Redox Flow Batteries (RFB) are electrochemical energy storage devices that converts chemical energy into electrical energy through reversible oxidation and reduction of the working fluids. Redox flow batteries are considered by many to be a promising technology for the storage of energy for days or even weeks. Other advantages of RFBs are modularly and the ability to change the output power and energy capacity independently, by changing the size and number of cells in a stack and by adjusting the volume of electrolyte. Also, RFBs show a long lifecycle compared to lithium-ion batteries.
- redox flow batteries
- energy storage
- batteries
- stationary energy storage
1. Introduction
2. Redox Flow Batteries (RFB)
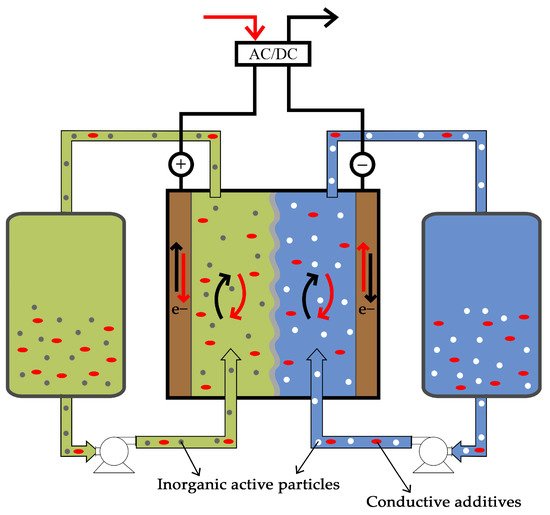
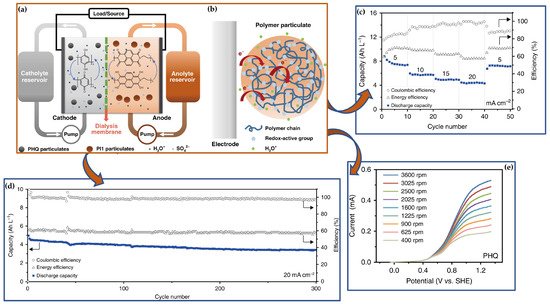
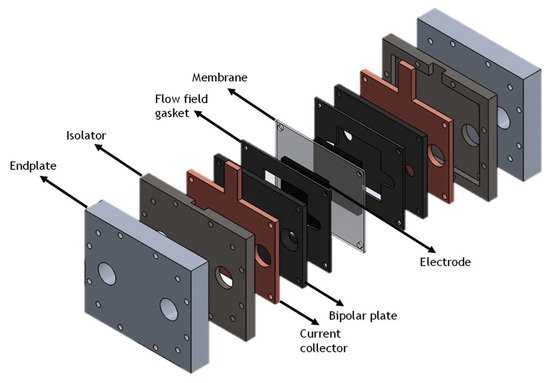
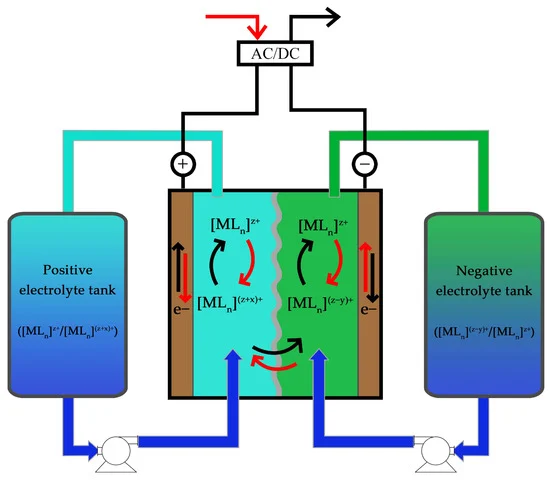
RFBs comprise three components: two tanks and a cell (vide Figure 1). The tanks are used to store electrolyte, the solution where the energy is stored, while the cell is where the redox reactions occur. This property of RFB is one of the main advantages of the technology since it is the reason why the quantity of energy stored is decoupled from the power output. By increasing the volume of electrolyte in the tanks, it is possible to store more energy; however, if the objective is to increase the power output, only the cell needs to be changed. Stacking cells in series increases the potential of the battery and amplifying the active area of the cell increases the current produced in the cell. The highly customizable nature of RFBs is generally rare in energy storage systems, but extremely versatile and interesting.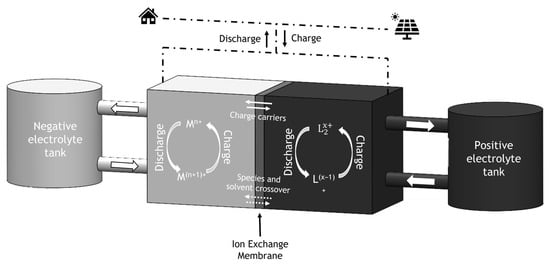
Year | Ref. |
G1 | G1 | Authors | Discussed Subject Matter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
G2 | G2 |
G3 | G3 | ||||||||||||
2006 | |||||||||||||||
|
Name |
[9] |
Ponce de Léon et al. | Name |
All-Vanadium | All-Vanadium |
Vanadium-Polyhalide | The different RFB systems were compared considering the OCP, power density, EE and charge–discharge behaviour. | ||||||||
| Vanadium-Polyhalide | Mixed Acid Vanadium | Mixed Acid Vanadium | 2011 | ||||||||||||
|
Positive Couple | Positive Couple |
[10] |
V(III)/V(II) | V(III)/V(II) | Weber et al. |
V(III)/V(II) | V(III)/V(II) | RFB chemistries, kinetics and transport of RFBs were discussed. The electrode/cell modeling and designs were reviewed as well as future research needs. | |||||||
V(III)/V(II) | V(III)/V(II) |
[11] |
|||||||||||||
|
Negative Couple | Li et al. | Negative Couple |
V(IV)/V(V) | The requirements for ion exchange membranes for VRFBs were reviewed, as well as the development prospects for next-generation materials. | |||||||||||
| V(IV)/V(V) | Cl−/ClBr2− | Cl−/ClBr2− |
V(IV)/V(V) | V(IV)/V(V) |
[12] |
||||||||||
|
Supporting Electrolyte | Supporting Electrolyte | Skyllas-Kazacos et al. |
| 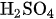 | Discussion focused on the technology in general. An historical review was also considered as well as the latest commercial developments and large-scale field testing. | ||||||||||
| and | 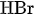 and and 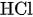 |
| 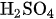 and and 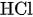 |
2012 | ||||||||||
|
Vanadium Concentration (M) | [13] |
Vanadium Concentration (M) |
1.5–2 [76] | 1.5–2 [76] | Kear et al. |
2.0–3.0 [77] | 2.0–3.0 [77] | Development, commercialisation history, and current performance properties of intermediate- and large-scale VRFBs were reviewed. The potential for VRFB systems to meet the economic requirements was compared to the economic performance of thermal-based generators. | |||||||
2.5–3.0 | [ | 78] | 2.5–3.0 [78] |
[14] |
Leung et al. | ||||||||||
|
Temperature Range (°C) | Temperature Range (°C) |
10–40 [76] | 10–40 [76] | The development of RFB systems was reviewed. It was concluded that fundamental studies on chemistry and kinetics are necessary for many RFB technologies. | |||||||||||
0–50 | [79] | 0–50 [79] | −5–50 [4,78,80] |
[15] |
|||||||||||
|
Specific Energy (Wh/L) | Wang et al. | Specific Energy (Wh/L) |
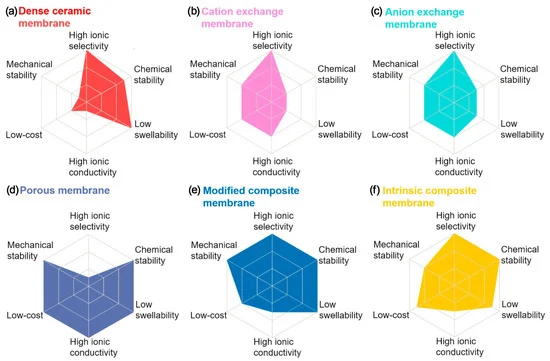
20–33 [77] | 20–33 [77 | The chemistries and progresses of Li-ion and RFB were reviewed and compared. The authors discussed the research status of a Li–RFB hybrid system and concluded that it was still in its infancy. | ||||||||||
| ] | 35–70 [77] | 35–70 [77] | 22–40 [ | 2013 |
[16] |
Wang et al. | Review of the main developments, particullarly new chemistries reported since 2010. The field of NA-RFBs was also included (i.e., redox chemistries, new RFB configurations) and was limited to R&D on cell-level components, excluding stack system, e.g., flow-field simulations, shunt-current analysis, and bipolar plate development. | ||||||||
| 4 | , | 78 | ,80] |
[17] |
Shin et al. | Non-aqueous RFB (NA-RFB) systems were compared to aqueous RFBs in terms of the current and power density through membranes. | |||||||||
2014 |
[18] |
Chakrabarti et al. | The application of ionic liquids (ILs) and deep eutetic solvents (DESs) in different RFB configurations was reviewed. The prospect of applying DESs in RFBs was discussed using the results reported in the literature considering the electrochemical engineering aspects of these solvents. | ||||||||||||
|
[19] |
Alotto et al. | The state-of-the-art of the most important plants in service and programs development were discussed. The most relevant research issues were debated. | |||||||||||||
2015 |
[20] |
Pan and Wang | The redox species of RFB were discussed. It was concluded that most of the non-aqueous electrolytes were focused on the catholyte, that the anodic species were limited, and that to fabricate a NA-RFB with high energy density, the development of anodic species was necessary. | ||||||||||||
|
[21] |
Soloveichik | A discussion on the different types of flow batteries was conducted. Technical and economical issues were also approached. | |||||||||||||
|
[22] |
Kim et al. | The technical trends in the selection, characterization, evaluation, and modification of electrodes for VRFBs were reviewed between 1985 and 2015. | |||||||||||||
|
[23] |
Xu and Zhao | The various issues associated with flow batteries were summarized and a critical review on the numerical investigations of each issue was performed. | |||||||||||||
|
[24] |
Huang et al. | NA-RFBs were compared with aqueous systems. The parameters included wider voltage windows, intrinsically faster electron-transfer kinetics, and more extended working temperature ranges. | |||||||||||||
2016 |
[25] |
Winsber et al. | Overview focused on different flow-battery systems ranging from the classical inorganic to organic/inorganic to RFBs with organic redox-active cathode and anode materials in terms of technical, economic, and environmental aspects. | ||||||||||||
|
[26] |
Kowalski et al. | Review focused on describing the main advances in the developments of redox active organic molecules for all-organic flow batteries. | |||||||||||||
|
[27] |
Park et al. | Review on the development of flow batteries focused on materials and chemistries, i.e., conventional aqueous RFBs and the next-generation flow batteries. Despite progress, next-generation battery systems based on organic, iodine, polysulfide or semi-solid materials are still uncertain. | |||||||||||||
2017 |
[28] |
Arenas et al. | Review focused on the engineering aspects of RFBs. An approach to RFB design and scale-up was performed in order to reduce the gap in technological and research awareness between the academic literature and the industry. | ||||||||||||
|
[29] |
Leung et al. | Review of organic based RFB. Emphasis was given to electrode reactions in both aqueous and non-aqueous electrolytes. It was concluded that organic RFB containing materials of high solubilities and multi-electron-transfers meet the cost target for practical applications at the grid scale and in the automotive industry. | |||||||||||||
|
[30] |
Ye et al. | The impact on the voltage efficiency, CE, and EE of the types and properties of membranes on the VRFBs were reviewed. Material modification of carbon-based electrodes, catalyst application, and electrolytes using solid redox-active compounds in semi-solid RFB systems were also discussed. | |||||||||||||
|
[31] |
Choi et al. | Vanadium electrolyte technologies from the viewpoint of VRFB design was reviewed providing a logical understanding of how the electrolyte design influences battery performance. | |||||||||||||
|
[32] |
Li and Liu | Review comparing the future of RFB technology with Li-ion batteries. The questions regarding breakthroughs needed to enable large-scale deployment of RFBs remain. It was concluded that finding a low-cost, highly soluble aqueous system was the most attractive approach. | |||||||||||||
|
[33] |
Musbaudeen et al. | Membraneless cell designs for RFBs were reviewed considering the evolutionary trend of membraneless flow cell design concepts. | |||||||||||||
|
[34] |
Zhou et al. | The progress in research on the transport phenomena of RFBs, as well as the critical transport issues, were reviewed. | |||||||||||||
2018 |
[35] |
Chen et al. | The review focused on the advantages of organic materials for RFBs compared with inorganic-based RFBs and on the recent progress in organic RFBs in redox active materials. The properties of the electrolyte and the design of the membrane, including polymeric and ceramic membranes, were also debated. | ||||||||||||
|
[36] |
Zhang et al. | The performance metrics of RFBs and the progress on the key components of RFBs, including the membranes and new redox-active electrolytes, were reviewed. | |||||||||||||
|
[37] |
Liu et al. | The state-of-the-art of several modification methods on the electrode materials for VRFB were reviewed. | |||||||||||||
|
[38] |
Cao et al. | Review focused on vanadium electrolyte additives studied for VRFB regarding its function, including precipitation inhibitors, immobilizing agents, kinetic enhancers, electrolyte impurities, and chemical reductants. | |||||||||||||
|
[39] |
Xu et al. | Review focus on understanding the evaluation criteria of energy efficiency for RFBs. | |||||||||||||
|
[40] |
Ke et al. | The first review focused on the influence on RFB cell performance linked with flow field designs, including their implementation in stacks. Several aspects were considered, e.g., flow field architecture types, flow distribution, cell performance, large-scale stack designs, stack performance, optimization of non-uniform flow distributions, shunt currents, and localized current distributions. | |||||||||||||
|
[41] |
Arenas et al. | Review focused on the four main types of RFB employing zinc electrodes, i.e., zinc–bromine, zinc–cerium, zinc–air and zinc–nickel. The main drawbacks linked with zinc deposition and dissolution, particularly in acid media, were also reviewed. | |||||||||||||
|
[42] |
Minke and Turek | The literature focused on the techno-economic assessment of VFB was reviewed. The data regarding materials, system designs, and modelling approaches were considred and critically analyzed. | |||||||||||||
2019 |
[43] |
Lourenssen et al. | The current state of the art of VRFB technology was discussed, including the design and working principles. The critical research areas were highlighted along with future developments. | ||||||||||||
|
[44] |
Narayan et al. | The authors discussed the basic requirements to be satisfied by next-generation aqueous RFBs and concluded that a safe, affordable, sustainable, and robust long-duration energy storage system was promising with next-generation RFBs. | |||||||||||||
|
[45] |
Hogue and Toghill | A review of the metal coordination complexes studied as electrolytes for NA-RFBs that were reported in the previous decade. | |||||||||||||
|
[46] |
Gubler | The review focused on the key requirements and current development trends for membranes and separators for the VRFB. | |||||||||||||
|
[47] |
Arenas et al. | The research needs were reviewed. It was concluded that most academic studies focus on the development of catalysts tested in small electrochemical cells was not realistic for advancing RFB technology; they are limited to short-term laboratory experiments. | |||||||||||||
2020 |
[48] |
Rhodes et al. | A review on NA-RFBs was conducted. It was concluded that these are predicted to be sustainable systems as the components are mostly organic materials and that NA-RFBs represent the next generation of RFB for green energy storage. | ||||||||||||
|
[49] |
Clemente and Costa-Castelló | A review focused on the RFB models and the main control strategies of RFB systems, as well as the main techniques to estimate the state of charge. | |||||||||||||
|
[50] |
Gencten and Sahin | The electrode materials for VRFBs were reviewed. It was concluded that graphene coatings, heteroatom doping, and metal oxide modified carbon-based electrodes were mainly used. Most of the work regarding VRFBs is focused on novel stack design, electrode, membrane, and electrolyte components. | |||||||||||||
|
[51] |
Kwabi et al. | The review focused on the electrolyte lifetime in aqueous organic RFB. It was concluded that RFBs are promising alternatives for surpassing lithium ion batteries and aqueous organic RFBs have potentially lower cost than their vanadium-based counterparts. | |||||||||||||
|
[52] |
Zhong et al. | The state of the art of organic electroactive molecules for aqueous and non-aqueous RFBs were reviewed. It was concluded that this field was still in its initial stage since no RFB has been deemed suitable to replace VRFBs. | |||||||||||||
|
[53] |
Gentil et al. | The challenges in the past five years for the development of next-generation RFBs were discussed. NA-RFBs were not included. The review addressed aqueous organic RFBs (AO-RFBs) and the technologies developed to increase the energy density of RFBs. | |||||||||||||
|
[54] |
Ortiz-Martínez | This work reviewed the advances in the application of ILs in RFBs. The authors showed that most of the studies focused on the use of Ils as supporting electrolytes and the latest studies showed their potential as electroactive species and electrolyte membranes. | |||||||||||||
|
[55] |
Ambrosi and Webster | The review focused on the most commonly used 3D printing fabrication methods as well as on the additive manufacturing technologies for the fabrication of RFB components that were classified according to the electrolyte nature used (i.e., aqueous and non-aqueous solvents). | |||||||||||||
|
[56] |
Aberoumand et al. | The review focused on VRFB technology methods developed to enhance the performance of the electrode and electrolyte as the main components. | |||||||||||||
|
[57] |
Esan et al. | The review focused on the modeling and simulation of RFB beyond the all-vanadium, including soluble lead–acid, semisolid, organic, zinc–nickel, zinc–bromine, hydrogen–bromine, sodium–air, and vanadium–cerium flow batteries. | |||||||||||||
|
[58] |
Tempelman et al. | A review on the most recent advancements in the structure design and optimization to improve the selectivity and conductivity of membranes. | |||||||||||||
2021 |
[5] |
Wang et al. | The research progress of insoluble flow batteries was reviewed. The key challenges from the fundamental research point of view and practical application perspectives were compared. | ||||||||||||
|
[3] |
Sánchez-Díez et al. | A review of the aqueous system technologies that potentially fulfill cost requirements and enable large scale storage. | |||||||||||||
|
[59] |
Zhang and Sun | The review focused on iron-based aqueous RFBs. The main achievements were highlightned and it was concluded that there is no “perfect chemistry”. | |||||||||||||
|
[60] |
Emmet and Roberts | The review focused on the advances in aqueous RFBs with lesser known chemistries than vanadium. The authors expect that these chemistries will become more viable than vanadium due to their lower material costs and less caustic nature. | |||||||||||||
|
[61] |
Symons | The review focused on the use of quinones for RFBs. It was concluded that most of the development on quinone-based RFBs is far from being commercially viable. The stability of quinones in high potential electrolytes is not enough and the attempts have led to very low overall cell voltages. | |||||||||||||
|
[62] |
Aramendia et al. | The studies and numerical models carried out by means of computational fluid dynamics (CFD) techniques were reviewed. Studies with stacks and approaches for VRFB optimization with CFD based models and different flow field designs to improve the electrochemical performance were discussed. | |||||||||||||
|
[63] |
Yuan et al. | The development of the membranes used in the three types of NA-RFBs were summarized and a comprehensive overview of the fundamentals, classification, and performance of the membranes applied in NA-RFBs was provided. |
2.1. Inorganic Aqueous
2.1.1. Vanadium Redox Flow Batteries (VRFB)
All-Vanadium Redox Flow Battery (G1 RFB)
Vanadium-Polyhalide Redox Flow Battery (G2 RFB)
Positive Side: \( 2\text{ Br}^-+ \text{Cl}^−⇌ \text{ClBr}^-_2 + 2\text{ e}^- \) (2)
Mixed Acid Vanadium Redox Flow Battery (G3 RFB)
Another way to optimize the solubility of redox species is to use electrolyte additives to thermodynamically increase the solubility threshold and therefore to improve the kinetics, stability, and energy density of redox flow batteries. It is known that vanadium ions \( \text{V}^{2+} \), \( \text{V}^{3+} \), and \( \text{VO}^{2+} \)(i.e., \( \text{V}^{4+} \)) precipitate as its sulfate salts at low temperatures through the exothermal reactions represented by Equations (4)–(6) [4][38][105][4,38,105]:2.1.2. Polyoxometalates
2.2. Organic Aqueous
2.3. Non-Aqueous Solvents
3. Other RFB Configurations
3.1. Membraneless
3.2. Metal–Air Flow Batteries (MAFB) and Metal–Air Fuel Cells (MAFC)
3.3. Zinc–Bromine Flow Batteries
3.4. Semi-Solid (Slurry Flow Batteries)
3.4.1. Without Redox Mediator