Jung, S.-K.; Ku, K.; Kim, J.; Park, S.Y.; Kwon, J.E.; et al. Bio-Inspired Molecular Redesign of a Multi-Redox Catholyte for High-Energy Non-Aqueous Organic Redox Flow Batteries. Chem 2019, 5, 2642–2656.
- Mirle, C.R.; Raja, M.; Vasudevarao, P.; Sankararaman, S.; Kothandaraman, R. Functionalised Carbazole as a Cathode for High Voltage Non-Aqueous Organic Redox Flow Batteries. New J. Chem. 2020, 44, 14401–14410.
- Chai, J.; Lashgari, A.; Wang, X.; Williams, C.K.; Jiang, J. “Jimmy” All-PEGylated Redox-Active Metal-Free Organic Molecules in Non-Aqueous Redox Flow Battery. J. Mater. Chem. A 2020, 8, 15715–15724.
- Back, J.; Kwon, G.; Byeon, J.E.; Song, H.; Kang, K.; Lee, E. Tunable Redox-Active Triazenyl–Carbene Platforms: A New Class of Anolytes for Non-Aqueous Organic Redox Flow Batteries. ACS Appl. Mater. Interfaces 2020, 12, 37338–37345.
- Silcox, B. Stability and Cyclability Predictions of Redox Active Organic Molecules for Non-Aqueous Redox Flow Batteries. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2021.
- Armstrong, C.G.; Hogue, R.W.; Toghill, K.E. Application of the Dianion Croconate Violet for Symmetric Organic Non-Aqueous Redox Flow Battery Electrolytes. J. Power Sources 2019, 440, 227037.
- Sharma, S.; Andrade, G.A.; Maurya, S.; Popov, I.A.; Batista, E.R.; Davis, B.L.; Mukundan, R.; Smythe, N.C.; Tondreau, A.M.; Yang, P.; et al. Iron-Iminopyridine Complexes as Charge Carriers for Non-Aqueous Redox Flow Battery Applications. Energy Storage Mater. 2021, 37, 576–586.
- Sharma, S.; Rathod, S.; Prakash Yadav, S.; Chakraborty, A.; Shukla, A.K.; Aetukuri, N.; Patil, S. Electrochemical Evaluation of Diketopyrrolopyrrole Derivatives for Nonaqueous Redox Flow Batteries. Chem. Eur. J. 2021.
- Rahimi, M.; Kim, T.; Gorski, C.A.; Logan, B.E. A Thermally Regenerative Ammonia Battery with Carbon-Silver Electrodes for Converting Low-Grade Waste Heat to Electricity. J. Power Sources 2018, 373, 95–102.
- Zhang, H.; Zhang, H.; Li, X.; Mai, Z.; Zhang, J. Nanofiltration (NF) Membranes: The next Generation Separators for All Vanadium Redox Flow Batteries (VRBs)? Energy Environ. Sci. 2011, 4, 1676–1679.
- Qiao, L.; Zhang, H.; Lu, W.; Dai, Q.; Li, X. Advanced Porous Membranes with Tunable Morphology Regulated by Ionic Strength of Nonsolvent for Flow Battery. ACS Appl. Mater. Interfaces 2019, 11, 24107–24113.
- Su, L.; Zhang, D.; Peng, S.; Wu, X.; Luo, Y.; He, G. Orientated Graphene Oxide/Nafion Ultra-Thin Layer Coated Composite Membranes for Vanadium Redox Flow Battery. Int. J. Hydrog. Energy 2017, 42, 21806–21816.
- Zhang, M.; Moore, M.; Watson, J.S.; Zawodzinski, T.A.; Counce, R.M. Capital Cost Sensitivity Analysis of an All-Vanadium Redox-Flow Battery. J. Electrochem. Soc. 2012, 159, A1183.
- Navalpotro, P.; Neves, C.M.S.S.; Palma, J.; Freire, M.G.; Coutinho, J.A.P.; Marcilla, R. Pioneering Use of Ionic Liquid-Based Aqueous Biphasic Systems as Membrane-Free Batteries. Adv. Sci. 2018, 5, 1800576.
- Park, H.B.; Lee, K.H.; Sung, H.J. Performance of H-Shaped Membraneless Micro Fuel Cells. J. Power Sources 2013, 226, 266–271.
- López-Montesinos, P.O.; Yossakda, N.; Schmidt, A.; Brushett, F.R.; Pelton, W.E.; Kenis, P.J.A. Design, Fabrication, and Characterization of a Planar, Silicon-Based, Monolithically Integrated Micro Laminar Flow Fuel Cell with a Bridge-Shaped Microchannel Cross-Section. J. Power Sources 2011, 196, 4638–4645.
- Marschewski, J.; Jung, S.; Ruch, P.; Prasad, N.; Mazzotti, S.; Michel, B.; Poulikakos, D. Mixing with Herringbone-Inspired Microstructures: Overcoming the Diffusion Limit in Co-Laminar Microfluidic Devices. Lab Chip 2015, 15, 1923–1933.
- Chang, M.-H.; Chen, F.; Fang, N.-S. Analysis of Membraneless Fuel Cell Using Laminar Flow in a Y-Shaped Microchannel. J. Power Sources 2006, 159, 810–816.
- Park, H.B.; Ahmed, D.H.; Lee, K.H.; Sung, H.J. An H-Shaped Design for Membraneless Micro Fuel Cells. Electrochim. Acta 2009, 54, 4416–4425.
- Kjeang, E.; Michel, R.; Harrington, D.A.; Djilali, N.; Sinton, D. A Microfluidic Fuel Cell with Flow-Through Porous Electrodes. J. Am. Chem. Soc. 2008, 130, 4000–4006.
- Kjeang, E.; Proctor, B.T.; Brolo, A.G.; Harrington, D.A.; Djilali, N.; Sinton, D. High-Performance Microfluidic Vanadium Redox Fuel Cell. Electrochim. Acta 2007, 52, 4942–4946.
- Ferrigno, R.; Stroock, A.D.; Clark, T.D.; Mayer, M.; Whitesides, G.M. Membraneless Vanadium Redox Fuel Cell Using Laminar Flow. J. Am. Chem. Soc. 2002, 124, 12930–12931.
- Ibáñez, S.E.; Quintero, A.E.; García-Salaberri, P.A.; Vera, M. Effects of the Diffusive Mixing and Self-Discharge Reactions in Microfluidic Membraneless Vanadium Redox Flow Batteries. Int. J. Heat Mass Transf. 2021, 170, 121022.
- Ibrahim, O.A.; Goulet, M.-A.; Kjeang, E. In-Situ Characterization of Symmetric Dual-Pass Architecture of Microfluidic Co-Laminar Flow Cells. Electrochim. Acta 2016, 187, 277–285.
- Lee, J.W.; Goulet, M.-A.; Kjeang, E. Microfluidic Redox Battery. Lab Chip 2013, 13, 2504.
- Marschewski, J.; Ruch, P.; Ebejer, N.; Huerta Kanan, O.; Lhermitte, G.; Cabrol, Q.; Michel, B.; Poulikakos, D. On the Mass Transfer Performance Enhancement of Membraneless Redox Flow Cells with Mixing Promoters. Int. J. Heat Mass Transf. 2017, 106, 884–894.
- Navalpotro, P.; Palma, J.; Anderson, M.; Marcilla, R. A Membrane-Free Redox Flow Battery with Two Immiscible Redox Electrolytes. Angew. Chem. 2017, 129, 12634–12639.
- Bamgbopa, M.O.; Shao-Horn, Y.; Hashaikeh, R.; Almheiri, S. Cyclable Membraneless Redox Flow Batteries Based on Immiscible Liquid Electrolytes: Demonstration with All-Iron Redox Chemistry. Electrochim. Acta 2018, 267, 41–50.
- Navalpotro, P.; Trujillo, C.; Montes, I.; Neves, C.M.S.S.; Palma, J.; Freire, M.G.; Coutinho, J.A.P.; Marcilla, R. Critical Aspects of Membrane-Free Aqueous Battery Based on Two Immiscible Neutral Electrolytes. Energy Storage Mater. 2020, 26, 400–407.
- Navalpotro, P.; Sierra, N.; Trujillo, C.; Montes, I.; Palma, J.; Marcilla, R. Exploring the Versatility of Membrane-Free Battery Concept Using Different Combinations of Immiscible Redox Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 41246–41256.
- Molina-Osorio, A.F.; Gamero-Quijano, A.; Peljo, P.; Scanlon, M.D. Membraneless Energy Conversion and Storage Using Immiscible Electrolyte Solutions. Curr. Opin. Electrochem. 2020, 21, 100–108.
- Peljo, P.; Bichon, M.; Girault, H.H. Ion Transfer Battery: Storing Energy by Transferring Ions across Liquid–Liquid Interfaces. Chem. Commun. 2016, 52, 9761–9764.
- Han, X.; Li, X.; White, J.; Zhong, C.; Deng, Y.; Hu, W.; Ma, T. Metal–Air Batteries: From Static to Flow System. Adv. Energy Mater. 2018, 8, 1801396.
- Yu, W.; Shang, W.; Tan, P.; Chen, B.; Wu, Z.; Xu, H.; Shao, Z.; Liu, M.; Ni, M. Toward a New Generation of Low Cost, Efficient, and Durable Metal–Air Flow Batteries. J. Mater. Chem. A 2019, 7, 26744–26768.
- Risbud, M.; Menictas, C.; Skyllas-Kazacos, M.; Noack, J. Vanadium Oxygen Fuel Cell Utilising High Concentration Electrolyte. Batteries 2019, 5, 24.
- Charvát, J.; Mazúr, P.; Paidar, M.; Pocedič, J.; Vrána, J.; Mrlík, J.; Kosek, J. The Role of Ion Exchange Membrane in Vanadium Oxygen Fuel Cell. J. Membr. Sci. 2021, 629, 119271.
- Chen, P.-T.; Sangeetha, T.; Hsu, T.-W.; Yang, C.-J.; Yung, T.-Y.; Yan, W.-M.; Huang, K.D. Improved Performance of a Zn-Air Fuel Cell by Coupling Zn Particle Fuel and Flowing Electrolyte. Chem. Phys. Lett. 2019, 728, 160–166.
- Pei, P.; Huang, S.; Chen, D.; Li, Y.; Wu, Z.; Ren, P.; Wang, K.; Jia, X. A High-Energy-Density and Long-Stable-Performance Zinc-Air Fuel Cell System. Appl. Energy 2019, 241, 124–129.
- Sangeetha, T.; Chen, P.-T.; Yan, W.-M.; Huang, K.D. Enhancement of Air-Flow Management in Zn-Air Fuel Cells by the Optimization of Air-Flow Parameters. Energy 2020, 197, 117181.
- Zhang, N.; Deng, C.; Tao, S.; Guo, L.; Cheng, Y. Bifunctional Oxygen Electrodes with Gradient Hydrophilic/Hydrophobic Reactive Interfaces for Metal Air Flow Batteries. Chem. Eng. Sci. 2020, 224, 115795.
- Yang, T.-F.; Lu, J.-H.; Yan, W.-M.; Ghalambaz, M. Optimization of Pulse Current on Energy Storage of Zinc-Air Flow Batteries. J. Power Sources 2019, 442, 227253.
- Yu, W.; Shang, W.; Xiao, X.; Ma, Y.; Chen, Z.; Chen, B.; Xu, H.; Ni, M.; Tan, P. Elucidating the Mechanism of Discharge Performance Improvement in Zinc-Air Flow Batteries: A Combination of Experimental and Modeling Investigations. J. Energy Storage 2021, 40, 102779.
- Huang, J.; Faghri, A. Capacity Enhancement of a Lithium Oxygen Flow Battery. Electrochim. Acta 2015, 174, 908–918.
- Guang Zhu, Y.; Jia, C.; Yang, J.; Pan, F.; Huang, Q.; Wang, Q. Dual Redox Catalysts for Oxygen Reduction and Evolution Reactions: Towards a Redox Flow Li–O2 Battery. Chem. Commun. 2015, 51, 9451–9454.
- Zhu, Y.G.; Wang, X.; Jia, C.; Yang, J.; Wang, Q. Redox-Mediated ORR and OER Reactions: Redox Flow Lithium Oxygen Batteries Enabled with a Pair of Soluble Redox Catalysts. ACS Catal. 2016, 6, 6191–6197.
- Ruggeri, I.; Arbizzani, C.; Soavi, F. Carbonaceous Catholyte for High Energy Density Semi-Solid Li/O2 Flow Battery. Carbon 2018, 130, 749–757.
- Guo, L.; Guo, H.; Huang, H.; Tao, S.; Cheng, Y. Inhibition of Zinc Dendrites in Zinc-Based Flow Batteries. Front. Chem. 2020, 8, 557.
- Xu, Z.; Fan, Q.; Li, Y.; Wang, J.; Lund, P.D. Review of Zinc Dendrite Formation in Zinc Bromine Redox Flow Battery. Renew. Sustain. Energy Rev. 2020, 127, 109838.
- Wu, M.C.; Zhao, T.S.; Jiang, H.R.; Zeng, Y.K.; Ren, Y.X. High-Performance Zinc Bromine Flow Battery via Improved Design of Electrolyte and Electrode. J. Power Sources 2017, 355, 62–68.
- Wu, M.C.; Zhao, T.S.; Wei, L.; Jiang, H.R.; Zhang, R.H. Improved Electrolyte for Zinc-Bromine Flow Batteries. J. Power Sources 2018, 384, 232–239.
- Jiang, H.R.; Wu, M.C.; Ren, Y.X.; Shyy, W.; Zhao, T.S. Towards a Uniform Distribution of Zinc in the Negative Electrode for Zinc Bromine Flow Batteries. Appl. Energy 2018, 213, 366–374.
- Archana, K.S.; Naresh, R.p.; Enale, H.; Rajendran, V.; Mohan, A.M.V.; Bhaskar, A.; Ragupathy, P.; Dixon, D. Effect of Positive Electrode Modification on the Performance of Zinc-Bromine Redox Flow Batteries. J. Energy Storage 2020, 29, 101462.
- Lu, W.; Xu, P.; Shao, S.; Li, T.; Zhang, H.; Li, X. Multifunctional Carbon Felt Electrode with N-Rich Defects Enables a Long-Cycle Zinc-Bromine Flow Battery with Ultrahigh Power Density. Adv. Funct. Mater. 2021, 31, 2102913.
- Mariyappan, K.; Velmurugan, R.; Subramanian, B.; Ragupathy, P.; Ulaganathan, M. Low Loading of Felt for Enhancing Multifunctional Activity towards Achieving High Energy Efficiency of Zn–Br2 Redox Flow Battery. J. Power Sources 2021, 482, 228912.
- Lee, J.-N.; Do, E.; Kim, Y.; Yu, J.-S.; Kim, K.J. Development of Titanium 3D Mesh Interlayer for Enhancing the Electrochemical Performance of Zinc–Bromine Flow Battery. Sci. Rep. 2021, 11, 4508.
- Yuan, X.; Mo, J.; Huang, J.; Liu, J.; Liu, C.; Zeng, X.; Zhou, W.; Yue, J.; Wu, X.; Wu, Y. An Aqueous Hybrid Zinc-Bromine Battery with High Voltage and Energy Density. ChemElectroChem 2020, 7, 1531–1536.
- Hua, L.; Lu, W.; Li, T.; Xu, P.; Zhang, H.; Li, X. A Highly Selective Porous Composite Membrane with Bromine Capturing Ability for a Bromine-Based Flow Battery. Mater. Today Energy 2021, 21, 100763.
- Adith, R.V.; Naresh, R.p.; Mariyappan, K.; Ulaganathan, M.; Ragupathy, P. An Optimistic Approach on Flow Rate and Supporting Electrolyte for Enhancing the Performance Characteristics of Zn-Br2 Redox Flow Battery. Electrochim. Acta 2021, 388, 138451.
- Yu, F.; Zhang, C.; Wang, F.; Gu, Y.; Zhang, P.; Waclawik, E.R.; Du, A.; Ostrikov, K.; Wang, H. A Zinc Bromine “Supercapattery” System Combining Triple Functions of Capacitive, Pseudocapacitive and Battery-Type Charge Storage. Mater. Horiz. 2020, 7, 495–503.
- Wang, Z.; Tam, L.-Y.S.; Lu, Y.-C. Flexible Solid Flow Electrodes for High-Energy Scalable Energy Storage. Joule 2019, 3, 1677–1688.
- Qi, Z.; Koenig, G.M. Review Article: Flow Battery Systems with Solid Electroactive Materials. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2017, 35, 040801.
- Ventosa, E.; Buchholz, D.; Klink, S.; Flox, C.; Chagas, L.G.; Vaalma, C.; Schuhmann, W.; Passerini, S.; Morante, J.R. Non-Aqueous Semi-Solid Flow Battery Based on Na-Ion Chemistry. P2-Type NaxNi0.22Co0.11Mn0.66O2–NaTi2(PO4)3. Chem. Commun. 2015, 51, 7298–7301.
- Liu, Y.; Hu, Q.; Zhong, J.; Wang, Z.; Guo, H.; Yan, G.; Li, X.; Peng, W.; Wang, J. A Renewable Sedimentary Slurry Battery: Preliminary Study in Zinc Electrodes. iScience 2020, 23, 101821.
- Percin, K.; Rommerskirchen, A.; Sengpiel, R.; Gendel, Y.; Wessling, M. 3D-Printed Conductive Static Mixers Enable All-Vanadium Redox Flow Battery Using Slurry Electrodes. J. Power Sources 2018, 379, 228–233.
- Petek, T.J.; Hoyt, N.C.; Savinell, R.F.; Wainright, J.S. Slurry Electrodes for Iron Plating in an All-Iron Flow Battery. J. Power Sources 2015, 294, 620–626.
- Chen, H.; Liu, Y.; Zhang, X.; Lan, Q.; Chu, Y.; Li, Y.; Wu, Q. Single-Component Slurry Based Lithium-Ion Flow Battery with 3D Current Collectors. J. Power Sources 2021, 485, 229319.
- Ye, J.; Xia, L.; Wu, C.; Ding, M.; Jia, C.; Wang, Q. Redox Targeting-Based Flow Batteries. J. Phys. D Appl. Phys. 2019, 52, 443001.
- Chayambuka, K.; Fransaer, J.; Dominguez-Benetton, X. Modeling and Design of Semi-Solid Flow Batteries. J. Power Sources 2019, 434, 226740.
- Li, Z.; Smith, K.C.; Dong, Y.; Baram, N.; Fan, F.Y.; Xie, J.; Limthongkul, P.; Carter, W.C.; Chiang, Y.-M. Aqueous Semi-Solid Flow Cell: Demonstration and Analysis. Phys. Chem. Chem. Phys. 2013, 15, 15833–15839.
- Zhu, Y.G.; Narayanan, T.M.; Tulodziecki, M.; Sanchez-Casalongue, H.; Horn, Q.C.; Meda, L.; Yu, Y.; Sun, J.; Regier, T.; McKinley, G.H.; et al. High-Energy and High-Power Zn–Ni Flow Batteries with Semi-Solid Electrodes. Sustain. Energy Fuels 2020, 4, 4076–4085.
- Mourshed, M.; Niya, S.M.R.; Ojha, R.; Rosengarten, G.; Andrews, J.; Shabani, B. Carbon-Based Slurry Electrodes for Energy Storage and Power Supply Systems. Energy Storage Mater. 2021, S2405829721002440.
- Yan, W.; Wang, C.; Tian, J.; Zhu, G.; Ma, L.; Wang, Y.; Chen, R.; Hu, Y.; Wang, L.; Chen, T.; et al. All-Polymer Particulate Slurry Batteries. Nat. Commun. 2019, 10, 2513.
- Yan, R.; Wang, Q. Redox-Targeting-Based Flow Batteries for Large-Scale Energy Storage. Adv. Mater. 2018, 30, 1802406.
- Lohaus, J.; Rall, D.; Kruse, M.; Steinberger, V.; Wessling, M. On Charge Percolation in Slurry Electrodes Used in Vanadium Redox Flow Batteries. Electrochem. Commun. 2019, 101, 104–108.
- Brunini, V.E.; Chiang, Y.-M.; Carter, W.C. Modeling the Hydrodynamic and Electrochemical Efficiency of Semi-Solid Flow Batteries. Electrochim. Acta 2012, 69, 301–307.
- Páez, T.; Martínez-Cuezva, A.; Palma, J.; Ventosa, E. Mediated Alkaline Flow Batteries: From Fundamentals to Application. ACS Appl. Energy Mater. 2019, 2, 8328–8336.
- Zanzola, E.; Dennison, C.R.; Battistel, A.; Peljo, P.; Vrubel, H.; Amstutz, V.; Girault, H.H. Redox Solid Energy Boosters for Flow Batteries: Polyaniline as a Case Study. Electrochim. Acta 2017, 235, 664–671.
- Zhou, M.; Huang, Q.; Pham Truong, T.N.; Ghilane, J.; Zhu, Y.G.; Jia, C.; Yan, R.; Fan, L.; Randriamahazaka, H.; Wang, Q. Nernstian-Potential-Driven Redox-Targeting Reactions of Battery Materials. Chem 2017, 3, 1036–1049.
- Moghaddam, M.; Sepp, S.; Wiberg, C.; Bertei, A.; Rucci, A.; Peljo, P. Thermodynamics, Charge Transfer and Practical Considerations of Solid Boosters in Redox Flow Batteries. Molecules 2021, 26, 2111.
- Choi, N.H.; del Olmo, D.; Milian, D.; El Kissi, N.; Fischer, P.; Pinkwart, K.; Tübke, J. Use of Carbon Additives towards Rechargeable Zinc Slurry Air Flow Batteries. Energies 2020, 13, 4482.
- Dmello, R.; Milshtein, J.D.; Brushett, F.R.; Smith, K.C. Cost-Driven Materials Selection Criteria for Redox Flow Battery Electrolytes. J. Power Sources 2016, 330, 261–272.


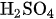
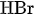 and
and