| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mickaël Dollé | + 991 word(s) | 991 | 2021-02-04 10:34:22 | | | |
| 2 | Mickaël Dollé | + 8 word(s) | 999 | 2021-02-10 17:21:39 | | | | |
| 3 | Vicky Zhou | -106 word(s) | 893 | 2021-02-12 10:12:50 | | | | |
| 4 | Vicky Zhou | -210 word(s) | 789 | 2021-02-12 10:14:07 | | |
Video Upload Options
With the ever-growing energy storage notably due to the electric vehicle market expansion and stationary applications, one of the challenges of lithium batteries lies in the cost and environmental impacts of their manufacture. The main process employed is the solvent-casting method, based on a slurry casted onto a current collector. The disadvantages of this technique include the use of toxic and costly solvents as well as significant quantity of energy required for solvent evaporation and recycling. A solvent-free manufacturing method would represent significant progress in the development of cost-effective and environmentally friendly lithium-ion and lithium metal batteries.
1. Introduction
Li-ion batteries are a well-established and mature technology that has spread to almost, if not all, of our portable devices. Li-ion batteries are based on the insertion/deintercalation of lithium ions inside the electrode, more specifically into inorganic materials commonly called active materials due to their role inside electrodes. Such batteries have conquered the battery market as they present several advantages compared to other battery types like alkaline or lead-acid batteries. Li-ion batteries have higher specific power and specific energy and show an increased cycle life [1]. For these reasons, this technology is one of the most promising for use in the developing electric vehicle (EV) market. Mostly due to the high demand in the automotive industry for EVs, the fabrication of Li-ion batteries has drastically increased and is predicted to rise even more in coming years. The creation of batteries requires the formulation and manufacturing of an electrolyte and electrodes, the latter being made via the solvent-casting method. For an electrode, this classical process is based on pre-dissolving a polymer into an organic, usually toxic, solvent. The different particles, which are the conductive carbons and the active material are then added in order to make a slurry. This slurry is casted onto a current collector thanks to the doctor-blade technic. A good representation of this technic can be seen in Marks et al.’s publication [2]. The final step to obtain a solid electrode is to evaporate the remaining solvent, usually thanks to heat and vacuum. The increasing production of batteries therefore raises the issue of using more environmentally friendly and cheaper processing methods that do not require the use of solvents.
2. Safety Issues Related to the Use of Liquid Electrolytes
Another concern regarding the lithium batteries are the safety issues related to the use of liquid electrolytes. The organic solvents needed to make these electrolytes are highly flammable and, when used with Li metal anodes, can result in the formation of Li dendrites which can cause the device to short-circuit. To circumvent these constraints, solid electrolytes can be used. Promising solid electrolytes include solid polymer electrolytes (SPE), ceramics [3], and hybrid polymer electrolytes that contain inorganic fillers [4]. The use of flexible polymer-based electrolytes leads to improved surface contact at the interface with the electrode which can be an issue with brittle ceramic systems. Polymer-based electrolytes can also act as separators decreasing the battery’s weight and will mostly allow the use of Li metal as the anode. Device safety also increases because no flammable organic solvent is needed and the mechanical strength of the polymer-based electrolyte is generally sufficient to act as a physical barrier to limit dendrite growth [5]. Polymer electrolytes and hybrid polymer electrolytes are now under intense investigation to find the most efficient component which could replace definitively liquid electrolytes in commercial Li-ion batteries.
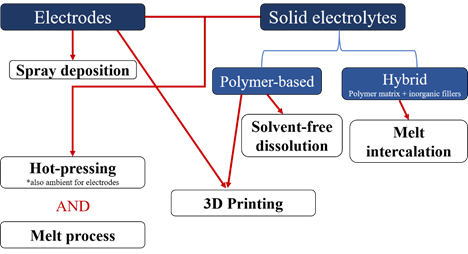
Hence, research on all-solid-state batteries is very active due to the high potential of these devices to be the batteries of tomorrow. Although the race has been centered on creating new high-performance polymer-based electrolytes or electrodes, the other concern in the community is the processes that are used to make these components. Though the solvent-casting method is the most common process for making porous and non-porous electrodes as well as polymer-based electrolytes [6][7][8], several groups have been working on new processes to replace the use of solvents. The solvent-casting technique raises the potential issues of a time consuming drying step [7] and the presence of residual solvent [9][10][11] which can be detrimental to battery performance and safety (presence of water with Li metal or coordination of water at the surface of ceramics that are in the hybrid electrolyte [12]). Moreover, this process is limited to making thick electrodes and the long drying time can lead to the agglomeration/sedimentation of particles resulting in non-homogenous samples, decreasing the performance of the electrolyte or the electrode [13]. Also, solvent evaporation leaves pores which are unnecessary in solid electrolytes and electrodes used in all-solid-state batteries [14]. Solvent-free preparation of polymer-based electrolytes and electrodes has emerged as a response to these limitations. (Figure 1)
Figure 1. Flow chart summarizing the different solvent-free processes recently developed to make electrodes and/or solid electrolytes for lithium-ion batteries.
References
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-art and energy management system of lithium-ion batteries in electric vehicle applications: Issues and recommendations. IEEE Access 2018, 6, 19362–19378.
- Marks, T.; Trussler, S.; Smith, A.; Xiong, D.; Dahn, J. A guide to Li-ion coin-cell electrode making for academic researchers. J. Electrochem. Soc. 2010, 158, A51.
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569.
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174.
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396–A404.
- Wright, P.V. Electrical conductivity in ionic complexes of poly(ethylene oxide). Br. Polym. J. 1975, 7, 319–327.
- Das, S.; Ghosh, A. Ion conduction and relaxation in PEO-LiTFSI-Al2O3 polymer nanocomposite electrolytes. J. Appl. Phys. 2015, 117, 174103.
- Gong, L.; Nguyen, M.H.T.; Oh, E.-S. High polar polyacrylonitrile as a potential binder for negative electrodes in lithium ion batteries. Electrochem. Commun. 2013, 29, 45–47.
- Mankovsky, D.; Lepage, D.; Lachal, M.; Caradant, L.; Aymé-Perrot, D.; Dollé, M. Water content in solid polymer electrolytes: The lost knowledge. Chem. Commun. 2020, 56, 10167–10170.
- Zhou, C.; Bag, S.; Lv, B.; Thangadurai, V. Understanding the Role of Solvents on the Morphological Structure and Li-Ion Conductivity of Poly (vinylidene fluoride)-Based Polymer Electrolytes. J. Electrochem. Soc. 2020, 167, 070552.
- Foran, G.; Mankovsky, D.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. The Impact of Absorbed Solvent on the Performance of Solid Polymer Electrolytes for Use in Solid-State Lithium Batteries. iScience 2020, 101597. [PubMed]
- González, F.; Garcia-Calvo, O.; Tiemblo, P.; García, N.; Fedeli, E.; Thieu, T.; Urdampilleta, I.; Kvasha, A. Synergy of Inorganic Fillers in Composite Thermoplastic Polymer/Ionic Liquid/LiTFSI Electrolytes. J. Electrochem. Soc. 2020, 167, 070519.
- Huang, Z.; Pang, W.; Liang, P.; Jin, Z.; Grundish, N.; Li, Y.; Wang, C.-A. A dopamine modified Li6.4La3Zr1.4Ta0.6O12/PEO solid-state electrolyte: Enhanced thermal and electrochemical properties. J. Mater. Chem. A 2019, 7, 16425–16436.
- Xi, J.; Qiu, X.; Ma, X.; Cui, M.; Yang, J.; Tang, X.; Zhu, W.; Chen, L. Composite polymer electrolyte doped with mesoporous silica SBA-15 for lithium polymer battery. Solid State Ionics 2005, 176, 1249–1260.