Your browser does not fully support modern features. Please upgrade for a smoother experience.
Experimental Evidence for Double Quaternary Azeotropy’s Existence
- Subjects: Chemistry, Physical
- |
- Contributors:
- Anastasia Frolkova ,
- Valeriy Zhuchkov ,
- Alla Frolkova
03:31
13:18
04:36
Playlist
03:31
13:18
04:36
- double azeotropy
- phase diagram
- vapor‒liquid equilibrium
- quaternary system
- singular points
- azeotropy rule
Video Introduction
This video is adapted from 10.3390/e25070980
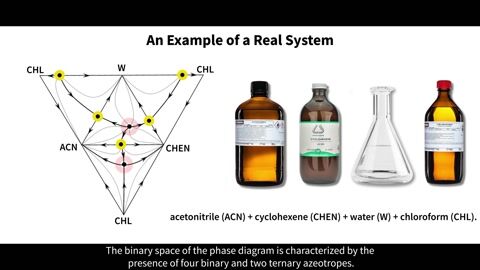
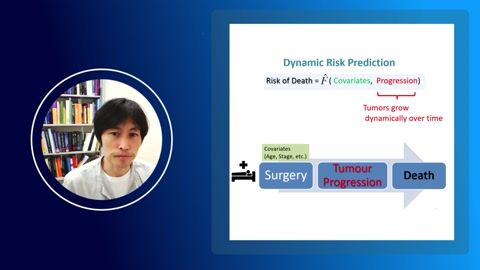
The phase equilibrium in an acetonitrile + water + cyclohexene + chloroform system was studied at 101.3 kPa. A prediction regarding the internal structure of the composition tetrahedron (presence/absence of one/two internal singular points) was made using thermodynamic modeling in AspenPlus V.10.0. The existence of two internal quaternary azeotropes (of node type with a minimum boiling point and of saddle type) was confirmed as a result of a full-scale experiment. Thermodynamic-topological analysis of the structure of phase equilibrium diagrams was carried out to confirm the correctness of the diagram construction.
Full Transcript
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Frolkova, A.; Zhuchkov, V.; Frolkova, A. Experimental Evidence for Double Quaternary Azeotropy’s Existence. Encyclopedia. Available online: https://encyclopedia.pub/video/video_detail/1003 (accessed on 28 February 2026).
Frolkova A, Zhuchkov V, Frolkova A. Experimental Evidence for Double Quaternary Azeotropy’s Existence. Encyclopedia. Available at: https://encyclopedia.pub/video/video_detail/1003. Accessed February 28, 2026.
Frolkova, Anastasia, Valeriy Zhuchkov, Alla Frolkova. "Experimental Evidence for Double Quaternary Azeotropy’s Existence" Encyclopedia, https://encyclopedia.pub/video/video_detail/1003 (accessed February 28, 2026).
Frolkova, A., Zhuchkov, V., & Frolkova, A. (2023, November 27). Experimental Evidence for Double Quaternary Azeotropy’s Existence. In Encyclopedia. https://encyclopedia.pub/video/video_detail/1003
Frolkova, Anastasia, et al. "Experimental Evidence for Double Quaternary Azeotropy’s Existence." Encyclopedia. Web. 27 November, 2023.
Copy Citation