With the ever-growing energy storage notably due to the electric vehicle market expansion and stationary applications, one of the challenges of lithium batteries lies in the cost and environmental impacts of their manufacture. The main process employed is the solvent-casting method, based on a slurry casted onto a current collector. The disadvantages of this technique include the use of toxic and costly solvents as well as significant quantity of energy required for solvent evaporation and recycling. A solvent-free manufacturing method would represent significant progress in the development of cost-effective and environmentally friendly lithium-ion and lithium metal batteries.This review provides an overview of solvent-free processes used to make solid polymer electrolytes and composite electrodes. Two methods can be described: heat-based (hot-pressing, melt processing,dissolution into melted polymer, the incorporation of melted polymer into particles) and spray-based (electrospray deposition or high-pressure deposition). Heat-based processes are used for solid electrolyte and electrode manufacturing, while spray-based processes are only used for electrodeprocessing. Amongst these techniques, hot-pressing and melt processing were revealed to be themost used alternatives for both polymer-based electrolytes and electrodes. These two techniques are versatile and can be used in the processing of fillers with a wide range of morphologies and loadings
- Li-ion batteries
- electrodes
1. Introduction
Li-ion batteries are a well-established and mature technology that has spread to almost, if not all, of our portable devices. Li-ion batteries are based on the insertion/deintercalation of lithium ions inside the electrode, more specifically into inorganic materials commonly called active materials due to their role inside electrodes. Such batteries have conquered the battery market as they present several advantages compared to other battery types like alkaline or lead-acid batteries. Li-ion batteries have higher specific power and specific energy and show an increased cycle life [1]. For these reasons, this technology is one of the most promising for use in the developing electric vehicle (EV) market. Mostly due to the high demand in the automotive industry for EVs, the fabrication of Li-ion batteries has drastically increased and is predicted to rise even more in coming years. The creation of batteries requires the formulation and manufacturing of an electrolyte and electrodes, the latter being made via the solvent-casting method. For an electrode, this classical process is based on pre-dissolving a polymer into an organic, usually toxic, solvent. The different particles, which are the conductive carbons and the active material are then added in order to make a slurry. This slurry is casted onto a current collector thanks to the doctor-blade technic. A good representation of this technic can be seen in Marks et al.’s publication [2]. The final step to obtain a solid electrode is to evaporate the remaining solvent, usually thanks to heat and vacuum. The increasing production of batteries therefore raises the issue of using more environmentally friendly and cheaper processing methods that do not require the use of solvents.
2. Safety Issues Related to the Use of Liquid Electrolytes
Another concern regarding the lithium batteries are the safety issues related to the use of liquid electrolytes. The organic solvents needed to make these electrolytes are highly flammable and, when used with Li metal anodes, can result in the formation of Li dendrites which can cause the device to short-circuit. To circumvent these constraints, solid electrolytes can be used. Promising solid electrolytes include solid polymer electrolytes (SPE), ceramics [3], and hybrid polymer electrolytes that contain inorganic fillers [4]. The use of flexible polymer-based electrolytes leads to improved surface contact at the interface with the electrode which can be an issue with brittle ceramic systems. Polymer-based electrolytes can also act as separators decreasing the battery’s weight and will mostly allow the use of Li metal as the anode. Device safety also increases because no flammable organic solvent is needed and the mechanical strength of the polymer-based electrolyte is generally sufficient to act as a physical barrier to limit dendrite growth [5]. Polymer electrolytes and hybrid polymer electrolytes are now under intense investigation to find the most efficient component which could replace definitively liquid electrolytes in commercial Li-ion batteries.
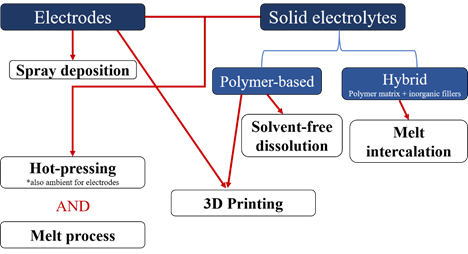
Hence, research on all-solid-state batteries is very active due to the high potential of these devices to be the batteries of tomorrow. Although the race has been centered on creating new high-performance polymer-based electrolytes or electrodes, the other concern in the community is the processes that are used to make these components. Though the solvent-casting method is the most common process for making porous and non-porous electrodes as well as polymer-based electrolytes [6][7][8] [6,7,8], several groups have been working on new processes to replace the use of solvents. The solvent-casting technique raises the potential issues of a time consuming drying step [7] and the presence of residual solvent [9][10][11][9,10,11] which can be detrimental to battery performance and safety (presence of water with Li metal or coordination of water at the surface of ceramics that are in the hybrid electrolyte [12]). Moreover, this process is limited to making thick electrodes and the long drying time can lead to the agglomeration/sedimentation of particles resulting in non-homogenous samples, decreasing the performance of the electrolyte or the electrode [13]. Also, solvent evaporation leaves pores which are unnecessary in solid electrolytes and electrodes used in all-solid-state batteries [14]. Solvent-free preparation of polymer-based electrolytes and electrodes has emerged as a response to these limitations. (Figure 1)
This review reports on different solvent-free processes that have been developed to make polymer-based electrolytes and electrodes (non-porous and porous) for Li-ion and Li-metal batteries. The different processes mentioned in this review are summarized in Figure 1. The first part of this review focuses on processes that are used to make solid polymer electrolytes (polymer and salt) and hybrid polymer electrolytes (polymer, salt and ionically conductive or non-conductive fillers), both being promising alternatives for replacing classical liquid organic electrolytes. The second part of this review emphasizes electrode manufacturing with an overview of processes that can be used at high particle loadings. Both porous and non-porous electrodes will be discussed in this part.
Figure 1. Flow chart summarizing the different solvent-free processes recently developed to make electrodes and/or solid electrolytes for lithium-ion batteries.
Flow chart summarizing the different solvent-free processes recently developed to make electrodes and/or solid electrolytes for lithium-ion batteries.
The entry is from 10.3390/polym13030323.