Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sebastian Yu | -- | 2031 | 2024-02-06 16:35:38 | | | |
| 2 | Jessie Wu | + 1 word(s) | 2032 | 2024-02-07 01:42:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chu, Y.; Yu, S. Therapeutic Agents Targeting Immune Mediators in Hidradenitis Suppurativa. Encyclopedia. Available online: https://encyclopedia.pub/entry/54807 (accessed on 07 February 2026).
Chu Y, Yu S. Therapeutic Agents Targeting Immune Mediators in Hidradenitis Suppurativa. Encyclopedia. Available at: https://encyclopedia.pub/entry/54807. Accessed February 07, 2026.
Chu, Yi-Lun, Sebastian Yu. "Therapeutic Agents Targeting Immune Mediators in Hidradenitis Suppurativa" Encyclopedia, https://encyclopedia.pub/entry/54807 (accessed February 07, 2026).
Chu, Y., & Yu, S. (2024, February 06). Therapeutic Agents Targeting Immune Mediators in Hidradenitis Suppurativa. In Encyclopedia. https://encyclopedia.pub/entry/54807
Chu, Yi-Lun and Sebastian Yu. "Therapeutic Agents Targeting Immune Mediators in Hidradenitis Suppurativa." Encyclopedia. Web. 06 February, 2024.
Copy Citation
Hidradenitis suppurativa (HS), also known as acne inversa (AI), is a chronic, recurrent, debilitating skin disease thought to involve occlusion of the hair follicle at the pilosebaceous unit (PSU) such as axillary, inguinal, and anogenital regions. Clinically, HS manifests as painful inflammatory nodules, abscesses, and interconnected tunnels emitting malodorous discharge and results in disfiguring scarring that has a considerable impact on a patient’s quality of life.
hidradenitis suppurativa
inflammatory skin disease
NCSTN
γ-secretase
TNF-α
IL-17
IL-1β
IL-12
IL-23
1. Anti-TNF-α Therapy
Currently, there are some TNF-α inhibitors for hidradenitis suppurativa.
In 2015, the FDA approved the first biotechnology drug for HS: adalimumab. It is a fully human recombinant anti-TNF-α immunoglobulin (Ig) G1 monoclonal antibody that binds and neutralizes sTNF-α and tmTNF-α to inhibit the inflammatory cascade leading to HS skin lesions [1][2]. The administration of adalimumab involves an initial subcutaneous dose of 160 mg, followed by an 80 mg dose after 2 weeks, and subsequently, a maintenance dose of 40 mg weekly [3]. Although substantial evidence supports its efficacy and safety [4], patients who are younger than four years old and weigh less than 15 kg are not advised to use it due to there being no adequate study about its safety for this group.
Infliximab is a chimeric IgG monoclonal antibody protein derived from recombinant DNA, incorporating components from both murine and human sources. Despite its off-label use to treat HS, it targets TNF-α and also has presumed efficacy [5]. For patients who received an initial dose of 7.5 mg/kg followed by a maintenance frequency every 4 weeks, 47.6% at week 4 and 70.8% at week 12 achieved a clinical response. If those patients have an incomplete initial response, the dosage escalates to 10 mg/kg, which can achieve a clinical response of 37.5% at week 4 and 50% at week 12 [6].
Etanercept, an FDA-approved anti-inflammatory agent for several autoimmune diseases, is a fusion protein consisting of the p75-Fc region of the human TNF-α receptor. It functions by competitively attaching to membrane-bound TNF-α receptors [7]. Although some small case series and an open-label phase II trial illustrated the efficacy of etanercept in early improvement of disease activity and local pain when given 50 mg once a week subcutaneously [8][9][10], it failed to show any benefit in other clinical trials [11]. So far, there is no adequate evidence to support the benefit and optimal dosage of etanercept in HS.
Certolizumab is a recombinant humanized IgG4 that contains only polyethylene glycol antigen-binding fragments. Without a fragment-crystallizable region, this agent cannot pass through the placenta [7], indicating that usage on pregnant individuals is relatively acceptable. Six case reports demonstrated the effectiveness of certolizumab in treating HS patients in which certolizumab was given 200 or 400 mg every other week [12]. However, it is not yet sanctioned for treating HS. The effectiveness and safety of certolizumab for HS treatment remain inadequately established, warranting further exploration through larger-scale trials.
Regarding golimumab, another monoclonal anti-TNF-α antibody that binds both forms of TNF-α, it has been under study for its efficacy and safety in treating patients with HS. One case report demonstrated that a patient received a subcutaneous dose of 200 mg, followed by 100 mg subcutaneously every 4 weeks, combined with antibiotic therapy, resulting in the disappearance of HS lesions with no recurrence [13]. In another case, a dosage of 50 mg subcutaneously once a month for 8 months showed no benefits in improving the disease [14]. This suggests that more research is needed to confirm the effectiveness of this drug in HS treatment, and a higher dosage might be necessary for the treatment of HS.
2. Anti-IL-17 Therapy
Secukinumab is a fully human monoclonal antibody designed to specifically target IL-17A. It functions by inhibiting the binding of IL-17A with IL-17R, consequently impeding keratinocyte hyperproliferation and T-cell infiltration and thus attenuating the immune response. Though currently used off-label for HS [1], two phase 3 trials, SUNRISE and SUNSHINE, showed its efficacy in patients with moderate-to-severe HS. It is effective at rapidly improving clinical presentation with a favorable safety profile when given as a subcutaneous dosage 300 mg every 2 weeks [15].
Instead of targeting cytokines, brodalumab is a fully human monoclonal IgG2 antibody that inhibits the function of IL-17 by directly binding to its receptor, IL-17RA [16]. By acting as an antagonist, it inhibits interactions with cytokines and is thus explored as an off-label therapy for HS. An open-label cohort study administered brodalumab to ten patients with a dose of 210 mg/1.5 mL subcutaneously at weeks 0, 1, and 2 and every 2 weeks until week 24. All of the ten patients achieved hidradenitis suppurativa clinical response (HiSCR) with no serious adverse effects [17].
Unlike secukinumab, which targets IL-17A only, bimekizumab, an IgG1 monoclonal antibody, is created to inhibit both IL-17A and IL-17F driving inflammatory processes [18]. The data of BE HEARD I and BE HEARD II phase 3 studies were presented recently by UCB, a biopharmaceutical company, revealing that bimekizumab achieved substantial and clinically significant responses in patients with HS. Over 55% of patients achieved HiSCR50 by week 16. Until week 48, over 60% of patients attained HiSCR75, and around 30% reached HiSCR100.
Ixekizumab has emerged as a prominent subject in recent studies exploring its efficacy in treating HS. As a monoclonal antibody belonging to the IgG4 class, it has been humanized to target and neutralize both soluble IL-17A and IL-17 A/F [19]. Presently, several case reports highlight its effectiveness. Patients in these studies received a dosage of 60 mg initially, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12. In one of the reports, 80% of patients achieved HiSCR at week 12 [20]. Additional case reports also demonstrated a substantial improvement in the disease, suggesting that ixekizumab holds promise as a potential therapeutic agent for HS. However, further research is imperative to validate these findings.
Also, two novel nanobody drugs, sonelokimab and izokibep, are in phase II clinical trials to evaluate their efficacy in patients with moderate to severe HS [21].
3. Anti-IL-1 Therapy
Anakinra, a recombinant human IL-1Ra, acts as a receptor antagonist by competitively suppressing the binding of both IL-1α and IL-1β to their receptors [19]. It is primarily employed in the treatment of rheumatoid arthritis. Two studies have investigated its impact on HS patients. In an open-label study, six patients underwent anakinra therapy, resulting in a significant mean decrease in their modified Sartorius score at week 8 among five patients [22]. Another double-blind, randomized clinical trial involved 20 patients divided into two groups: one was prescribed a subcutaneous 100 mg dose of anakinra, and the other received a placebo once daily. Following a 12-week follow-up, 78% of patients receiving anakinra showed a reduction in disease activity scores compared to 20% in the placebo group. Additionally, there was a decrease in the production of interferon-γ and an increase in the production of interleukin 22 [23]. These findings indicate that anakinra holds promise as a therapeutic agent beneficial to HS patients.
Canakinumab, a human monoclonal antibody specified for the blockage of IL-1β signal, has obtained approval from the US FDA for treating familial cold auto-inflammatory syndrome and Muckle–Wells syndrome. Numerous clinical studies have established its potential therapeutic benefits in conditions such as rheumatoid arthritis, systemic-onset juvenile idiopathic arthritis, and gout arthritis [24]. However, as of now, the evidence supporting its effectiveness in HS patients is limited to case reports and series, where it was administered as a 150 mg subcutaneously dose once a week. Further research is required to thoroughly investigate and confirm its efficacy in the context of HS treatment.
As IL-1α and IL-1β share similar physiological functions, biopharmaceuticals targeting IL-1α have been off-labeled and used for HS. A phase II open-label study of bermekimab indicated its safety and efficacy in patients with moderate-to-severe HS. Bermekimab is a true human monoclonal antibody that has a high affinity with IL-1α. It was administered subcutaneously at a dose of 400 mg per week for 13 weeks to two groups of patients with naïve or failed anti-TNF therapy previously. After 12 weeks of treatment, 61% and 63% of the two groups, respectively, achieved HiSCR. Significant reductions in abscesses and inflammatory nodules, as well as in patients experiencing pain, were seen in both groups, with the only adverse effect being injection site reactions [25].
4. Anti-IL-12/23 Therapy and Anti-IL-23 Therapy
Ustekinumab is a human monoclonal IgG1 antibody employed for the management and treatment of various inflammatory conditions. This antagonist blocks the p40 subunit of IL-12 and IL-23, thus suppressing the interaction of these cytokines with the IL-12Rβ1 receptor on the surface of natural killer cells and T cells. In doing so, ustekinumab can result in downregulation of the immune system [26]. A case series enrolled ten patients to evaluate the therapeutic outcomes of ustekinumab. Improvements in the Physician Global Assessment Score and Numerical Pain Rating Scale were observed in 70% and 80% of patients, respectively, with no severe adverse effects reported [27].
Guselkumab is a human IgG1λ monoclonal antibody that selectively targets the p19 subunit of IL-23, thus blocking the IL-23-mediated signaling pathway [28]. A phase IIa trial was carried out for patients with moderate-to-severe HS [29]. Twenty patients were subcutaneously administered guselkumab 200 mg every 4 weeks for 16 weeks. A total of 65% of patients achieved HiSCR at the end of the trial, with a statistically significant decrease in the median international hidradenitis suppurativa severity score system (IHS4) score. However, a lower HiSCR response of 45–50.8% was reported in another phase IIb NOVA trial, indicating that guselkumab seems only to be beneficial to a minor group of HS patients [29].
Risankizumab, another humanized IgG1 monoclonal antibody that impedes immune signaling by targeting the p19 subunit of IL-23 [30], was investigated for its efficacy and safety in patients with moderate-to-severe HS. A phase II trial allocated patients randomly to receive risankizumab 180 mg, risankizumab 360 mg, or placebo subcutaneously. HiSCR was achieved by 46.8%, 43.4%, and 41.5% at week 16, respectively, indicating that risankizumab does not appear to be an effective treatment for moderate-to-severe HS [31].
5. Janus Kinase Inhibitors
As the JAK pathway plays a crucial role in the pathophysiology of HS, the blockade of this pathway is considered to offer a novel treatment option for HS. INCB054707 is an example that has undergone two clinical trials to assess its efficacy and safety in the treatment of HS. In these trials, 10, 9, 9, 8, and 9 patients who received INCB054707 at doses of 15, 30, 60, 90 mg, or placebo, respectively, once daily, achieved HiSCR at rates of 43%, 56%, 56%, 88%, and 57% at week 8. Although improvement in life quality, IHS4, and skin pain was observed, several patients experienced treatment-emergent side effects, including upper respiratory tract infection and thrombocytopenia [32].
Tofacitinib and upadacitinib are two additional JAK inhibitors, and a case report highlights the potential benefits of tofacitinib in HS. In this research, two patients received a dosage of 5 mg twice daily in conjunction with other medications, such as antibiotics and immunosuppressants [33]. Moreover, an ongoing phase II trial, including 68 patients, is currently assessing the efficacy of upadacitinib in treating individuals with moderate to severe HS. Prescribed doses include upadacitinib 30 mg orally or a placebo, followed by upadacitinib 15 mg. The primary outcome is the percentage of participants achieving HiSCR at Week 12.
Furthermore, various drugs are currently undergoing clinical trials for HS treatment, including PF 06650833, PF 06700841, PF 06826647, PF-06650833, brepocitinib, ropsacitinib, and KT-474 [19].
The current and potential therapeutic agents and their targeting immune mediators are summarized in Figure 1.
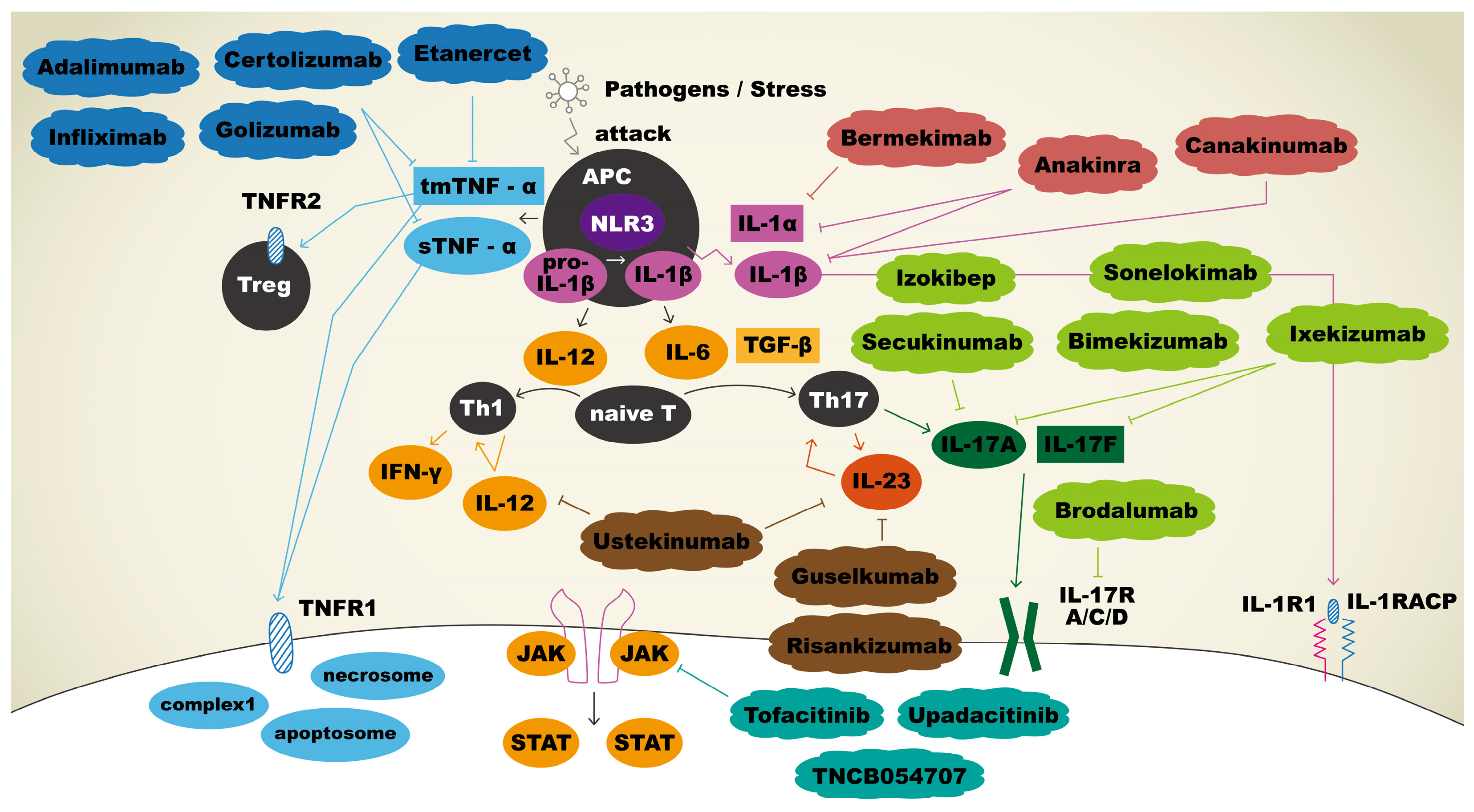
Figure 1. Immunopathogenesis and therapeutic agents targeting immune mediators in hidradenitis suppurativa. Upon APCs sensing pathogens, tmTNF-α and sTNF-α were secreted, and both bound to TNFR1 to facilitate apoptosis, necroptosis, and inflammation reactions. tmTNF-α will also bind to TNFR2 to play a role in regulation of cell activation, migration, and proliferation. Adalimumab, certolizumab, golimumab, and infliximab bind and neutralize sTNF-α and tmTNF-α, thus inhibiting the inflammatory cascade. IL-1β is initially produced as a precursor and then cleaved by NLR3 to become a mature form. Mature IL-1β binds IL-1R1 with co-receptor IL-1RAcP to promote keratinocyte proliferation. Anakinra inhibits both IL-1α and IL-1β, whereas sonelokimab targets IL-1β, and bermekimab targets IL-1α specifically to use off-label for HS. APCs release IL-12 for the differentiation of naïve T cells into Th1 and IL-6 for the differentiation of naïve T cells into Th17 cells. Th17 cells produce IL-23, which promotes the production of IL-17. IL-17 binds to its receptor, contributing to the proliferation of keratinocytes. Sekukinumab, bimekizumab, izokibep, sonelokimab, ixekizumab, and brodalumab are biologic agents that block IL-17 pathway and thus have been explored as off-label treatments for HS. Ustekinumab is an antagonist that blocks both IL-12 and IL-23 to result in the downregulation of immune system. Guselkumab and risankizumab bind selectively to IL-23 only. Three biologics act as JAK inhibitors: tofacitinib, upadacitinib, and TNCB054707.
References
- Martora, F.; Megna, M.; Battista, T.; Potestio, L.; Annunziata, M.C.; Marasca, C.; Villani, A.; Fabbrocini, G. Adalimumab, Ustekinumab, and Secukinumab in the Management of Hidradenitis Suppurativa: A Review of the Real-Life Experience. Clin. Cosmet. Investig. Dermatol. 2023, 16, 135–148.
- Reddy, S.P.; Lin, E.J.; Shah, V.V.; Wu, J.J. Chapter 10—Adalimumab. In Therapy for Severe Psoriasis; Wu, J.J., Feldman, S.R., Lebwohl, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 111–126.
- Huang, Q.; Yang, J.; Lin, Y.; Walker, C.; Cheng, J.; Liu, Z.G.; Su, B. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat. Immunol. 2004, 5, 98–103.
- Kobayashi, S.; Kashiwagi, T.; Kimura, J. Real-world effectiveness and safety of adalimumab for treatment of ankylosing spondylitis in Japan. Mod. Rheumatol. 2019, 29, 1007–1012.
- Efficacy of high dose infliximab in hidradenitis suppurativa. J. Am. Acad. Dermatol. 2019, 81, AB54.
- Ghias, M.H.; Johnston, A.D.; Kutner, A.J.; Micheletti, R.G.; Hosgood, H.D.; Cohen, S.R. High-dose, high-frequency infliximab: A novel treatment paradigm for hidradenitis suppurativa. J. Am. Acad. Dermatol. 2020, 82, 1094–1101.
- Savage, K.T.; Flood, K.S.; Porter, M.L.; Kimball, A.B. TNF-α inhibitors in the treatment of hidradenitis suppurativa. Ther. Adv. Chronic Dis. 2019, 10, 2040622319851640.
- Giamarellos-Bourboulis, E.J.; Pelekanou, E.; Antonopoulou, A.; Petropoulou, H.; Baziaka, F.; Karagianni, V.; Stavrianeas, N.; Giamarellou, H. An open-label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br. J. Dermatol. 2008, 158, 567–572.
- Cusack, C.; Buckley, C. Etanercept: Effective in the management of hidradenitis suppurativa. Br. J. Dermatol. 2006, 154, 726–729.
- Sotiriou, E.; Apalla, Z.; Ioannidos, D. Etanercept for the treatment of hidradenitis suppurativa. Acta Derm. Venereol. 2009, 89, 82–83.
- Lee, R.A.; Dommasch, E.; Treat, J.; Sciacca-Kirby, J.; Chachkin, S.; Williams, J.; Shin, D.B.; Leyden, J.J.; Vittorio, C.; Gelfand, J.M. A prospective clinical trial of open-label etanercept for the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2009, 60, 565–573.
- Shadid, A.; Alobaida, S.; Binamer, Y. Certolizumab to treat hidradenitis suppurativa. Dermatol. Rep. 2023, 15, 9566.
- Tursi, A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig. Liver Dis. 2016, 48, 1511–1512.
- van der Zee, H.H.; Prens, E.P. Failure of anti-interleukin-1 therapy in severe hidradenitis suppurativa: A case report. Dermatology 2013, 226, 97–100.
- Kimball, A.B.; Jemec, G.B.E.; Alavi, A.; Reguiai, Z.; Gottlieb, A.B.; Bechara, F.G.; Paul, C.; Giamarellos Bourboulis, E.J.; Villani, A.P.; Schwinn, A.; et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): Week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet 2023, 401, 747–761.
- Chima, M.A.; Lebwohl, M.G. 28—Interleukin 17 Inhibitors. In Comprehensive Dermatologic Drug Therapy, 4th ed.; Wolverton, S.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 312–320.e2.
- Frew, J.W.; Navrazhina, K.; Grand, D.; Sullivan-Whalen, M.; Gilleaudeau, P.; Garcet, S.; Ungar, J.; Krueger, J.G. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: An open-label cohort study. J. Am. Acad. Dermatol. 2020, 83, 1341–1348.
- Merola, J.F.; Landewé, R.; McInnes, I.B.; Mease, P.J.; Ritchlin, C.T.; Tanaka, Y.; Asahina, A.; Behrens, F.; Gladman, D.D.; Gossec, L.; et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: A randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023, 401, 38–48.
- Świerczewska, Z.; Lewandowski, M.; Surowiecka, A.; Barańska-Rybak, W. Immunomodulatory Drugs in the Treatment of Hidradenitis Suppurativa-Possibilities and Limitations. Int. J. Mol. Sci. 2022, 23, 9716.
- Esme, P.; Botsali, A.; Akoglu, G.; Caliskan, E. An Anti-Interleukin-17A Monoclonal Antibody, Ixekizumab, in the Treatment of Resistant Hidradenitis Suppurativa: A Case Series. Skin. Appendage Disord. 2022, 8, 342–345.
- Ocker, L.; Abu Rached, N.; Seifert, C.; Scheel, C.; Bechara, F.G. Current Medical and Surgical Treatment of Hidradenitis Suppurativa—A Comprehensive Review. J. Clin. Med. 2022, 11, 7240.
- Leslie, K.S.; Tripathi, S.V.; Nguyen, T.V.; Pauli, M.; Rosenblum, M.D. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J. Am. Acad. Dermatol. 2014, 70, 243–251.
- Tzanetakou, V.; Kanni, T.; Giatrakou, S.; Katoulis, A.; Papadavid, E.; Netea, M.G.; Dinarello, C.A.; van der Meer, J.W.M.; Rigopoulos, D.; Giamarellos-Bourboulis, E.J. Safety and Efficacy of Anakinra in Severe Hidradenitis Suppurativa: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 52–59.
- Dhimolea, E. Canakinumab. MAbs 2010, 2, 3–13.
- Gottlieb, A.; Natsis, N.E.; Kerdel, F.; Forman, S.; Gonzalez, E.; Jimenez, G.; Hernandez, L.; Kaffenberger, J.; Guido, G.; Lucas, K.; et al. A Phase II Open-Label Study of Bermekimab in Patients with Hidradenitis Suppurativa Shows Resolution of Inflammatory Lesions and Pain. J. Investig. Dermatol. 2020, 140, 1538–1545.E2.
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and mechanism of ustekinumab: A human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs 2011, 3, 535–545.
- Montero-Vilchez, T.; Pozo-Román, T.; Sánchez-Velicia, L.; Vega-Gutiérrez, J.; Arias-Santiago, S.; Molina-Leyva, A. Ustekinumab in the treatment of patients with hidradenitis suppurativa: Multicenter case series and systematic review. J. Dermatol. Treat. 2022, 33, 348–353.
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Angileri, R.G.; Bardazzi, F.; Bernardini, N.; Burlando, M.; Carrera, C.G.; Chiricozzi, A.; Dapavo, P.; et al. Real-life effectiveness and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: A 104-week multicenter retrospective study—IL PSO (ITALIAN LANDSCAPE PSORIASIS). J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1017–1027.
- Dudink, K.; Bouwman, K.; Chen, Y.; DePrimo, S.E.; Munoz-Elias, E.J.; Aarts, P.; Schappin, R.; Florencia, E.F.; van Heeswijk, B.; Prens, L.M.; et al. Guselkumab for hidradenitis suppurativa: A phase II, open-label, mode-of-action study. Br. J. Dermatol. 2023, 188, 601–609.
- Baeten, D.; Østergaard, M.; Wei, J.C.-C.; Sieper, J.; Järvinen, P.; Tam, L.-S.; Salvarani, C.; Kim, T.-H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 2018, 77, 1295–1302.
- Kimball, A.B.; Prens, E.P.; Passeron, T.; Maverakis, E.; Turchin, I.; Beeck, S.; Drogaris, L.; Geng, Z.; Zhan, T.; Messina, I.; et al. Efficacy and Safety of Risankizumab for the Treatment of Hidradenitis Suppurativa: A Phase 2, Randomized, Placebo-Controlled Trial. Dermatol. Ther. 2023, 13, 1099–1111.
- Alavi, A.; Hamzavi, I.; Brown, K.; Santos, L.L.; Zhu, Z.; Liu, H.; Howell, M.D.; Kirby, J.S. Janus kinase 1 inhibitor INCB054707 for patients with moderate-to-severe hidradenitis suppurativa: Results from two phase II studies. Br. J. Dermatol. 2022, 186, 803–813.
- Savage, K.T.; Santillan, M.R.; Flood, K.S.; Charrow, A.; Porter, M.L.; Kimball, A.B. Tofacitinib shows benefit in conjunction with other therapies in recalcitrant hidradenitis suppurativa patients. JAAD Case Rep. 2020, 6, 99–102.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
411
Revisions:
2 times
(View History)
Update Date:
07 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




