
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peter Taylor | + 3372 word(s) | 3372 | 2021-02-08 10:51:25 | | | |
| 2 | Vivi Li | Meta information modification | 3372 | 2021-04-25 03:45:43 | | | | |
| 3 | Rita Xu | -36 word(s) | 3336 | 2024-12-17 09:30:07 | | |
Video Upload Options
Comorbidities in patients with rheumatoid arthritis (RA) are often associated with poor health outcomes and increased mortality. Treatment decisions should consider these comorbidities due to known or suspected associations with certain drug classes. In clinical practice, it is critical to balance potential treatment benefit against the possible risks for comorbidities as well as the articular manifestations of RA. This entry summarises the current literature relating to prevalence and risk factors for the important comorbidities of cardiovascular disease, infections, lymphomas and nonmelanoma skin cancers in patients with RA. The impact on patient outcomes and the interplay between these comorbidities and the therapeutic options currently available, including tumour necrosis factor inhibitors and newer biological therapies, are also explored. As newer RA therapies are developed, and patients gain wider and earlier access to advanced therapies, in part due to the emergence of biosimilars, it is important to consider the prevention or treatment of comorbidities as part of the overall management of RA.
1. Introduction
The relationship between rheumatoid arthritis (RA) and a wide range of comorbid conditions and extra-articular manifestations is well known [1][2][3]. The development of comorbidities is associated with poor health outcomes, including decreased function, reduced quality of life, and increased morbidity and mortality [1][2][4]. The identification of tumour necrosis factor (TNF) as a therapeutic target and its subsequent validation in clinical trials led to the approval of biologic TNF inhibitors for the treatment of RA over two decades ago, greatly improving the outlook for many patients [5][6][7]. The success of this therapeutic class was followed by the introduction of other biologic disease-modifying antirheumatic drugs (bDMARDs) and, more recently, small molecule targeted synthetic disease-modifying antirheumatic drugs [7]. However, high costs of originator drugs have resulted in varying degrees of access restrictions across national health care economies [7][8]. Furthermore, although all targeted therapies have a broadly similar efficacy, they differ in mechanisms of action and associated target-related benefits and toxicities [9]. In the face of a wide range of available contemporary treatment options and management strategies for RA [10][11], careful consideration needs to be given to certain comorbidities with respect to treatment decisions [10][11][12]. The large international population-based, cross-sectional COMOrbidities in Rheumatoid Arthritis (COMORA) study evaluated the prevalence of comorbidities in 3920 patients with RA from 17 countries [1]. The most commonly observed comorbidities (past or current) were depression (15%), asthma (7%), cardiovascular (CV) events (myocardial infarction (MI), stroke; 6%), solid-organ malignancies (5%) and chronic obstructive pulmonary disease (4%) [1]. Results from the COMORA study demonstrated considerable intercountry variability for the prevalence of these comorbidities; for example, the prevalence of depression was 2% in Morocco compared with 33% in the USA [1]. For patients with RA, CV disease (CVD), infections and malignancies are important comorbidities as they may lead to an increased risk of mortality [1]. Furthermore, they are also impacted by at least one of the therapeutic options currently available for the treatment of RA [13][14][15] and may therefore affect treatment decisions in clinical practice. Notably, patients with congestive heart failure, active hepatitis B or a history of other serious infections or malignancy are classified as high risk in the current American College of Rheumatology treatment guidelines for RA, and they have separate treatment recommendations [10].
2. CV Comorbidities in RA
2.1. Prevalence of CVD in Patients with RA
CVD is a major comorbidity affecting patients with RA and a leading cause of morbidity and mortality in this population [16]. Multiple recent studies have highlighted the extent to which patients with RA are affected by CVD, although comparisons between studies are often hindered by differences in the definition of events. Patients with RA have been shown to be at higher risk of CVD compared with healthy controls. Using a large database of primary care patients across England, patients with early RA had a 33% higher CVD risk (composite endpoint of MI, stroke or heart failure) than matched controls without RA after adjustment for baseline differences including inflammatory markers, seropositivity and use of glucocorticoids (GCs) at diagnosis [17]. Similarly, in the CARdiovascular research and RhEumatoid arthritis (CARRÉ) long-term, prospective cohort study, patients with RA had a risk of CV events (coronary heart disease, cerebral arterial disease or peripheral arterial disease) that was almost double that of the general population. In the same study, the increased risk of CVD associated with RA was even higher than that associated with diabetes mellitus [18]. An increased risk of heart failure in patients with RA has also been highlighted recently in a systematic review that included over 5 million patients; in this study, the incidence of heart failure was almost two-fold higher in patients with RA than in matched controls [19]. It is important to note that the increased risk of CVD associated with RA may already be present at an early stage of the disease, with an excess of stroke and heart failure reported prior to RA diagnosis compared with matched controls in England [17].
Imaging studies have also demonstrated the increased risk of CV in patients with RA. A study of patients referred for computed tomography (CT) angiography due to chest pain reported a higher prevalence of coronary artery calcification in RA patients than in controls [20]. The strongest associations were reported in seropositive RA patients and in patients requiring treatment with GCs for relapse or flare; treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), but not bDMARDs, was also associated with a tendency for increased risk of obstructive coronary artery disease (CAD). Consistent with these findings, a recent meta-analysis of CT studies reported that asymptomatic CAD is more prevalent in patients with RA than in controls [21]. Although the evidence was limited, it was also noted that patients with RA had a higher prevalence of moderate-to-severe CAD and more multivessel CAD [21]. CV abnormalities in patients without overt cardiac disease have also been detected by echocardiography in patients with RA. In a prospective study in Italy, a five-fold increased risk of abnormal changes to the structure and function of the left ventricle was observed in patients with RA compared with matched controls [22]. In this study, left ventricular strain was independently associated with a 2.4-fold increased risk of all-cause hospitalisation and a 6.6-fold increased risk of CV-related hospitalisation.
2.2. Impact of CV Comorbidities in Patients with RA
Patients with comorbid CVD and RA tend to have worse long-term health outcomes than patients with CVD alone. In a large population cohort study in Denmark of patients undergoing coronary angiography, the 10-year risk of MI was higher in patients with RA and coincident CAD compared with non-RA patients with CAD (12.2% vs. 9.9%). Similar associations were observed for major adverse CV events (MACE) and all-cause mortality [23]. Worse outcomes were also noted in a recent meta-analysis, which demonstrated that patients with co-existing CAD and RA had significantly increased risks of all-cause mortality, cardiac death and congestive heart failure compared with CAD patients without RA. In patients who underwent percutaneous coronary intervention, all-cause mortality rates remained significantly higher in patients with RA and CAD than in non-RA patients with CAD [24]. Similarly, in a large cohort study of patients who experienced an acute coronary syndrome (ACS) event, patients with RA had a 27% and 50% increased risk of ACS recurrence or mortality, respectively, after a mean 2-year follow-up compared with controls, which remained statistically significant even after adjustment for baseline comorbidities. This increased risk could not be explained by differences in the use of standard-of-care secondary preventative drugs [25].
The presence of RA can also have a negative impact on short-term outcomes following CV events. In a recent database study in Taiwan, patients with RA had a higher risk of in-hospital mortality after acute MI, intracranial haemorrhage or ischaemic stroke compared with patients without RA [26]. Similarly, a large Swedish study demonstrated that mortality rates within 1 week and 1 month following an ACS event were significantly higher in patients with RA compared with a matched cohort from the general population, even after adjustment for age, sex, pre-existing comorbidities and pharmacotherapies, and ACS type. Patients with RA also had higher troponin levels, and higher frequencies of in-hospital complications and ST-segment elevation MIs compared with the control cohort [27]. In contrast, a small study in Israel found that the outcome and prognosis of patients with ACS were not affected by the coexistence of inflammatory rheumatic diseases. The authors of this study argued that excess mortality in patients with rheumatic diseases reflects the higher prevalence of CVD in this population rather than worse outcomes for CV events [28]. However, it should be noted that this study included only 20 patients with ACS and inflammatory rheumatic conditions, of whom only 11 had RA.
2.3. Risk Factors for CVD in Patients with RA
The increased CVD risk observed in patients with RA is likely to be multifactorial, reflecting an increased prevalence of traditional CVD risk factors, the impact of systemic inflammation and potential side effects from medications used to treat RA.
The burden of traditional risk factors for CVD among patients with RA has been extensively documented, with multiple large studies reporting a high prevalence of current tobacco use (ranging from 19 to 29%), hypertension (19 to 61%), diabetes mellitus (5 to 14%) and hyperlipidaemia (10 to 32%) [1][3][17][18][29][30]. In addition, many patients were reported to be overweight or obese (mean body mass index (BMI) ranged from 27 to 29 kg/m2) [17][18][29][31]. In the COMORA study, around half of the enrolled patients were overweight or obese (51%), and almost half (43%) were considered to have a high 10-year risk of CVD based on the Framingham score [1]. Compared with the general population, the prevalence of traditional CVD risk factors is often higher amongst patients with RA. For example, in a large study of primary care patients in England, patients with RA had a higher BMI and were significantly more likely to have diabetes and to be current or former smokers than age- and gender-matched controls without RA [17]. A similar pattern was reported in the CARRÉ study, with patients with RA having a significantly higher prevalence of hypertension and tobacco use than a control population [18]. Interestingly, in both studies, total and low-density lipoprotein (LDL) cholesterol levels were lower in patients with RA than in controls [17][18].
To date, numerous studies have shown that traditional risk factors contribute to the increased CVD risk faced by patients with RA. In a large, international cohort study, traditional risk factors were responsible for 49% of the CV risk in RA patients, with current smoking status and hypertension being major modifiable risk factors [29]. Similar conclusions were drawn by Nikiphorou et al., who reported that current smoking, BMI and diabetes were associated with a higher rate of CVD among patients with RA [17]. At a subclinical level, traditional risk factors (age, mean arterial pressure and diabetes) have also been shown to be independently associated with worsening atherosclerosis in patients with RA who had not experienced previous CV events [32].
The presence of traditional risk factors does not fully account for the increased CVD risk in patients with RA, with many studies reporting that a considerable residual risk remains even after adjustment for traditional risk factors [17][18][29]. Systemic inflammation may, at least in part, explain the remaining risk, and this theory is supported by a multitude of studies demonstrating an association between high RA disease activity and increased CVD risk [29][30][32][33][34][35][36][37]. The relationship between chronic inflammation and CVD remains to be fully delineated, but it has been reported that the proinflammatory mechanisms underlying the pathogenesis of RA may contribute to the development of atherosclerosis [38][39][40], promotion of cardiac remodelling [41], alterations in lipid blood profiles [42] and changes to the morphology of red blood cells [43] (Figure 1). RA-related inflammation can also be exacerbated by cytomegalovirus infection and is linked to coronary artery damage through the actions of a population of cytotoxic T cells (reviewed in detail by Broadley et al. [44]). Autoimmune mechanisms that drive RA progression have also been linked to abnormal fibrin clot formation and increased CVD risk. However, the extent to which haemostasis is affected in RA remains unclear (reviewed in detail by Bezuidenhout et al. [45]).
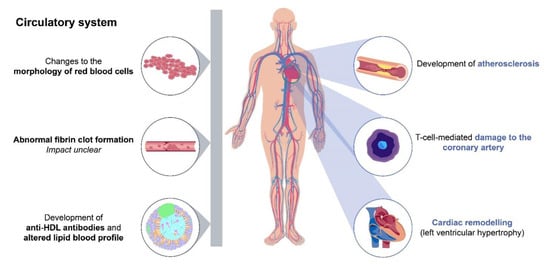
Figure 1. Chronic inflammation in rheumatoid arthritis as a risk factor for cardiovascular disease. The proinflammatory mechanisms underlying RA may contribute to the development of atherosclerosis [38][39][40], promotion of cardiac remodelling [41], alterations in lipid blood profiles [42] and changes to the morphology of red blood cells [43]. RA can be exacerbated by cytomegalovirus infection and is linked to coronary artery damage via cytotoxic T cells [44]. The autoimmune mechanisms that drive RA have been linked to abnormal fibrin clot formation and increased CVD risk; however, the extent to which this is affected in RA remains unclear [45]. Grey bar indicates nonlocalised effects. Abbreviations: CVD, cardiovascular disease; HDL, high-density lipoprotein; RA, rheumatoid arthritis.
Targeted CVD risk management is an important part of the overall clinical management of patients with inflammatory joint disorders, including RA [46]. Guidance based on expert opinion and scientific evidence was issued by the European League Against Rheumatism (EULAR) in 2017 and includes the importance of optimal control of disease activity, CVD risk assessment every 5 years and lifestyle recommendations [46]. CVD risk prediction models should incorporate a multiplication factor of 1.5 for patients with RA (if not already included) and screening for asymptomatic atherosclerotic plaques by carotid ultrasound should be considered; however, this has not yet been assessed in a clinical setting [46]. In terms of treatment, nonsteroidal anti-inflammatory drugs (NSAIDs) should be used with caution in patients with documented CVD or with CVD risk factors, and the dose of GCs should be kept to a minimum for prolonged treatments [46]. The guidelines also emphasised the important role of the rheumatologist in CVD risk management [46]. Fortunately, physicians appear to be aware of the need to monitor CVD risk in patients with active RA: a study of 14,503 patients in world-wide data from the SUrvey of cardiovascular disease Risk Factor management in Rheumatoid Arthritis (SURF-RA) database demonstrated that positivity for rheumatoid factor and anticitrullinated protein antibodies, longer disease duration and higher disease activity (measured by Disease Activity Score 28 joint count-C reactive protein [CRP]) was associated with a higher likelihood of lipid and blood pressure assessments [47].
In order to improve the management of comorbidities in chronic inflammatory rheumatic diseases in daily practice, an initiative supported by EULAR aimed to standardise reporting and screening of comorbidities [12]. For CVD, this included the use of a standardised form for reporting a history of ischaemic CV diseases, risk factors and CVD-related treatments [12].
2.4. Effect of RA Treatments on CV Risk
In addition to traditional CV risk factors and inflammatory processes, CVD risk may also be altered by some of the common medications used for the treatment of RA. For example, corticosteroids and NSAIDs, particularly COX-2 inhibitors, are generally associated with an increase in CVD risk in patients with RA (reviewed by Jagpal et al. [48] and DiMizio et al. [16]). Conversely, nonbiologic DMARDs, such as methotrexate, are associated with an improved CVD risk [49].
As chronic inflammation is considered to be a modifiable risk factor for the development of CVD, targeting systemic inflammation with TNF inhibitors has the potential to reduce CVD risk in patients with RA [50]. This is supported by a meta-analysis from 2015, which demonstrated that TNF inhibitors and methotrexate significantly reduced the risk of CV events compared with no treatment; however, treatment with NSAIDs or corticosteroids led to an increase in risk [51]. Similarly, a large study of patients with RA recruited to the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) demonstrated that treatment with TNF inhibitors significantly reduced the risk of MI compared with csDMARDs, although no differences in MI severity or mortality were observed between treatment groups [52]. The benefits of TNF inhibitors and other biologics were also demonstrated in a prospective cohort study from Australia, which concluded that, after adjustment for a number of risk factors, the risk of CV events was significantly reduced following the use of these agents in patients with RA, psoriatic arthritis or ankylosing spondylitis [53]. Length of treatment may also have an impact on CV risk, with a large retrospective insurance claims database study in the USA reporting a positive correlation between duration of treatment with TNF inhibitors and reduction in CV risk. This study concluded that cumulative use of 1, 2 or 3 years of anti-TNF therapy is expected to reduce CV risk by 21%, 38% and 51%, respectively, compared with non-use [54].
Consistent with the reduced risk of clinical events, treatment with TNF inhibitors may also lead to improvements in CV abnormalities detected by imaging. For example, patients with early RA and no CVD history have been shown to have abnormal vascular stiffness, evidence of diffuse myocardial fibrosis and reduced left ventricular mass compared with controls. Treatment with methotrexate and etanercept (either concomitantly or following a step-up strategy) resulted in significant improvements in vascular stiffness after 1 year [55]. Similarly, patients with RA have been reported to have impaired left ventricle longitudinal strain, which improved after treatment with TNF inhibitors [56].
The benefits of TNF inhibitors on CVD risk correlate with their impact on RA disease control, with data from the Swedish biologics register demonstrating that the 1-year risk of ACS for patients with a good EULAR response was approximately half that of patients with no EULAR response [57]. Similarly, improvements in the apolipoprotein profile, a biomarker of CVD risk, were observed in patients with RA who exhibited a good or moderate EULAR response to etanercept but not in EULAR nonresponders [58]. The relationship between RA disease control and the impact of TNF inhibitors on CV risk suggests that these agents may exert their effects through reducing systemic inflammation rather than by modifying traditional CV risk factors. This is supported by a phase IV, randomised, double-blind, placebo-controlled study, which demonstrated that etanercept did not affect levels of traditional metabolic risk factors (including glucose, insulin, lipid and apolipoprotein parameters) despite reducing RA severity, as indicated by decreases in CRP [59]. Additional support for this theory includes a molecular profiling study, which demonstrated that patients with RA at the greatest risk of CVD expressed high levels of biomolecules, which are known to be mediators of autoimmunity, inflammation and oxidative damage. Importantly, bDMARDs, such as TNF inhibitors and cluster of differentiation 20 (CD20) inhibitors, were shown to re-establish normal levels of these circulating biomolecules and, therefore, reduce CVD risk [60].
The impact of newer classes of therapy on CVD risk is also of considerable interest for the management of patients with RA. Concerns have previously been raised regarding lipid elevations associated with the interleukin-6 inhibitor tocilizumab, including significantly increased levels of total and LDL cholesterol [61][62][63]. However, this does not appear to translate to increased clinical risks, with a number of studies showing no significant differences between TNF inhibitors and tocilizumab in terms of CV risk [64][65][66]. This included the ENTRACTE randomised controlled trial (RCT), which demonstrated no significant differences in the risk of MACE between tocilizumab and etanercept in a large population of patients with RA and at least one CV risk factor [64]. Conversely, there is some limited evidence that tocilizumab may offer CV benefits compared with TNF inhibitors, including improvements in lipoprotein markers [67][68] and a lower risk of MACE [69]. Similarly, a large study utilising data from Taiwan’s National Health Insurance claims database reported that TNF inhibitor nonresponders who received tocilizumab had a lower risk of CV events compared with patients who received rituximab [70]. The disconnect between clinical CVD risk and elevated lipids remains to be fully elucidated but may relate to protective changes to other surrogates of CV risk following treatment with tocilizumab. For example, in the MEASURE and ADACTA trials, tocilizumab treatment altered high-density lipoprotein particles towards an anti-inflammatory composition (reduced serum amyloid A content) and induced a significant reduction in secretory phospholipase A2-IIA, lipoprotein(a), fibrinogen and D-dimers [61][63]. Abatacept, which inhibits the activation of T cells, has also been associated with modest decreases in the risk of CV events compared with TNF inhibitors [71][72] and rituximab [70].
Janus kinase (JAK) inhibitors and, in particular, tofacitinib, have been associated with an increased risk of venous thromboembolisms (VTEs) in postmarketing surveillance studies [73][74]. However, it should be noted that this increased risk was only observed when tofacitinib was used at a dose of 10 mg twice a day, which is higher than the dose approved for RA in most countries [75][76]. Moreover, a recent meta-analysis of 26 RCTs, comprising 11,799 patients, indicated that treatment with JAK inhibitors as a class or as individual therapies did not affect the risk of VTEs, CV events or MACE in patients with RA, at least in the short term [77].
References
- Dougados, M.; Soubrier, M.; Antunez, A.; Balint, P.; Balsa, A.; Buch, M.H.; Casado, G.; Detert, J.; El-Zorkany, B.; Emery, P.; et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: Results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 2014, 73, 62–68.
- Norton, S.; Koduri, G.; Nikiphorou, E.; Dixey, J.; Williams, P.; Young, A. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology 2013, 52, 99–110.
- Radner, H.; Lesperance, T.; Accortt, N.A.; Solomon, D.H. Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res. 2017, 69, 1510–1518.
- Ramos, A.L.; Redeker, I.; Hoffmann, F.; Callhoff, J.; Zink, A.; Albrecht, K. Comorbidities in patients with rheumatoid arthritis and their association with patient-reported outcomes: Results of claims data linked to questionnaire survey. J. Rheumatol. 2019, 46, 564–571.
- FDA. Highlights of Prescribing Information: Enbrel® (Etanercept). Available online: (accessed on 2 November 2020).
- Burmester, G.R.; Landewé, R.; Genovese, M.C.; Friedman, A.W.; Pfeifer, N.D.; Varothai, N.A.; Lacerda, A.P. Adalimumab long-term safety: Infections, vaccination response and pregnancy outcomes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 414–417.
- Massalska, M.; Maslinski, W.; Ciechomska, M. Small molecule inhibitors in the treatment of rheumatoid arthritis and beyond: Latest updates and potential strategy for fighting COVID-19. Cells 2020, 9, 1876.
- Putrik, P.; Ramiro, S.; Kvien, T.K.; Sokka, T.; Pavlova, M.; Uhlig, T.; Boonen, A. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann. Rheum. Dis. 2014, 73, 198–206.
- Benjamin, O.; Bansal, P.; Goyal, A.; Lappin, S.L. Disease Modifying Anti-Rheumatic Drugs (DMARD) [Updated 6 January 2020]; StatPearls Publishing: Treasure Island, FL, USA, 2019.
- Singh, J.A.; Saag, K.G.; Bridges, S.L.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 1–26.
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; Van Vollenhoven, R.F.; De Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, S685–S699.
- Baillet, A.; Gossec, L.; Carmona, L.; De Wit, M.; Van Eijk-Hustings, Y.; Bertheussen, H.; Alison, K.; Toft, M.; Kouloumas, M.; Ferreira, R.J.O.; et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: A EULAR initiative. Ann. Rheum. Dis. 2016, 75, 965–973.
- Murdaca, G.; Negrini, S.; Pellecchio, M.; Greco, M.; Schiavi, C.; Giusti, F.; Puppo, F. Update upon the infection risk in patients receiving TNF alpha inhibitors. Expert Opin. Drug Saf. 2019, 18, 219–229.
- Harigai, M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology 2019, 58, i34–i42.
- Bongartz, T.; Sutton, A.J.; Sweeting, M.J.; Buchan, I.; Matteson, E.L.; Montori, V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. J. Am. Med. Assoc. 2006, 295, 2275–2285.
- DeMizio, D.J.; Geraldino-Pardilla, L.B. Autoimmunity and inflammation link to cardiovascular disease risk in rheumatoid arthritis. Rheumatol. Ther. 2020, 7, 19–33.
- Nikiphorou, E.; de Lusignan, S.; Mallen, C.D.; Khavandi, K.; Bedarida, G.; Buckley, C.D.; Galloway, J.; Raza, K. Cardiovascular risk factors and outcomes in early rheumatoid arthritis: A population-based study. Heart 2020, 106, 1566–1572.
- Agca, R.; Hopman, L.H.G.A.; Laan, K.J.C.; van Halm, V.P.; Peters, M.J.L.; Smulders, Y.M.; Dekker, J.M.; Nijpels, G.; Stehouwer, C.D.A.; Voskuyl, A.E.; et al. Cardiovascular event risk in rheumatoid arthritis compared with type 2 diabetes: A 15-year longitudinal study. J. Rheumatol. 2020, 47, 316–324.
- Khalid, Y.; Dasu, N.; Shah, A.; Brown, K.; Kaell, A.; Levine, A.; Dasu, K.; Raminfard, A. Incidence of congestive heart failure in rheumatoid arthritis: A review of literature and meta-regression analysis. ESC Hear. Fail. 2020, ehf2.12947.
- Tinggaard, A.B.; de Thurah, A.; Andersen, I.T.; Riis, A.H.; Therkildsen, J.; Winther, S.; Hauge, E.M.; Bøttcher, M. Rheumatoid arthritis as a risk factor for coronary artery calcification and obstructive coronary artery disease in patients with chest pain: A registry based cross-sectional study. Clin. Epidemiol. 2020, 12, 679–689.
- Hansen, P.R.; Feineis, M.; Abdulla, J. Rheumatoid arthritis patients have higher prevalence and burden of asymptomatic coronary artery disease assessed by coronary computed tomography: A systematic literature review and meta-analysis. Eur. J. Intern. Med. 2019, 62, 72–79.
- Cioffi, G.; Ognibeni, F.; Dalbeni, A.; Giollo, A.; Orsolini, G.; Gatti, D.; Rossini, M.; Viapiana, O. High prevalence of occult heart disease in normotensive patients with rheumatoid arthritis. Clin. Cardiol. 2018, 41, 736–743.
- Løgstrup, B.B.; Olesen, K.K.W.; Masic, D.; Gyldenkerne, C.; Thrane, P.G.; Ellingsen, T.; Bøtker, H.E.; Maeng, M. Impact of rheumatoid arthritis on major cardiovascular events in patients with and without coronary artery disease. Ann. Rheum. Dis. 2020, 79, 1182–1188.
- Wang, H.; Li, X.; Gong, G. Cardiovascular outcomes in patients with co-existing coronary artery disease and rheumatoid arthritis: A systematic review and meta-analysis. Medicine (Baltimore) 2020, 99, e19658.
- Mantel, Ä.; Holmqvist, M.; Jernberg, T.; Wållberg-Jonsson, S.; Askling, J. Long-term outcomes and secondary prevention after acute coronary events in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 2017–2024.
- Lai, C.H.; Hsieh, C.Y.; Barnado, A.; Huang, L.C.; Chen, S.C.; Tsai, L.M.; Shyr, Y.; Li, C.Y. Outcomes of acute cardiovascular events in rheumatoid arthritis and systemic lupus erythematosus: A population-based study. Rheumatology 2020, 59, 1355–1363.
- Mantel, Ä.; Holmqvist, M.; Jernberg, T.; Wållberg-Jonsson, S.; Askling, J. Rheumatoid arthritis is associated with a more severe presentation of acute coronary syndrome and worse short-term outcome. Eur. Heart J. 2015, 36, 3413–3422.
- Ben-Zvi, I.; Goldenberg, I.; Matetzky, S.; Grossman, C.; Elis, A.; Gavrielov-Yusim, N.; Livneh, A. The impact of inflammatory rheumatic diseases on the presentation, severity, and outcome of acute coronary syndrome. Clin. Rheumatol. 2016, 35, 233–237.
- Crowson, C.S.; Rollefstad, S.; Ikdahl, E.; Kitas, G.D.; van Riel, P.; Gabriel, S.E.; Matteson, E.L.; Kvien, T.K.; Douglas, K.; Sandoo, A.; et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 48–54.
- Solomon, D.; Reed, G.; Kremer, J.; Curtis, J.; Farkouh, M.; Harrold, L.; Hochberg, M.; Tsao, P.; Greenberg, J. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015, 67, 1449–1455.
- Solomon, A.; Stanwix, A.E.; Castañeda, S.; Llorca, J.; Gonzalez-Juanatey, C.; Hodkinson, B.; Romela, B.; Ally, M.; Maharaj, A.B.; Van Duuren, E.M.; et al. Points to consider in cardiovascular disease risk management among patients with rheumatoid arthritis living in South Africa, an unequal middle income country. BMC Rheumatol. 2020, 4, 42.
- Dalbeni, A.; Giollo, A.; Bevilacqua, M.; Cioffi, G.; Tagetti, A.; Cattazzo, F.; Orsolini, G.; Ognibeni, F.; Minuz, P.; Rossini, M.; et al. Traditional cardiovascular risk factors and residual disease activity are associated with atherosclerosis progression in rheumatoid arthritis patients. Hypertens. Res. 2020, 43, 922–928.
- Arts, E.E.A.; Fransen, J.; Den Broeder, A.A.; Van Riel, P.L.C.M.; Popa, C.D. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: A time-dependent Cox regression analysis in a large cohort study. Ann. Rheum. Dis. 2017, 76, 1693–1699.
- Mantel, Ä.; Holmqvist, M.; Nyberg, F.; Tornling, G.; Frisell, T.; Alfredsson, L.; Askling, J. Risk factors for the rapid increase in risk of acute coronary events in patients with new-onset rheumatoid arthritis: A nested case-control study. Arthritis Rheumatol. 2015, 67, 2845–2854.
- Ahlers, M.J.; Lowery, B.D.; Farber-Eger, E.; Wang, T.J.; Bradham, W.; Ormseth, M.J.; Chung, C.P.; Stein, C.M.; Gupta, D.K. Heart failure risk associated with rheumatoid arthritis-related chronic inflammation. J. Am. Heart Assoc. 2020, 9, e014661.
- Bajraktari, I.H.; Rexhepi, S.; Berisha, I.; Lahu, A.; Kryeziu, A.; Durmishi, B.; Bajraktari, H.; Bahtiri, E. Prevalence of asymptomatic arterial hypertension and its correlation with inflammatory activity in early rheumatoid arthritis. Open Access Maced. J. Med. Sci. 2017, 5, 641–644.
- Berendsen, M.L.T.; van Maaren, M.C.; Arts, E.E.A.; den Broeder, A.A.; Popa, C.D.; Fransen, J. Anticyclic citrullinated peptide antibodies and rheumatoid factor as risk factors for 10-year cardiovascular morbidity in patients with rheumatoid arthritis: A large inception cohort study. J. Rheumatol. 2017, 44, 1325–1330.
- Wahlin, B.; Meedt, T.; Jonsson, F.; Henein, M.Y.; Wållberg-Jonsson, S. Coronary artery calcification is related to inflammation in rheumatoid arthritis: A long-term follow-up study. Biomed Res. Int. 2016, 2016, 1261582.
- Chan, Y.H.; Ngai, M.C.; Chen, Y.; Wu, M.Z.; Yu, Y.J.; Zhen, Z.; Lai, K.; Cheung, T.; Ho, L.M.; Chung, H.Y.; et al. Cumulative rheumatic inflammation modulates the bone-vascular axis and risk of coronary calcification. J. Am. Heart Assoc. 2019, 8, e011540.
- Karpouzas, G.A.; Ormseth, S.R.; Hernandez, E.; Budoff, M.J. Impact of cumulative inflammation, cardiac risk factors, and medication exposure on coronary atherosclerosis progression in rheumatoid arthritis. Arthritis Rheumatol. 2020, 72, 400–408.
- Pan, L.; Wang, T. Features of cardiac remodeling in patients with acute coronary syndrome complicated with rheumatoid arthritis. Sci. Rep. 2017, 7, 1–6.
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; Ballina-García, F.J.; Abal, F.; Suárez, A. Antibodies to high-density lipoproteins are associated with inflammation and cardiovascular disease in rheumatoid arthritis patients. Transl. Res. 2015, 166, 529–539.
- Olumuyiwa-Akeredolu, O.O.; Soma, P.; Buys, A.V.; Debusho, L.K.; Pretorius, E. Characterizing pathology in erythrocytes using morphological and biophysical membrane properties: Relation to impaired hemorheology and cardiovascular function in rheumatoid arthritis. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2381–2391.
- Broadley, I.; Pera, A.; Morrow, G.; Davies, K.A.; Kern, F. Expansions of cytotoxic CD4(+)CD28(-) T cells drive excess cardiovascular mortality in rheumatoid arthritis and other chronic inflammatory conditions and are triggered by CMV infection. Front. Immunol. 2017, 8, 195.
- Bezuidenhout, J.A.; Pretorius, E. The central role of acute phase proteins in rheumatoid arthritis: Involvement in disease autoimmunity, inflammatory responses, and the heightened risk of cardiovascular disease. Semin. Thromb. Hemost. 2020, 46, 465–483.
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28.
- Myasoedova, E.; Crowson, C.; Sexton, J.; Rollefstad, S.; Grete Semb, A. Sex Differences in Cardiovascular Disease Prevention in Patients with Rheumatoid Arthritis: World-Wide Data from the SURF-RA [Abstract]. Available online: (accessed on 9 November 2020).
- Jagpal, A.; Navarro-Millán, I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: A narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol. 2018, 2, 10.
- Widdifield, J.; Abrahamowicz, M.; Paterson, J.M.; Huang, A.; Thorne, J.C.; Pope, J.E.; Kuriya, B.; Beauchamp, M.E.; Bernatsky, S. Associations between methotrexate use and the risk of cardiovascular events in patients with elderly-onset rheumatoid arthritis. J. Rheumatol. 2019, 46, 467–474.
- Gualtierotti, R.; Ughi, N.; Marfia, G.; Ingegnoli, F. Practical management of cardiovascular comorbidities in rheumatoid arthritis. Rheumatol. Ther. 2017, 4, 293–308.
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 480–489.
- Low, A.S.L.; Symmons, D.P.M.; Lunt, M.; Mercer, L.K.; Gale, C.P.; Watson, K.D.; Dixon, W.G.; Hyrich, K.L. Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 654–660.
- Lee, J.L.; Sinnathurai, P.; Buchbinder, R.; Hill, C.; Lassere, M.; March, L. Biologics and cardiovascular events in inflammatory arthritis: A prospective national cohort study. Arthritis Res. Ther. 2018, 20, 171.
- Nurmohamed, M.; Bao, Y.; Signorovitch, J.; Trahey, A.; Mulani, P.; Furst, D.E. Longer durations of antitumour necrosis factor treatment are associated with reduced risk of cardiovascular events in patients with rheumatoid arthritis. RMD Open 2015, 1, e000080.
- Plein, S.; Erhayiem, B.; Fent, G.; Horton, S.; Dumitru, R.B.; Andrews, J.; Greenwood, J.P.; Emery, P.; Hensor, E.M.; Baxter, P.; et al. Cardiovascular effects of biological versus conventional synthetic disease-modifying antirheumatic drug therapy in treatment-naïve, early rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 1414–1422.
- Atzeni, F.; Gianturco, L.; Boccassini, L.; Sarzi-Puttini, P.; Bonitta, G.; Turiel, M. Noninvasive imaging methods for evaluating cardiovascular involvement in patients with rheumatoid arthritis before and after anti-TNF drug treatment. Futur. Sci. OA 2019, 5, FSO396.
- Ljung, L.; Rantapää-Dahlqvist, S.; Jacobsson, L.T.; Askling, J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 2087–2094.
- Jamnitski, A.; Visman, I.M.; Peters, M.J.L.; Dijkmans, B.A.C.; Voskuyl, A.E.; Nurmohamed, M.T. Beneficial effect of 1-year etanercept treatment on the lipid profile in responding patients with rheumatoid arthritis: The ETRA study. Ann. Rheum. Dis. 2010, 69, 1929–1933.
- Deodhar, A.; Bitman, B.; Yang, Y.; Collier, D.H. The effect of etanercept on traditional metabolic risk factors for cardiovascular disease in patients with rheumatoid arthritis. Clin. Rheumatol. 2016, 35, 3045–3052.
- Luque-Tevar, M.; Pérez-Sánchez, C.; Font, P.; Maria Patiño-Trives, A.; Romero-Gomez, M.; Ruiz-Vilchez, D.; Remuzgo-Martínez, S.; López-Mejías, R.; Arias de la Rosa, I.; Torres-Granados, C.; et al. Unsupervised Molecular Profile Clustering in the Serum of Rheumatoid Arthritis Patients Identifies Groups with Differential CV-Risk SCORE: Modulation by Biological Therapies [Abstract]. Available online: (accessed on 2 November 2020).
- Gabay, C.; McInnes, I.B.; Kavanaugh, A.; Tuckwell, K.; Klearman, M.; Pulley, J.; Sattar, N. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 1806–1812.
- Souto, A.; Salgado, E.; Maneiro, J.R.; Mera, A.; Carmona, L.; Gõmez-Reino, J.J. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: A systematic review and meta-analysis. Arthritis Rheumatol. 2015, 67, 117–127.
- McInnes, I.B.; Thompson, L.; Giles, J.T.; Bathon, J.M.; Salmon, J.E.; Beaulieu, A.D.; Codding, C.E.; Carlson, T.H.; Delles, C.; Lee, J.S.; et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. 2015, 74, 694–702.
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: A randomized controlled trial. Arthritis Rheumatol. 2020, 72, 31–40.
- Xie, F.; Yun, H.; Levitan, E.B.; Muntner, P.; Curtis, J.R. Tocilizumab and the risk of cardiovascular disease: Direct comparison among biologic disease-modifying antirheumatic drugs for rheumatoid arthritis patients. Arthritis Care Res. 2019, 71, 1004–1018.
- Kim, S.C.; Solomon, D.H.; Rogers, J.R.; Gale, S.; Klearman, M.; Sarsour, K.; Schneeweiss, S. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: A multi-database cohort study. Arthritis Rheumatol. 2017, 69, 1154–1164.
- Virone, A.; Bastard, J.P.; Fellahi, S.; Capeau, J.; Rouanet, S.; Sibilia, J.; Ravaud, P.; Berenbaum, F.; Gottenberg, J.E.; Sellam, J. Comparative effect of tumour necrosis factor inhibitors versus other biological agents on cardiovascular risk-associated biomarkers in patients with rheumatoid arthritis. RMD Open 2019, 5, e000897.
- García-Gómez, C.; Martín-Martínez, M.A.; Castañeda, S.; Sanchez-Alonso, F.; Uriarte-Ecenarro, M.; González-Juanatey, C.; Romera-Baures, M.; Santos-Rey, J.; Pinto-Tasende, J.A.; Quesada-Masachs, E.; et al. Lipoprotein(a) concentrations in rheumatoid arthritis on biologic therapy: Results from the CARdiovascular in rheuMAtology study project. J. Clin. Lipidol. 2017, 11, 749–756.e3.
- Singh, S.; Fumery, M.; Singh, A.G.; Singh, N.; Prokop, L.J.; Dulai, P.S.; Sandborn, W.J.; Curtis, J.R. Comparative risk of cardiovascular events with biologic and synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: A systematic review and meta-analysis. Arthritis Care Res. 2020, 72, 561–576.
- Hsieh, M.J.; Lee, C.H.; Tsai, M.L.; Kao, C.F.; Lan, W.C.; Huang, Y.T.; Tseng, W.Y.; Wen, M.S.; Chang, S.H. Biologic agents reduce cardiovascular events in rheumatoid arthritis not responsive to tumour necrosis factor inhibitors: A national cohort study. Can. J. Cardiol. 2020, 36, 1739–1746.
- Jin, Y.; Kang, E.H.; Brill, G.; Desai, R.J.; Kim, S.C. Cardiovascular (CV) risk after initiation of abatacept versus TNF inhibitors in rheumatoid arthritis patients with and without baseline CV disease. J. Rheumatol. 2018, 45, 1240–1248.
- Kang, E.H.; Jin, Y.; Brill, G.; Lewey, J.; Patorno, E.; Desai, R.J.; Kim, S.C. Comparative cardiovascular risk of abatacept and tumor necrosis factor inhibitors in patients with rheumatoid arthritis with and without diabetes mellitus: A multidatabase cohort study. J. Am. Heart Assoc. 2018, 7, e007393.
- Mease, P.; Charles-Schoeman, C.; Cohen, S.; Fallon, L.; Woolcott, J.; Yun, H.; Kremer, J.; Greenberg, J.; Malley, W.; Onofrei, A.; et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann. Rheum. Dis. 1400, 79, 1400–1413.
- EMA. Xeljanz-EMEA-H-A-20-1485-Assessment Report. Available online: (accessed on 3 November 2020).
- Pfizer. XELJANZ 5 mg Film-Coated Tablets-Summary of Product Characteristics (SmPC)-Print Friendly-(EMC). Available online: (accessed on 3 November 2020).
- FDA. Highlights of Prescribing Information: Xeljanz. Available online: (accessed on 10 November 2020).
- Xie, W.; Huang, Y.; Xiao, S.; Sun, X.; Fan, Y.; Zhang, Z. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: Systematic review and meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2019, 78, 1048–1054.




