Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Szücs-Bencze | -- | 3328 | 2023-10-26 16:23:33 | | | |
| 2 | Catherine Yang | Meta information modification | 3328 | 2023-10-27 02:48:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Szücs-Bencze, L.; Vékony, T.; Pesthy, O.; Szabó, N.; Kincses, T.Z.; Turi, Z.; Nemeth, D. Determining Factors in rTMS on Sequence Learning. Encyclopedia. Available online: https://encyclopedia.pub/entry/50844 (accessed on 07 February 2026).
Szücs-Bencze L, Vékony T, Pesthy O, Szabó N, Kincses TZ, Turi Z, et al. Determining Factors in rTMS on Sequence Learning. Encyclopedia. Available at: https://encyclopedia.pub/entry/50844. Accessed February 07, 2026.
Szücs-Bencze, Laura, Teodóra Vékony, Orsolya Pesthy, Nikoletta Szabó, Tamás Zsigmond Kincses, Zsolt Turi, Dezso Nemeth. "Determining Factors in rTMS on Sequence Learning" Encyclopedia, https://encyclopedia.pub/entry/50844 (accessed February 07, 2026).
Szücs-Bencze, L., Vékony, T., Pesthy, O., Szabó, N., Kincses, T.Z., Turi, Z., & Nemeth, D. (2023, October 26). Determining Factors in rTMS on Sequence Learning. In Encyclopedia. https://encyclopedia.pub/entry/50844
Szücs-Bencze, Laura, et al. "Determining Factors in rTMS on Sequence Learning." Encyclopedia. Web. 26 October, 2023.
Copy Citation
Sequence learning is a fundamental ability of the human brain. It forms the basis of many cognitive, social, and motor skills. Repetitive transcranial magnetic stimulation (rTMS) is an increasingly used non-invasive brain stimulation (NIBS) tool to examine the functional role of cortical areas and brain networks. In addition to neuroimaging methods, rTMS might contribute to a better understanding of the functional and neural underpinnings of visuomotor sequence learning.
non-invasive brain stimulation
sequence learning
repetitive TMS
1. Stimulated Brain Regions
First, the researchers examined the stimulated brain regions and found that the two most frequent targets were M1 (nine out of 17 studies) and the DLPFC (eight out of 17 studies). In addition, three studies targeted the SMA and four the parietal cortex. One study stimulated the Broca area, and another one the cerebellum (see Figure 1).
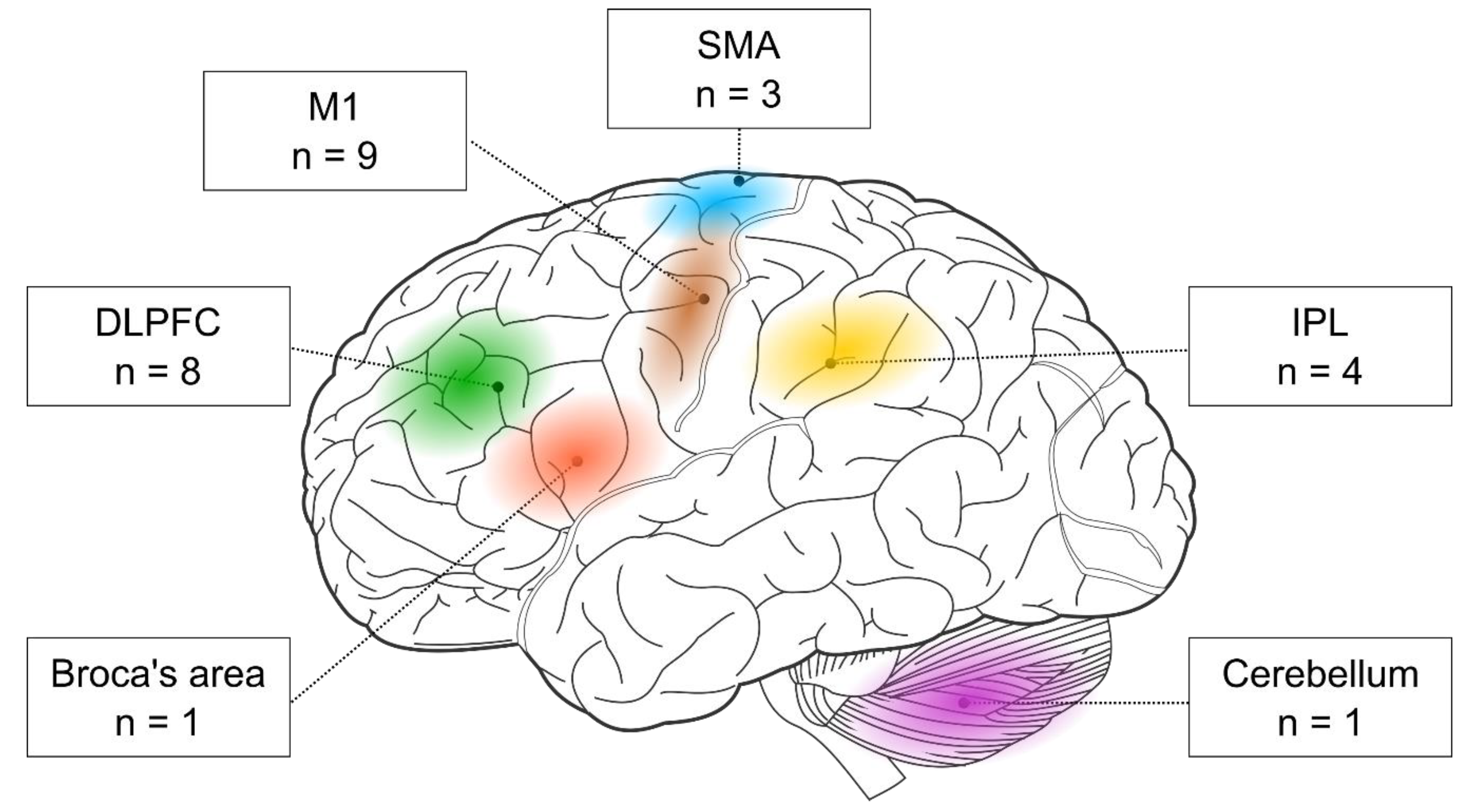
Figure 1. The cortical targets of rTMS-SRTT studies. Brain image was adapted from Hugh Guiney (https://commons.wikimedia.org/wiki/File:Human-brain.SVG; CC BY-SA 3.0; accessed on 25 July 2021).
M1. The role of M1 in motor consolidation goes beyond sequence learning as it engages in the early motor consolidation of elementary motor behavior (Buetefisch et al. 2015; Bütefisch et al. 2004; Muellbacher et al. 2002). The popularity of M1 as a stimulation target is attributed to its implicated role in the initial encoding of sequences and the early consolidation of already learned sequences (Seidler et al. 2005). In the reviewed rTMS studies, typically, low-frequency rTMS or cTBS was applied over M1, resulting in the weakening of the learning process (Clark et al. 2019; Rosenthal et al. 2009; Steel et al. 2016; Wilkinson et al. 2015), or the prevention of offline improvements (Breton and Robertson 2017; Robertson et al. 2005). Interestingly, two studies found an increase in visuomotor skills following low-frequency rTMS or cTBS over M1. In one of them, low-frequency rTMS affected the SRTT indirectly through the prevention of interference with a declarative task (Cohen and Robertson 2011). In the other study, cTBS abolished the decrease in corticospinal excitability, which allowed for offline improvements on an explicit SRTT (Tunovic et al. 2014). Only one of the nine studies attempted to use iTBS over M1, but it did not find any effect on implicit sequence learning (Wilkinson et al. 2010).
DLPFC. The DLPFC has traditionally been identified as a brain area supporting executive functions and working memory (Miller and Cohen 2001; Yuan and Raz 2014). Plasticity changes in the DLPFC seem to be associated with sequence learning (Cao et al. 2022). Lesion studies—where patients with prefrontal lesions show decreased sequence learning on the SRTT (Beldarrain et al. 1999, 2002)—highlight its importance in visuomotor sequence learning, too. However, its functional role is still controversial (Janacsek and Nemeth 2013, 2015). Based on recent models, the DLPFC may act as a neural switch between competitive memory processes (Ambrus et al. 2020; Daw et al. 2005; Lee et al. 2014). On the one hand, it may favor declarative learning and memory (e.g., memory for events and facts), as well as top-down processes. However, if the situation requires acquiring new regularities (e.g., a completely new pattern or sequence), it recedes. A potential mediator role of the DLPFC is also supported by the findings of a TBS study, where the learning of linguistic sequences was enhanced due to the disruptive stimulation of the DLPFC (Smalle et al. 2022). Out of the eight identified studies, three studies found that rTMS over the DLPFC weakened implicit sequence learning (Cohen and Robertson 2011; Pascual-Leone et al. 1996; Robertson et al. 2001). In one of them, DLPFC stimulation reduced learning on the SRTT indirectly by interfering with a declarative task (Cohen and Robertson 2011). Examining explicit sequence learning, two of the six studies found an enhancement for cTBS over the DLPFC (Galea et al. 2010; Tunovic et al. 2014). However, one recent study found no effect of DLPFC stimulation on explicit the SRTT (Gann et al. 2021). Two additional studies used probabilistic instead of deterministic sequences. One of them found that low-frequency rTMS over the DLPFC led to better performance on this sequence type (Ambrus et al. 2020), while the other study found no effect of DLPFC stimulation on performance (Wilkinson et al. 2010).
SMA. Some neuroimaging studies reveal that, besides M1, another motor area, the SMA also appears to be involved during the SRTT (Hazeltine et al. 1997; Seidler et al. 2005). According to an fMRI study, SMA activation is associated with the performance of sequential movements (Hikosaka et al. 1996). Additionally, a PET study suggests that the SMA is involved in the execution of previously learned sequences rather than in the acquisition of sequences (Honda et al. 1998). In more recent studies, the SMA was found to be involved in the automatization of sequential movements (Shimizu et al. 2020) and the consolidation of implicit sequence knowledge (Verwey et al. 2022).
Parietal cortex. According to previous behavioral studies, a motor response is not strictly necessary for the acquisition of complex sequences; monitoring in itself can lead to learning (Nemeth et al. 2009; Song et al. 2008; Zolnai et al. 2022). Based on functional neuroimaging studies, the inferior parietal lobule (IPL) encodes the sequence at a general, abstract level, independently of the response mode (Grafton et al. 1998; Hikosaka et al. 1999).
Broca’s area. In the field of sequence learning, Broca’s area has been primarily tested on artificial grammar learning tasks (De Vries et al. 2010; Uddén et al. 2017), where participants need to extract rules from artificially generated grammatical sequences (Reber 1967, 1989). Because the acquisition of the grammar of a language is connected to sequence learning (Nemeth et al. 2011), it is an interesting question whether this brain area is also involved in the acquisition of non-linguistic visuomotor sequences. Only one study has examined the role of Broca’s area and showed that cTBS over the BA 44 prevented the learning on an implicit SRTT (Clerget et al. 2012).
Cerebellum. The role of the cerebellum in sequence learning is highly uncertain in the literature (Baetens et al. 2020; Janacsek et al. 2020). While the detrimental effect of cerebellar damage on sequence learning assumes its essential role (Dirnberger et al. 2013; Doyon et al. 1997; Gomez-Beldarrain et al. 1998; Shin and Ivry 2003), neuroimaging studies do not always support this hypothesis (Janacsek et al. 2020; Kóbor et al. 2022; Seidler et al. 2002; van der Graaf et al. 2006). However, a recent fMRI study revealed that the cerebellum is involved in the early phase of task performance and coordination since its activity diminishes as the task becomes well practiced (Hermsdorf et al. 2020). On the other hand, the functional role of the cerebellum has been mainly studied in motor adaptation tasks (Doppelmayr et al. 2016; Galea et al. 2011; Jayaram et al. 2012). Only one study investigated the causal role of the cerebellum in visuomotor sequence learning and showed that low-frequency rTMS over the lateral cerebellum resulted in a significant weakening in sequence learning (Torriero et al. 2004).
2. ‘Inhibitory’ and ‘Facilitatory’ rTMS Protocols
It is common to assume a linear relationship between the direction of the produced aftereffects on cortical excitability and the behavioral effects of rTMS. According to this view, low-frequency rTMS and cTBS (i.e., the ‘inhibitory’ protocols) might induce functional inhibition/disruption, whereas high-frequency rTMS and iTBS (i.e., the ‘excitatory’ protocols) might lead to functional improvements/enhancement. Although this generally accepted dichotomy between the stimulation frequency and the direction of the produced cognitive aftereffects is likely oversimplified, several studies discuss the results in this framework.
In the following, the researchers delineate several reasons that it is challenging to predict the functional aftereffects of rTMS solely based on the protocol type (i.e., ‘inhibitory’ or ‘excitatory’). First, there is substantial interindividual variability when inducing corticospinal excitability changes in M1. While group-level data might show frequency-dependent modulatory effects, they can vary significantly across individuals (Hamada et al. 2013; Maeda et al. 2000), and even within individuals (Goldsworthy et al. 2021). Biological (e.g., age, time of the day, genetics, brain state) and methodological factors (e.g., stimulation parameters, measures for the effect) both may be responsible for the intra- and interindividual variability of the rTMS effect (Huang et al. 2017). One possible strategy to decrease variability effects is increasing the specificity of the stimulation. This can be achieved by a novel rTMS technique, called quadripulse stimulation (QPS), that uses repetitive monophasic pulses, instead of biphasic pulses resulting in smaller variability in the after-effects (Simeoni et al. 2016; Tiksnadi et al. 2020).
Second, it is unclear whether a given protocol that may decrease the cortical excitability in M1 produces the same physiological effects in other cortical areas. For instance, some authors speculate that there might be overlaps in the produced aftereffects at least within the frontal cortex (e.g., M1 and DLPFC, as discussed in de Jesus et al. 2014). Third, this view may miss the brain’s endogenous and dynamic compensatory mechanisms to external perturbations. For example, due to the interhemispheric compensation, decreasing the excitability level of the left DLPFC with low-frequency rTMS may lead to the compensatory recruitment of the right DLPFC (Ambrus et al. 2020). Fourth, the stimulation frequency is only one of many crucial stimulation parameters that can shape the direction of aftereffects. For example, the facilitating effect of high-frequency rTMS requires inter-train intervals; otherwise, it is more likely to produce an inhibitory effect (Rothkegel et al. 2010).
Based on these arguments, it is conceivable to expect that the behavioral effects of rTMS may not always match the alterations in cortical excitability. Consequently, ‘facilitatory’ protocols may not always enhance, and ‘inhibitory’ protocols may not necessarily weaken the performance.
3. Stimulated Hemisphere(s)
Many studies have used rTMS to better understand the hemispheric involvement of a given brain region when performing the SRTT. These studies typically ask whether the left or right brain area (e.g., M1) is causally involved in a specific task phase (e.g., learning phase). To this aim, most studies have stimulated the left or the right hemisphere at a time and studied whether rTMS could modulate the performance.
Performing the SRTT may require using only one hand or both hands. When the participants perform the SRTT with only one hand (e.g., the right hand), the stimulation may target the contralateral (i.e., left) or ipsilateral (i.e., right) hemisphere. Eleven of the 17 studies targeted the contralateral hemisphere (see Table 1). In studies where only the left hemisphere was stimulated, only right-handed participants were included (see Table 1).
Table 1. The effect of different rTMS protocols on visuomotor sequence learning according to the target area, sequence type, and timing of the stimulation.
| Target Area | Authors and Year | rTMS Protocol | Hemisphere | Timing of Stimulation | Type of Sequence | Outcome |
|---|---|---|---|---|---|---|
| M1 | ||||||
| (Robertson et al. 2005) | 1 Hz rTMS | Left only | After learning | 12-item implicit deterministic | Blocked offline improvements over the day, but not overnight | |
| (Breton and Robertson 2017) | 1 Hz rTMS | Left only | After learning | 12-item implicit/explicit deterministic | Blocked offline improvements in explicit, but not in implicit task | |
| (Tunovic et al. 2014) (Experiment 3) | cTBS | Right only | After learning | 12-item explicit deterministic | Offline improvements after cTBS | |
| (Cohen and Robertson 2011) (Experiment 2) |
1 Hz rTMS | Right only | After learning | 12-item implicit deterministic | Increased learning after 12 h consolidation by preventing interference with a declarative task | |
| (Wilkinson et al. 2010) | cTBS, iTBS | Left only | Before learning | 12-item implicit probabilistic | Learning was prevented by cTBS | |
| (Wilkinson et al. 2015) | cTBS | Left only | Before learning | 12-item implicit probabilistic | Decreased initial sequence learning and recall | |
| (Steel et al. 2016) | cTBS | Left only | Before learning | 12-item implicit probabilistic | Learning was disrupted | |
| (Rosenthal et al. 2009) (Experiment 1, 2) |
cTBS | Contralateral to dominant hand | Before learning | 12-item implicit probabilistic | Learning was disrupted in manual, but not in perceptual task | |
| (Clark et al. 2019) | cTBS | Left only | Before learning | 12-item implicit deterministic | Decreased learning in simple, but not in a more complex sequence | |
| DLPFC | ||||||
| (Pascual-Leone et al. 1996) | 5 Hz rTMS | Left or right in separate conditions | During learning | 12-item implicit deterministic | Learning was disrupted | |
| (Robertson et al. 2001) | 1 Hz rTMS | Contralateral to dominant hand | Before learning | 10-item implicit deterministic | Learning was prevented in spatial, but not in color cue guided task | |
| (Wilkinson et al. 2010) | cTBS | Left only | Before learning | 12-item implicit probabilistic | No effect on learning | |
| (Gann et al. 2021) | cTBS, iTBS | Left only | Before learning | 8-item explicit deterministic | No effect on learning | |
| (Galea et al. 2010) | cTBS | Left or right in separate groups | After learning | 12-item explicit deterministic | Improved learning after 8 h consolidation | |
| (Tunovic et al. 2014) (Experiment 2) |
cTBS | Right only | After learning | 12-item explicit deterministic | Offline improvements after cTBS | |
| (Cohen and Robertson 2011) (Experiment 2) |
1 Hz rTMS | Right only | After learning | 12-item implicit deterministic | Decreased learning after 12 h consolidation by failing to prevent interference with a declarative task | |
| (Ambrus et al. 2020) | 1 Hz rTMS | Bilaterally | Between learning blocks | 8-item implicit probabilistic | Improved learning after 24 h consolidation | |
| Broca’s area | ||||||
| (Clerget et al. 2012) | cTBS | Left only | Before learning | 20-item implicit deterministic | Learning was prevented | |
| SMA | ||||||
| (Pascual-Leone et al. 1996) | 5 Hz rTMS | Not applicable | During learning | 12-item implicit deterministic | No effect on learning | |
| (Wilkinson et al. 2010) | cTBS | Not applicable | Before learning | 12-item implicit probabilistic | No effect on learning | |
| (Perez et al. 2008) | 1 Hz rTMS | Not applicable | During learning | 12-item implicit deterministic | Blocked intermanual transfer of the skill | |
| IPL | ||||||
| (Robertson et al. 2001) | 1 Hz rTMS | Contralateral to the dominant hand | Before learning | 10-item implicit deterministic | No effect on learning | |
| (Rosenthal et al. 2009) (Experiment 1, 2) |
cTBS | Right only | Before learning | 12-item implicit probabilistic | Learning was disrupted in perceptual, but not in manual task | |
| (Breton and Robertson 2017) | 1 Hz rTMS | Left only | After learning | 12-item implicit, explicit deterministic | Blocked offline improvements in implicit, but not in explicit task | |
| (Clark et al. 2019) | cTBS | Left only | Before learning | 12-item implicit, deterministic | No effect on learning | |
| Cerebellum | ||||||
| (Torriero et al. 2004) | 1 Hz rTMS | Left or right in separate groups | Before learning | 12-item implicit, deterministic | Learning was disrupted |
However, studies targeting the dominant hemisphere (based on M1) may neglect the possibility that the non-stimulated hemisphere can take over the function of the stimulated one (Andoh and Martinot 2008; Sack et al. 2005), potentially influencing the results. Applying sequential bilateral stimulations (i.e., delivering the same rTMS protocol over a given cortical target consecutively on each hemisphere) may be a promising solution to overcome the possible interhemispheric compensatory mechanisms (Ambrus et al. 2020).
Considering the side of the stimulation, some studies have targeted both hemispheres in separate experimental groups. Using this method, Galea et al. (2010) successfully demonstrated that cTBS over the right DLPFC improved visuomotor sequence learning to a greater extent than the left DLPFC (Galea et al. 2010). Another study revealed a dissociation between cerebellar hemispheres: the stimulation of the right cerebellar hemisphere weakened sequence learning regardless of which hand was used, while the interference with the left cerebellar hemisphere affected only through the ipsilateral hand (Torriero et al. 2004). Therefore, this method is suitable for exploring potential lateralization effects as well.
For non-motor brain areas, it is worth targeting both hemispheres separately and applying sequential bilateral protocols. This approach can avoid hemispheric compensatory mechanisms and reveal the possible dissociation between hemispheres (Ambrus et al. 2020). Furthermore, the sequential bilateral stimulation for the two-handed version of the task may be a particularly good solution.
4. Timing of the Stimulation
Motor memory traces can be strengthened in two ways: when performance is gradually improving during practice (online learning) and when performance improves between two training sessions without any practice (offline learning) (Cohen et al. 2005; Robertson et al. 2004). Sequence learning is a multi-stage process: it consists of the learning, consolidation, and retrieval phases.
Learning phase. One can deliver rTMS immediately before or during the learning phase (see Figure 2). During the learning phase, rTMS may be applied simultaneously with the task performance or between the learning blocks. Only two studies have applied rTMS during the initial sequence learning process (so-called ‘online stimulation’). In one study, high-frequency rTMS over the DLPFC led to a performance decrease on the SRTT (Pascual-Leone et al. 1996). In a more recent study, the authors applied low-frequency rTMS over the DLPFC between the learning blocks and found a performance improvement on an alternating SRTT (Ambrus et al. 2020). Most research delivered stimulation immediately before task performance (so-called ‘offline stimulation’; see Table 1). Most of them have found decreased learning of visuomotor sequence learning (Clark et al. 2019; Clerget et al. 2012; Torriero et al. 2004; Wilkinson et al. 2015).

Figure 2. Timing of the stimulation in each task phase.
Consolidation phase. One may apply rTMS after the learning phase to verify its effect on memory consolidation (see Figure 2). In one study, cTBS over the DLPFC improved performance after an 8-h-long offline period (Galea et al. 2010). In contrast, low-frequency rTMS over M1 blocked offline improvements on an implicit SRTT over the day (Robertson et al. 2005), as well as on an explicit SRTT after sleep (Breton and Robertson 2017). rTMS over the DLPFC and M1 can influence the development of memory traces because their stimulation leads to changes in the consolidation process.
Retrieval phase. Finally, rTMS may be applied immediately before or during the recall phase. No studies have applied rTMS immediately before or during the recall phase as of the day of the literature search. Thus, it is unclear whether rTMS can modulate the recall of well-acquired sequence knowledge.
5. Type of the SRTT Sequence
The effect of stimulation may depend on the SRTT sequence type. Several new versions of the SRTT have emerged that can differ in three crucial dimensions: the sequence applied can be (1) implicit or explicit, (2) deterministic or probabilistic, and (3) first-order conditional (FOC) or second-order conditional (SOC) (as defined in section Different variations of the SRTT).
Implicit vs. explicit sequences. The most commonly used version of the SRTT uses implicit sequences (here, 13 out of the 17 reviewed studies used an implicit SRTT). However, its explicit version (i.e., the existence of the predetermined sequence is revealed to the participants before learning) can also be applied if the goal is to test intentional learning or declarative knowledge of the sequence. They examined the role of M1 and the IPL in both the implicit and explicit SRTT and revealed a double dissociation: low-frequency rTMS over the IPL prevented offline improvement in the implicit but not in the explicit task. On the other hand, the same stimulation over M1 prevented offline improvement in the explicit but not in the implicit task (Breton and Robertson 2017).
Deterministic vs. probabilistic sequences. In the classic SRTT, stimuli follow a fixed order, creating a deterministic sequence (Nissen and Bullemer 1987; Shanks 2005). Probabilistic types of the SRTT also exist, where the sequence is hidden in noise; therefore, learning is more likely to remain implicit (Howard et al. 2004; Song et al. 2007). Five out of 17 studies used probabilistic sequences, and 12 used deterministic sequences (Table 1). All of the studies employing probabilistic sequence learning tasks found behavioral effects of rTMS. In four studies, learning deteriorated (Rosenthal et al. 2009; Steel et al. 2016; Wilkinson et al. 2010, 2015), and, in one study, learning was improved (Ambrus et al. 2020). On the other hand, deterministic sequence learning performance was successfully manipulated in 11 out of the 12 studies: in eight studies, learning was disrupted (Breton and Robertson 2017; Clark et al. 2019; Clerget et al. 2012; Pascual-Leone et al. 1996; Perez et al. 2008; Robertson et al. 2005, 2001; Torriero et al. 2004), and, in three studies, learning was improved (Cohen and Robertson 2011; Galea et al. 2010; Tunovic et al. 2014). Therefore, it seems that rTMS can equally modify the learning of both probabilistic and deterministic sequences.
FOC vs. SOC sequences. Another critical factor is the statistical structure of the sequence. In the simpler first-order conditional (FOC) sequences, elements can be predicted by the preceding one. On the other hand, in the more complex second-order conditional (SOC) sequences, it is the combination of two consecutive elements that predicts the forthcoming one. Clark et al. (2019) investigated the role of M1 in the acquisition of simpler FOC and more complex SOC sequences. According to their findings, cTBS over M1 resulted in poorer learning of the FOC sequence compared to the SOC sequence. These findings support the hypothesis that the acquisition of FOC and SOC sequences may rely on different neural networks: simpler FOC sequences are processed by a circuitry involving M1, while more complex SOC sequences are associated with an expanded network, including Brodmann area 44 (BA44) and the DLPFC (Ashe et al. 2006; Lum et al. 2018). Although working with discrete sequence production task instead of the SRTT, an rTMS study also revealed the distinct role of the pre-SMA in more complex sequences compared to simpler ones (Ruitenberg et al. 2014). Based on these promising results, future studies may investigate FOC and SOC sequences targeting non-motor areas too.
References
- Buetefisch, Cathrin M., Cortney Howard, Christina Korb, Marc W. Haut, Linda Shuster, Paola Pergami, Cheryl Smith, and Gerald Hobbs. 2015. Conditions for enhancing the encoding of an elementary motor memory by rTMS. Clinical Neurophysiology 126: 581–93.
- Bütefisch, Cathrin M., Vikram Khurana, Leonid Kopylev, and Leonardo G. Cohen. 2004. Enhancing Encoding of a Motor Memory in the Primary Motor Cortex By Cortical Stimulation. Journal of Neurophysiology 91: 2110–16.
- Muellbacher, Wolf, Ulf Ziemann, Joerg Wissel, Nguyet Dang, Markus Kofler, Stefano Facchini, Babak Boroojerdi, Werner Poewe, and Mark Hallett. 2002. Early consolidation in human primary motor cortex. Nature 415: 640–44.
- Seidler, Raphael D., Arnie Purushotham, Seong-Gi Kim, Kaamil Ugurbil, Daniel Willingham, and James Ashe. 2005. Neural correlates of encoding and expression in implicit sequence learning. Experimental Brain Research 165: 114–24.
- Clark, Gillian M., Michael P. Barham, Anna T. Ware, James M. A. Plumridge, Bernadette O’Sullivan, Kristie Lyons, Tegan Fitzgibbon, Bree Buck, George J. Youssef, Michael T. Ullman, and et al. 2019. Dissociable implicit sequence learning mechanisms revealed by continuous theta-burst stimulation. Behavioral Neuroscience 133: 341–49.
- Rosenthal, Clive R., Emma E. Roche-Kelly, Masud Husain, and Christopher Kennard. 2009. Response-Dependent Contributions of Human Primary Motor Cortex and Angular Gyrus to Manual and Perceptual Sequence Learning. Journal of Neuroscience 29: 15115–25.
- Steel, Adam, Sunbin Song, Devin Bageac, Kristine M. Knutson, Aysha Keisler, Ziad S. Saad, Stephen J. Gotts, Eric M. Wassermann, and Leonora Wilkinson. 2016. Shifts in connectivity during procedural learning after motor cortex stimulation: A combined transcranial magnetic stimulation/functional magnetic resonance imaging study. Cortex 74: 134–48.
- Wilkinson, Leonora, Adam Steel, Eric Mooshagian, Trelawny Zimmermann, Aysha Keisler, Jeffrey D. Lewis, and Eric M. Wassermann. 2015. Online feedback enhances early consolidation of motor sequence learning and reverses recall deficit from transcranial stimulation of motor cortex. Cortex 71: 134–47.
- Breton, Jocelyn, and Edwin M. Robertson. 2017. Dual enhancement mechanisms for overnight motor memory consolidation. Nature Human Behaviour 1: 0111.
- Robertson, Edwin M., Daniel Z. Press, and Alvaro Pascual-Leone. 2005. Off-Line Learning and the Primary Motor Cortex. Journal of Neuroscience 25: 6372–78.
- Cohen, Daniel A., and Edwin M. Robertson. 2011. Preventing interference between different memory tasks. Nature Neuroscience 14: 953–55.
- Tunovic, Sanjin, Daniel Z. Press, and Edwin M. Robertson. 2014. A Physiological Signal That Prevents Motor Skill Improvements during Consolidation. Journal of Neuroscience 34: 5302–10.
- Wilkinson, Leonora, James T. Teo, Ignacio Obeso, John C. Rothwell, and Marjan Jahanshahi. 2010. The Contribution of Primary Motor Cortex is Essential for Probabilistic Implicit Sequence Learning: Evidence from Theta Burst Magnetic Stimulation. Journal of Cognitive Neuroscience 22: 427–36.
- Miller, Earl K., and Jonathan D. Cohen. 2001. An Integrative Theory of Prefrontal Cortex Function. Annual Review of Neuroscience 24: 167–202.
- Yuan, Peng, and Naftali Raz. 2014. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neuroscience & Biobehavioral Reviews 42: 180–92.
- Cao, Na, Yanling Pi, Fanghui Qiu, Yanqiu Wang, Xue Xia, Yu Liu, and Jian Zhang. 2022. Plasticity changes in dorsolateral prefrontal cortex associated with procedural sequence learning are hemisphere-specific. NeuroImage 259: 119406.
- Beldarrain, Marian Gómez, Jordan Grafman, Alvaro Pascual-Leone, and Juan C. Garcia-Monco. 1999. Procedural learning is impaired in patients with prefrontal lesions. Neurology 52: 1853–53.
- Beldarrain, Marian Gomez, Jordan Gafman, Ibone Ruiz de Velasco, Alvaro Pascual-Leone, and Juan Garcia-Monco. 2002. Prefrontal lesions impair the implicit and explicit learning of sequences on visuomotor tasks. Experimental Brain Research 142: 529–38.
- Janacsek, Karolina, and Dezso Nemeth. 2013. Implicit sequence learning and working memory: Correlated or complicated? Cortex 49: 2001–6.
- Janacsek, Karolina, and Dezso Nemeth. 2015. The puzzle is complicated: When should working memory be related to implicit sequence learning, and when should it not? (Response to Martini et al.). Cortex 64: 411–12.
- Ambrus, Géza Gergely, Teodóra Vékony, Karolina Janacsek, Anna B. C. Trimborn, Gyula Kovács, and Dezso Nemeth. 2020. When less is more: Enhanced statistical learning of non-adjacent dependencies after disruption of bilateral DLPFC. Journal of Memory and Language 114: 104144.
- Daw, Nathaniel D., Yael Niv, and Peter Dayan. 2005. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience 8: 1704–11.
- Lee, Sang Wan, Shinsuke Shimojo, and John P. O’doherty. 2014. Neural Computations Underlying Arbitration between Model-Based and Model-free Learning. Neuron 81: 687–99.
- Smalle, Eleonore H. M., Tatsuya Daikoku, Arnaud Szmalec, Wouter Duyck, and Riikka Möttönen. 2022. Unlocking adults’ implicit statistical learning by cognitive depletion. Proceedings of the National Academy of Sciences of the United States of America 119: e2026011119.
- Pascual-Leone, Alvaro, Eric M. Wassermann, Jordan Grafman, and Mark Hallett. 1996. The role of the dorsolateral prefrontal cortex in implicit procedural learning. Experimental Brain Research 107: 479–85.
- Robertson, Edwin M., Jose M. Tormos, Fumiko Maeda, and Alvaro Pascual-Leone. 2001. The Role of the Dorsolateral Prefrontal Cortex during Sequence Learning is Specific for Spatial Information. Cerebral Cortex 11: 628–35.
- Galea, Joseph M., Neil B. Albert, Thomas Ditye, and R. Chris Miall. 2010. Disruption of the Dorsolateral Prefrontal Cortex Facilitates the Consolidation of Procedural Skills. Journal of Cognitive Neuroscience 22: 1158–64.
- Gann, Mareike A., Bradley R. King, Nina Dolfen, Menno P. Veldman, Kimberly L. Chan, Nicolaas A. J. Puts, Richard A. E. Edden, Marco Davare, Stephan P. Swinnen, Dante Mantini, and et al. 2021. Hippocampal and striatal responses during motor learning are modulated by prefrontal cortex stimulation. NeuroImage 237: 118158.
- Hazeltine, Eliot, Scott T. Grafton, and Richard Ivry. 1997. Attention and stimulus characteristics determine the locus of motor- sequence encoding. A PET study. Brain 120: 123–40.
- Hikosaka, Okihide, Kuniyoshi Sakai, Satoru Miyauchi, Ryousuke Takino, Yuka Sasaki, and Benno Putz. 1996. Activation of human presupplementary motor area in learning of sequential procedures: A functional MRI study. Journal of Neurophysiology 76: 617–21.
- Honda, Manabu, Marie-Pierre Deiber, Vicente Ibánez, Alvaro Pascual-Leone, Ping Zhuang, and Mark Hallett. 1998. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain 121: 2159–73.
- Shimizu, Takahiro, Ritsuko Hanajima, Yuichiro Shirota, Ryosuke Tsutsumi, Nobuyuki Tanaka, Yasuo Terao, Masashi Hamada, and Yoshikazu Ugawa. 2020. Plasticity induction in the pre-supplementary motor area (pre-SMA) and SMA-proper differentially affects visuomotor sequence learning. Brain Stimulation 13: 229–38.
- Verwey, Willem B., Benedikt Glinski, Min-Fang Kuo, Mohammad Ali Salehinejad, and Michael A. Nitsche. 2022. Consolidation of motor sequence learning eliminates susceptibility of SMAproper to TMS: A combined rTMS and cTBS study. Experimental Brain Research 240: 1743–55.
- Nemeth, Dezso, Emese Hallgató, Karolina Janacsek, Timea Sándor, and Zsuzsa Londe. 2009. Perceptual and motor factors of implicit skill learning. NeuroReport 20: 1654–58.
- Song, Sunbin, James H. Howard, and Darlene V. Howard. 2008. Perceptual sequence learning in a serial reaction time task. Experimental Brain Research 189: 145–58.
- Zolnai, Tamás, Dominika Réka Dávid, Orsolya Pesthy, Marton Nemeth, Mariann Kiss, Márton Nagy, and Dezso Nemeth. 2022. Measuring statistical learning by eye-tracking. Experimental Results 3: e10.
- Grafton, Scott T., Eliot Hazeltine, and Richard B. Ivry. 1998. Abstract and Effector-Specific Representations of Motor Sequences Identified with PET. Journal of Neuroscience 18: 9420–28.
- Hikosaka, Okihide, Hiroyuki Nakahara, Miya K. Rand, Katsuyuki Sakai, Xiaofeng Lu, Kae Nakamura, Shigehiro Miyachi, and Kenji Doya. 1999. Parallel neural networks for learning sequential procedures. Trends in Neurosciences 22: 464–71.
- De Vries, Meinou H., Andre C. R. Barth, Sandra Maiworm, Stefan Knecht, Pienie Zwitserlood, and Agnes Flöel. 2010. Electrical Stimulation of Broca’s Area Enhances Implicit Learning of an Artificial Grammar. Journal of Cognitive Neuroscience 22: 2427–36.
- Uddén, Julia, Martin Ingvar, Peter Hagoort, and Karl Magnus Petersson. 2017. Broca’s region: A causal role in implicit processing of grammars with crossed non-adjacent dependencies. Cognition 164: 188–98.
- Reber, Arthur S. 1967. Implicit learning of artificial grammars. Journal of Verbal Learning and Verbal Behavior 6: 855–63.
- Reber, Arthur S. 1989. Implicit learning and tacit knowledge. Journal of Experimental Psychology: General 118: 219–35.
- Nemeth, Dezso, Karolina Janacsek, Gabor Csifcsak, Gabor Szvoboda, James H. Howard, and Darlene V. Howard. 2011. Interference between Sentence Processing and Probabilistic Implicit Sequence Learning. PLoS ONE 6: e17577.
- Clerget, Emeline, William Poncin, Luciano Fadiga, and Etienne Olivier. 2012. Role of Broca’s Area in Implicit Motor Skill Learning: Evidence from Continuous Theta-burst Magnetic Stimulation. Journal of Cognitive Neuroscience 24: 80–92.
- Baetens, Kris, Mahyar Firouzi, Frank Van Overwalle, and Natacha Deroost. 2020. Involvement of the cerebellum in the serial reaction time task (SRT) (Response to Janacsek et al.). NeuroImage 220: 117114.
- Janacsek, Karolina, Kyle F. Shattuck, Kaitlyn M. Tagarelli, Jarrad A. G. Lum, Peter E. Turkeltaub, and Michael T. Ullman. 2020. Sequence learning in the human brain: A functional neuroanatomical meta-analysis of serial reaction time studies. NeuroImage 207: 116387.
- Dirnberger, Georg, Judith Novak, and Christian Nasel. 2013. Perceptual Sequence Learning Is More Severely Impaired than Motor Sequence Learning in Patients with Chronic Cerebellar Stroke. Journal of Cognitive Neuroscience 25: 2207–15.
- Doyon, Julien, Danielle Gaudreau, Robert Laforce Jr., Martin Castonguay, Paul J. Bedard, Francois Bedard, and Jean-Pierre Bouchard. 1997. Role of the Striatum, Cerebellum, and Frontal Lobes in the Learning of a Visuomotor Sequence. Brain and Cognition 34: 218–45.
- Gomez-Beldarrain, Marian, Juan C. Garcia-Monco, Berta Rubio, and Alvaro Pascual-Leone. 1998. Effect of focal cerebellar lesions on procedural learning in the serial reaction time task. Experimental Brain Research 120: 25–30.
- Shin, Jacqueline C., and Richard B. Ivry. 2003. Spatial and Temporal Sequence Learning in Patients with Parkinson’s Disease or Cerebellar Lesions. Journal of Cognitive Neuroscience 15: 1232–43.
- Kóbor, Andrea, Karolina Janacsek, Petra Hermann, Zzófia Zavecz, Virág Varga, Valéria Csépe, Zoltán Vidnyánszki, Gyula Kovacs, and Dezso Nemeth. 2022. Finding pattern in the noise: Persistent implicit statistical knowledge impacts the processing of unpredictable stimuli. PsyArXiv.
- Seidler, Raphael D., Arnie Purushotham, Seong-Gi Kim, Kaamil Ugurbil, Daniel Willingham, and James Ashe. 2002. Cerebellum Activation Associated with Performance Change but Not Motor Learning. Science 296: 2043–46.
- van der Graaf, Ferdinand H. C. E., R. Paul Maguire, Klaus L. Leenders, and Bauke M. de Jong. 2006. Cerebral activation related to implicit sequence learning in a Double Serial Reaction Time task. Brain Research 1081: 179–90.
- Hermsdorf, Franz, Christopher Fricke, Anika Stockert, Joseph Classen, and Jost-Julian Rumpf. 2020. Motor Performance But Neither Motor Learning Nor Motor Consolidation Are Impaired in Chronic Cerebellar Stroke Patients. The Cerebellum 19: 275–85.
- Doppelmayr, Michael, Nils Henrik Pixa, and Fabian Steinberg. 2016. Cerebellar, but not Motor or Parietal, High-Density Anodal Transcranial Direct Current Stimulation Facilitates Motor Adaptation. Journal of the International Neuropsychological Society 22: 928–36.
- Galea, Joseph M., Alejandro Vazquez, Neel Pasricha, Jean-Jacques Orban de Xivry, and Pablo Celnik. 2011. Dissociating the Roles of the Cerebellum and Motor Cortex during Adaptive Learning: The Motor Cortex Retains What the Cerebellum Learns. Cerebral Cortex 21: 1761–70.
- Jayaram, Gowri, Byron Tang, Rani Pallegadda, Erin V. L. Vasudevan, Pablo Celnik, Jason Bouffard, Sauro E. Salomoni, Catherine Mercier, Kylie Tucker, Jean-Sébastien Roy, and et al. 2012. Modulating locomotor adaptation with cerebellar stimulation. Journal of Neurophysiology 107: 2950–57.
- Torriero, Sara, Massimiliano Oliveri, Giacomo Koch, Carlo Caltagirone, and Laura Petrosini. 2004. Interference of Left and Right Cerebellar rTMS with Procedural Learning. Journal of Cognitive Neuroscience 16: 1605–11.
- Hamada, Masashi, Nagako Murase, Alkomiet Hasan, Michelle Balaratnam, and John C. Rothwell. 2013. The Role of Interneuron Networks in Driving Human Motor Cortical Plasticity. Cerebral Cortex 23: 1593–605.
- Maeda, Fumiko, Julian P. Keenan, Jose M. Tormos, Helge Topka, and Alvaro Pascual-Leone. 2000. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Experimental Brain Research 133: 425–30.
- Goldsworthy, Mitchell R., Brenton Hordacre, John C. Rothwell, and Michael C. Ridding. 2021. Effects of rTMS on the brain: Is there value in variability? Cortex 139: 43–59.
- Huang, Ying-Zu, Ming-Kue Lu, Andrea Antal, Joseph Classen, Michael Nitsche, Ulf Ziemann, Michael Ridding, Masashi Hamada, Yoshikazu Ugawa, Shapour Jaberzadeh, and et al. 2017. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clinical Neurophysiology 128: 2318–29.
- Simeoni, Sara, Ricci Hannah, Daisuke Sato, Michiyuki Kawakami, John Rothwell, and Gian Luigi Gigli. 2016. Effects of Quadripulse Stimulation on Human Motor Cortex Excitability: A Replication Study. Brain Stimulation 9: 148–50.
- Tiksnadi, Amanda, Takenobu Murakami, Winnugroho Wiratman, Hideyuki Matsumoto, and Yoshikazu Ugawa. 2020. Direct comparison of efficacy of the motor cortical plasticity induction and the interindividual variability between TBS and QPS. Brain Stimulation 13: 1824–33.
- de Jesus, Danilo R., Gabriela Pereira de Souza Favalli, Sylco S. Hoppenbrouwers, Mera S. Barr, Robert Chen, Paul B. Fitzgerald, and Zafiris J. Daskalakis. 2014. Determining optimal rTMS parameters through changes in cortical inhibition. Clinical Neurophysiology 125: 755–62.
- Rothkegel, Holger, Martin Sommer, and Walter Paulus. 2010. Breaks during 5Hz rTMS are essential for facilitatory after effects. Clinical Neurophysiology 121: 426–30.
- Perez, Monica A., Satoshi Tanaka, Steven P. Wise, Daniel T. Willingham, and Leonardo G. Cohen. 2008. Time-Specific Contribution of the Supplementary Motor Area to Intermanual Transfer of Procedural Knowledge. Journal of Neuroscience 28: 9664–69.
- Andoh, Jamila, and Jean-Luc Martinot. 2008. Interhemispheric compensation: A hypothesis of TMS-induced effects on language-related areas. European Psychiatry 23: 281–88.
- Sack, Alexander T., Joan A. Camprodon, Alvaro Pascual-Leone, and Rainer Goebel. 2005. The Dynamics of Interhemispheric Compensatory Processes in Mental Imagery. Science 308: 702–4.
- Cohen, Daniel A., Alvaro Pascual-Leone, Daniel Z. Press, and Edwin M. Robertson. 2005. Off-line learning of motor skill memory: A double dissociation of goal and movement. Proceedings of The National Academy of Sciences 102: 18237–241.
- Robertson, Edwin M., Alvaro Pascual-Leone, and R. Chris Miall. 2004. Current concepts in procedural consolidation. Nature Reviews Neuroscience 5: 576–82.
- Nissen, Mary Jo, and Peter Bullemer. 1987. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology 19: 1–32.
- Shanks, David R. 2005. Implicit learning. In Handbook of Cognition. Thousand Oaks: SAGE Publications Ltd.
- Howard, Darlene V., James H. Howard, Karin Japikse, Cara DiYanni, Amanda Thompson, and Rachel Somberg. 2004. Implicit Sequence Learning: Effects of Level of Structure, Adult Age, and Extended Practice. Psychology and Aging 19: 79–92.
- Song, Sunbin, James H. Howard, and Darlene V. Howard. 2007. Sleep Does Not Benefit Probabilistic Motor Sequence Learning. Journal of Neuroscience 27: 12475–83.
- Ashe, James, Ovidiu V. Lungu, Alexandra T. Basford, and Xiaofeng Lu. 2006. Cortical control of motor sequences. Current Opinion in Neurobiology 16: 213–21.
- Lum, Jarrad A. G., Andrea Mills, James M. A. Plumridge, Nicole P. Sloan, Gillian M. Clark, Martina Hedenius, and Peter G. Enticott. 2018. Transcranial direct current stimulation enhances retention of a second (but not first) order conditional visuo-motor sequence. Brain and Cognition 127: 34–41.
- Ruitenberg, Marit F. L., Willem B. Verwey, Dennis J. L. G. Schutter, and Elger L. Abrahamse. 2014. Cognitive and neural foundations of discrete sequence skill: A TMS study. Neuropsychologia 56: 229–38.
More
Information
Subjects:
Behavioral Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
571
Revisions:
2 times
(View History)
Update Date:
27 Oct 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




