
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María Sonia Rodríguez-Cruz | + 5796 word(s) | 5796 | 2021-04-21 11:13:13 | | | |
| 2 | Lily Guo | Meta information modification | 5796 | 2021-04-29 08:54:58 | | |
Video Upload Options
The management of large volumes of organic residues generated in different livestock, urban, agricultural and industrial activities is a topic of environmental and social interest. The high organic matter content of these residues means that their application as soil organic amendments in agriculture is considered one of the more sustainable options, as it could solve the problem of the accumulation of uncontrolled wastes while improving soil quality and avoiding its irreversible degradation. However, the behavior of pesticides applied to increase crop yields could be modified in the presence of these amendments in the soil.
1. Introduction
The use of large quantities of pesticides in today’s intensive agricultural systems is a widespread practice for controlling pests, diseases and weeds. This increases the yield per hectare, ensuring the food supply for the world’s ever-growing population [1][2], which currently stands at over 7.7 billion people, and is estimated to rise above 9.6 billion by 2050, and reach nearly 11 billion around 2100 [3]. The application of a wide range of pesticides is considered a regular and required practice in agriculture, as almost 45% of annual food production is lost due to pest infestation or the competition between crops and weeds for soil nutrients [4]. In fact, 3.5 million tons of pesticides are being used, of which 47.5% are herbicides, 29.5% are insecticides, 17.5% are fungicides, and 5.5% are other pesticides [5]. The global pesticide market recorded a value of nearly USD 84.5 billion in 2019, increasing at an annual growth rate of 4.2% since 2015, and it is likely to reach 11.5% with a value of nearly USD 130.7 billion by 2023 [6]. The ten countries consuming the most pesticide in the world are China, USA, Argentina, Thailand, Brazil, Italy, France, Canada, Japan, and India [7].
However, this extensive use of pesticides over recent decades is now of considerable environmental concern because of the release of mobile and/or persistent pollutants into the environment, and the potential accumulation of these toxic substances in soils and/or waters [8][9][10]. The fate of pesticides and their degradation products determines the contamination of the soil, water and air ecosystems over time. Moreover, if agrochemicals remain in the crops, they could finally enter the food chain, posing a threat to human, animal, and plant welfare [11][12][13][14].
The contamination of agricultural soils with pesticides could lead to changes in their chemical and biological properties, affecting their quality and causing a negative impact on crop yields [15]. They may impair soil microbial biodiversity and enzymatic activity (a vital indicator of soil tolerance to pollutants), and the associated degradation of soil organic matter (OM) [16][17]. Many reports are available on these negative effects on soil microbial communities [17][18], and on the processes associated with microbial activities [19].
A recent study involving 317 agricultural topsoil samples from the European Union and 76 pesticide residues as target compounds has revealed that 83% of the soils have been contaminated by one or more residues [9]. The contamination of surface and ground waters by pesticides has also been detected in recent years, probably due to deficient pesticide management, and increased by precipitation and/or irrigation that give rise to the runoff or leaching process of these compounds through the soil [20][21][22][23]. In fact, the contamination of water by pesticides is increasing in agricultural areas across different countries, and a broad range of pesticide concentrations has been found, in some cases exceeding the limit established for drinking water by European Union (EU) legislation (0.1 µg·L−1) [24][25][26].
These environmental contamination data highlight the need to roll out strategies to optimize agricultural sustainability by maximizing crop productivity and reducing or preventing soil and water contamination by pesticides. This has been widely addressed in recent years due to the requirement to meet European Community regulations [27]. One of these strategies is based on the in-situ application of organic residues as organic amendments [28]. This method is a common agricultural practice which allows increasing soil OM content, and it can be used to control soil and water contamination by pesticides: (i) promoting the immobilization of pesticides in soil OM, enhancing their subsequent biodegradation, and preventing or reducing their potential mobility into water resources [28][29][30], and (ii) delivering nutrients to the soil by increasing OM content to promote soil fertility and plant growth and stimulate ecological restoration with concomitant benefits for the health of the soil ecosystem [11]. In addition, organic materials require minimal pre-treatment before their application to the soil because of their biological origin [31].
Large amounts of organic residues are generated from livestock, urban, agricultural and industrial activities, and their management is a topic of environmental and social interest in many countries today due to the problems surrounding their disposal [32][33]. In general, these wastes have a high OM content, and they could be used as organic amendments in agriculture, with this being one of the most sustainable options and with greater environmental advantages. Moreover, numerous organic residues could perform as possible sorbents for pesticides [34][35][36]. These studies have assessed the effects that organic carbon (OC) from exogenous sources have on the behavior and environmental fate of pesticides in soils due to the affinity of pesticides, which are generally hydrophobic substances, by these organic materials. The OC of the amendments, depending on their nature, composition and content, can modify the main physicochemical processes of pesticides (adsorption–desorption, dissipation and leaching) in soils. These processes determine their efficiency as well as the dissipation or persistence of these compounds in the soil and their effects as potential environmental contaminants of the soil and surface or ground waters [37].
2. Organic Residues as Soil Amendments
2.1. Origin, Characteristics and Impact on Soil Properties
2.2. European Legislation on the Use of Organic Residues as Soil Amendments
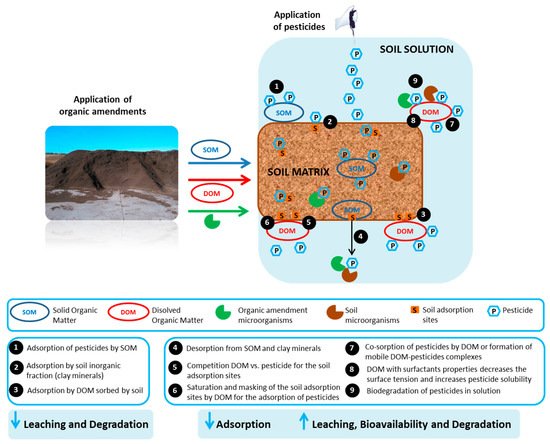
3. Effect of Organic Residues on the Fate of Pesticides in Soil
3.1. Effect of Organic Residues on the Adsorption-Desorption of Pesticides
| Pesticide | Soil Characteristics | Organic Amendment/Dose | Experimental Design | Results | Reference |
|---|---|---|---|---|---|
| Metalaxyl-M | Silt loam soil (pH 6.70, OC 2.90%) |
Biochar from grape vine pruning residues (BC–G) (pH 9.9, OC 75.1%) and spruce wood (BC–S) (pH 9.1, OC 83.8%). Vermicomposts (VC) from manure and olive mill wastewater (VC–M) (pH 7.9, OC 31.6%) and buffalo manure (VC–B) (pH 7.8, OC 36.6%) Biochar/soil: 2% (w w–1) |
Sorbent/Solution: 25 mg biochar/5 mL or 3 g soil/8 mL water solution Herbicide concentration: 1–20 mg L−1 Shaken: 24 h, T: 20 °C Analytical determination: HPLC |
Metalaxyl sorption order: non–amended soil < soil–VC–M ≤ soil–VC–B < soil–BC–S < soil–BC–G Much higher sorption efficiency by BC than by VC and a lower extent of metalaxyl desorption due to composition and structural differences of the organic matter of BC. |
Parlavecchia et al. [103] |
| Oxyfluorfen | Loamy clay soil (pH 4.85, OC 0.84%) Sandy loam soil (pH 7.55, OC 0.98%) Clay loam soil (pH 6.59, OC 2.23%) |
Biochar from peanut (BCP) (pH, 7.05, C 49.17%), chestnut (BCC) (pH 6.08, C 58.07%), bamboo (BCB) (pH 7.45, C 63.25%), maize straw (BCM) (pH 6.83, C 43.36%), rice hull (BCR) (pH 6.96, C 33.60%) BCR/soil: 0.5%, 1%, or 2% (w w–1) |
Sorbent/Solution: 0.1 g biochar/40 mL or 2 g soil/200 mL 0.01 M CaCl2 Herbicide concentration: 0.05–10 mg L−1 Shaken: 6 days, T: 25 °C Aging time of BCR-soil: 1, 3, 6 months Analytical determination: GC/MS |
BC sorption capacities followed the order: BCR > BCB > BCM > BCC > BCP owing to differences in physicochemical properties. BCR sorption capacity decreased with aging time. |
Wu et al. [89] |
| Atrazine | Krasnozem soil (pH 7.05, OC 0.89%, clay 28.2%, silt 37.8%) |
Biochar from cassava wastes (pH 9.55, C 62.38%) obtained at 750 °C (MS750). SSA: 430.4 m2/g, MP: 0.144 m3/g Biochar/soil: 0%, 0.1%, 0.5%, 1%, 3% and 5% (w w–1) |
Sorbent/Solution: 0.2–2 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.5–20 mg L−1 Shaken: 24 h T: 15, 25, 35 °C, pH: 3,5, 7, 9 Analytical determination: HPLC |
Great sorption capacity for atrazine of MS750 in soil due to high surface area and micropore volume. High degrees of aromaticity and hydrophobicity (H/C: 0.02, N + O/C: 0.09) of MS750 supplied numerous sorption sites. | Deng et al. [104] |
| Hexazinone Metribuzin Quinclorac |
Sandy loam soil (pH 6.9, OC 0.52%, clay 15.1%, silt 3.3%) |
Bone char (BC) (pH 9.72, C 11%) BC/soil: 5% (w w–1) or 60 t ha−1 |
Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.63–3.13 mgL−1, 1.60–8 mgL−1, 0.31–1.56 mgL−1 Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation |
High sorption of herbicides by BC, regardless of the application form of the material (topsoil or incorporated in the surface layer in leaching columns). | Mendes et al. [105] |
| Aminocyclopyrachlor Mesotrione |
Clay soil (pH 6.44, OC 2.73%), clay 50.9%, silt 19.6%) |
Bone Char (BC) (pH 9.72, C 11%) BC/soil: 0%, 1%, 5%, 10%, and 100% (w w−1) or 0, 12, 60, 120, and 1200 t ha−1 BC particle size groups: 0.3–0.6 and 0.15–0.3 mm |
Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.051 mg L−1 (0.32 Bq L−1) aminocyclopyrachlor 5.0 mg L−1 (1.13 Bq L−1) mesotrione Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation |
Higher BC rates (regardless of the particle size) increased both herbicides adsorption and decreased their desorption. | Mendes et al. [106] |
| Linuron Alachlor Metalaxyl |
Sandy loam soil (pH 6.3, OC 0.51%, clay 11.8, silt 13.6%), Sandy clay soil (pH 6.9, OC 1.04%, clay 38.1%, silt 5.8%) |
Pine Wood (OC 41.6%, DOM 1.62%, lignin 24.4%), oak wood (OC 38.5%, DOM 6.86%, lignin 18.2%) Wood/soil: 5% and 50% (w w–1) (40 and 400 t C ha–1) |
Sorbent/Solution: 5 g/10 mL water solution Herbicide concentration: 1–25 mg L−1 (100 kBq L−1) Shaken: 24 h, T: 20 °C Incubation times: 0, 5 and 12 months Analytical determination: Liquid scintillation |
Pesticide adsorption increased with high wood dose but OC nature was not relevant. Adsorption did not change after incubation times. The adsorption irreversibility decreased in presence of wood for alachlor and increased that of linuron and metalaxyl. | Marín–Benito et al. [107] |
| Aminocyclopyrachlor Mesotrione |
Clay soil (pH 6.0, OC 2.21%, clay 60.5%, silt 11.3%) |
Sewage sludge (SS) (pH 6.8, OC 16.64%) SS/soil: 0.1%, 1%, and 10% (w∙w–1) or 1.2, 12, and 120 t∙ha–1 |
Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.08–0.64 Bq·L−1 (aminocyclopyrachlor) 0.28–2.27 Bq·L−1 (mesotrione) Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation |
SS slightly affected sorption–desorption of both herbicides (lowest Kd at soil-SS1%). Kd for mesotrione was ~3.5–fold higher than for aminocyclopyrachlor (higher water solubility). | Mendes et al. [108] |
| Imazapic Atrazine Hexazinone Diuron Metribuzin |
Red Ferrusol (pH 7.1, OC 2.1%, clay 41%, silt 23%), Grey Dermosol (pH 5.7, OC 0.9%, clay 30%, silt 22%), Red Kandosol (pH 6.5, OC 3.5%, clay 22%, silt 8%) |
Eleven mill muds/ash from different sugar mills (pH 6.04–7.26, OC 27.7–37.8%) Mill muds/soil: 5–25% (w w–1) |
Sorbent/Solution: 1 g/5 mL 0.01 M CaCl2 Herbicide concentration: 0.5 mg L−1 Shaken: 24 h, T: 25 °C Analytical determination: Q-TOF |
Sorption order: diuron > atrazine = metribuzin > hexazinone = imazapic (consistent with herbicide properties). Mill muds at 5% dose increased herbicide retention up to tenfold. Amendments reduced desorption of mobile herbicides in low OC soils. | Duhan et al. [109] |
| MCPA Diuron Clomazone Terbuthylazine |
Sandy loam soil (pH 7.93, OC 0. 54%, clay 6.7%, silt 16.8%) Loam soil (pH 6.77, OC 1.77%, clay 22.1%, silt 34.2%) Clay loam soil (pH 8.14, OC 1.38%, clay 31.1%, silt 26.8%) |
Mucilage extracted from chia seeds (Salvia hispanica L.) Organic residue/soil: 10% (w w–1) |
Sorbent/Solution: 0.5 g unamended or amended soil/8 mL water solution Herbicide concentration: 1 mg L−1 Shaken: 24 h, T: 20 °C Analytical determination: HPLC |
Soil porosity decreased by mucilage amendment. Sorption of herbicides increased in amended soils (sandy–loam < loam < clay–loam). Diuron recorded the highest Kd value and desorption was observed only for terbuthylazine. | Marsico et al. [110] |
| Dichlorvos Chlorpyrifos |
Sandy soil (pH 8.52, OC 0.7%, clay + silt 9.3%) | Compost (C) from mixed wastes (pH 6.61, OC 29.5%, DOM 354 mg L−1), and dried goat organic manure (OM) (pH 8.67, OC 14.4%, DOM 620 mg L−1) Organic residues/soil: 2.5 and 5% (w w–1) |
Sorbent/Solution: 5 g soil/100 mL in C-DOM or 0.01 M CaCl2 Herbicide concentration: 0.1–10 mg L−1 (chlorpyrifos) 0.25–100 mg L−1 (dichlorvos) Shaken: 24 h, T: 25 °C Analytical determination: GC |
C–and OM–DOM increased dichlorvos sorption (S < S–OM–DOM< S–C–DOM) and decreased chlorpyrifos sorption (S > S–C–DOM> S–OM–DOM). Humified and aromatic nature of DOM determines the interactions with pesticides with different hydrophobic character. | Gaonkar et al. [111] |
| Triasulfuron Prosulfocarb Chlorotoluron Flufenacet |
Sandy loam soil (pH 7.36, OC 1.20%, clay 17%, silt 25%) Loamy sand soil (pH 7.61, OC 0.9%, clay 13%, silt 6%) |
Spent mushroom substrate (pH 7.9, C 26.4%, DOM 1.29%), green compost (pH 7.2, C 23.6%, DOM 0.69%), manure (C 18.5%, DOM 1.32%), sewage sludge (pH 7.6, C 28.9%, DOM 1.18%) Organic residues/soils: 10% (w w–1) |
Sorbent/Solution: 5 g soil or 0.1 g organic residues/10 mL 0.01 M CaCl2 Herbicide concentration: 1–25 mg L−1 (TSF, CTL, FNC) 0.25–10 mg L−1 (100 Bq mL−1) (PSC) Shaken: 24 h, T: 20 °C Analytical determination: HPLC/MS and Liquid scintillation |
Highest adsorption for prosulfocarb (lowest water solubility and highest Kow) in all materials. Aliphatic and aromatic structures optimize adsorption and O-alkyl and N-alkyl groups enhance desorption hysteresis. |
References
- Imfeld, G.; Vuilleumier, S. Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur. J. Soil Biol. 2012, 49, 22–30.
- Nyamwasa, I.; Li, K.; Rutikanga, A.; Rukazambuga, D.N.T.; Zhang, S.; Yin, J.; Ya-Zhong, C.; Zhang, X.X.; Sun, X. Soil insect crop pests and their integrated management in East Africa: A review. Crop Prot. 2018, 106, 163–176.
- United Nations. Global Issues. Our Global Population. 2020. Available online: (accessed on 10 September 2020).
- Ahmad, A.; Shahid, M.; Khalid, S.; Zaffar, H.; Naqvi, T.; Pervez, A.; Bilal, M.; Ali, M.A.; Abbas, G.; Nasim, W. Residues of endosulfan in cotton growing area of Vehari, Pakistan: An assessment of knowledge and awareness of pesticide use and health risks. Environ. Sci. Pollut. 2018, 26, 20079–20091.
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Preet, G.; Sidhu, S.; Handa, N. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1–16.
- Pesticide Reports. Pesticides Global Market Opportunities and Strategies to 2023. 2020. Available online: (accessed on 10 September 2020).
- Worldatlas. 2018. Available online: (accessed on 10 September 2020).
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613–614, 361–370.
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality. Sci. Total Environ. 2019, 653, 1532–1545.
- Picó, Y.; Alvarez-Ruiz, R.; Alfarhan, A.H.; El-Sheikh, M.A.; Alshahrani, H.O.; Barceló, D. Pharmaceuticals, pesticides, personal care products and microplastics contamination assessment of Al-Hassa irrigation network (Saudi Arabia) and its shallow lakes. Sci. Total Environ. 2020, 701, 135021.
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. 2009, 16, 765–794.
- Sánchez-González, S.; Pose-Juan, E.; Herrero-Hernández, E.; Álvarez-Martín, A.; Sánchez-Martín, M.J.; Rodríguez-Cruz, S. Pesticide residues in groundwaters and soils of agricultural areas in the Águeda River Basin from Spain and Portugal. Int. J. Environ. Anal. Chem. 2013, 93, 1585–1601.
- Pose-Juan, E.; Sánchez-Martín, M.J.; Andrades, M.S.; Rodríguez-Cruz, M.S.; Herrero-Hernández, E. Pesticide residues in vineyard soils from Spain: Spatial and temporal distributions. Sci. Total Environ. 2015, 514, 351–358.
- Herrero-Hernández, E.; Pose-Juan, E.; Sánchez-Martín, M.J.; Andrades, M.S.; Rodríguez-Cruz, M.S. Intra–annual trends of fungicide residues in waters from vineyard areas in La Rioja region of northern Spain. Environ. Sci. Pollut. Res. 2016, 23, 22924–22936.
- AL-Ahmadi, M.S. Pesticides, anthropogenic activities, and the health of our environment safety. IntechOpen 2019.
- Baxter, J.; Cummings, S.P. The degradation of the herbicide bromoxynil and its impact on bacterial diversity in a top soil. J. Appl. Microbiol. 2008, 104, 1605–1616.
- Arora, S.; Sahni, D.; Sehgal, M.; Srivastava, D.; Singh, A. Pesticides use and its effect on soil bacteria and fungal populations; microbial biomass carbon and enzymatic activity. Curr. Sci. 2019, 116, 643–649.
- Satapute, P.; Kamble, M.V.; Adhikari, S.S.; Jogaiah, S. Influence of triazole pesticides on tillage soil microbial populations and metabolic changes. Sci. Total Environ. 2019, 651, 2334–2344.
- Zhang, Q.; Saleem, M.; Wang, C. Effects of biochar on the earthworm (Eisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci. Total Environ. 2019, 671, 52–58.
- Li, Y.; Niu, J.; Shen, Z.; Zhang, C.; Wang, Z.; He, T. Spatial and seasonal distribution of organochlorine pesticides in the sediments of the Yangtze Estuary. Chemosphere 2014, 114, 233–240.
- Herrero-Hernández, E.; Rodríguez-Cruz, M.S.; Pose-Juan, E.; Sánchez-González, S.; Andrades, M.S.; Sánchez-Martín, M.J. Seasonal distribution of herbicide and insecticide residues in the water resources of the vineyard region of La Rioja (Spain). Sci. Total Environ. 2017, 609, 161–171.
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162.
- Barizon, R.R.M.; Figueiredo, R.O.; de Souza Dutra, D.R.C.; Regitano, J.B.; Ferracini, V.L. Pesticides in the surface waters of the Camanducaia River watershed, Brazil. J. Environ. Sci. Health B 2020, 55, 283–292.
- Kapsi, M.; Tsoutsi, C.; Paschalidou, A.; Albanis, T. Environmental monitoring and risk assessment of pesticide residues in surface waters of the Louros River (N.W. Greece). Sci. Total Environ. 2019, 650, 2188–2198.
- Herrero-Hernández, E.; Simón-Egea, A.B.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Andrades, M.S. Monitoring and environmental risk assessment of pesticide residues and some of their degradation products in natural waters of the Spanish vineyard region included in the Denomination of Origin Jumilla. Environ. Pollut. 2020, 264, 114666.
- Marsala, R.Z.; Capri, E.; Russo, E.; Bisagni, M.; Colla, R.; Lucini, L.; Gallo, A.; Suciu, N.A. First evaluation of pesticides occurrence in groundwater of Tidone Valley, an area with intensive viticulture. Sci. Total Environ. 2020, 736, 139730.
- Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. Off. J. Eur. Union L 2008, 348, 84–97.
- Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Impact of spent mushroom substrates on the fate of pesticides in soil, and their use for preventing and/or controlling soil and water contamination: A review. Toxics 2016, 4, 17.
- Álvarez-Martín, A.; Rodríguez-Cruz, M.S.; Andrades, M.S.; Sánchez-Martín, M.J. Application of a biosorbent to soil: A potential method for controlling water pollution by pesticides. Environ. Sci. Pollut. Res. 2016, 23, 9192–9203.
- Khorram, M.S.; Zhang, Q.; Lin, D.; Zheng, Y.; Fang, H.; Yu, Y. Biochar: A review of its impact on pesticide behavior in soil environments and its potential applications. J. Environ. Sci. 2016, 44, 269–279.
- Barrow, C.J. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28.
- Dickerson, G. A Sustainable Approach to Recycling Urban and Agricultural Organic Wastes; Guide H–159, Cooperative Extension Service; College of Agriculture and Home Economics: Belen, NM, USA, 2000.
- Moral, R.; Moreno-Caselles, J.; Perez-Murcia, M.; Perez-Espinosa, A.; Rufete, B.; Paredes, C. Characterization of the organic matter pool in manures. Bioresour. Technol. 2005, 96, 153–158.
- Marín-Benito, J.M.; Andrades, M.S.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in the sorption-desorption of fungicides over time in an amended sandy clay loam soil under laboratory conditions. J. Soils Sediments 2012, 12, 1111–1123.
- Rodríguez-Cruz, M.S.; Herrero-Hernández, E.; Ordax, J.M.; Marín-Benito, J.M.; Draoui, K.; Sánchez-Martín, M.J. Adsorption of pesticides by sewage sludge, grape marc, spent mushroom substrate and by amended soils. Int. J. Environ. Anal. Chem. 2012, 92, 933–948.
- Castillo, J.M.; Beguet, J.; Martin-Laurent, F.; Romero, E. Multidisciplinary assessment of pesticide mitigation in soil amended with vermicomposted agroindustrial wastes. J. Hazard. Mater. 2016, 304, 379–387.
- Worrall, F.; Fernandez-Perez, M.; Johnson, A.C.; Flores-Cesperedes, F.; Gonzalez-Pradas, E. Limitations on the role of incorporated organic matter in reducing pesticide leaching. J. Contam. Hydrol. 2001, 49, 241–262.
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A review of the use of organic amendments and the risk to human health. Adv. Agron. 2013, 120, 275–379.
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Plant. Nutr. Soil Sci. 2015, 15, 333–352.
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173–174, 152–161.
- Lugato, E.; Bampa, F.; Panagos, P.; Montanarella, L.; Jones, A. Potential carbon sequestration of European arable soils estimated by modelling a comprehensive set of management practices. Glob. Change Biol. 2014, 20, 3557–3567.
- Hijbeek, R.; Van Ittersum, M.K.; ten Berge, H.F.; Gort, G.; Spiegel, H.; Whitmore, A.P. Do organic inputs matter—A meta–analysis of additional yield effects for arable crops in Europe. Plant Soil 2017, 411, 293–303.
- UNFCCC, United Nations Framework Convention on Climate Change. Join the 4/1000 Initiative. In Soils for Food Security and Climate; UNFCCC: Rio de Janeiro, Brazil; New York, NY, USA, 2015; Available online: (accessed on 10 September 2020).
- Donn, S.; Wheatley, R.E.; McKenzie, B.M.; Loades, K.W.; Hallett, P.D. Improved soil fertility from compost amendment increases root growth and reinforcement of surface soil on slope. Ecol. Eng. 2014, 71, 458–465.
- Singh, R.P.; Singh, P.; Ibrahim, M.H.; Hashim, R. Land Application of sewage sludge: Physicochemical and microbial response. Rev. Environ. Contam. Toxicol. 2011, 214, 41–61.
- Gómez-Sagasti, M.T.; Hernández, A.; Artetxe, U.; Garbisu, C.; Becerril, J.M. How Valuable Are Organic Amendments as Tools for the Phytomanagement of Degraded Soils? The Knowns, Known Unknowns, and Unknowns. Front. Sustain. Food Syst. 2018, 2, 68.
- Fließbach, A.; Oberholzer, H.R.; Gunst, L.; Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ. 2007, 118, 273–284.
- Montiel-Rozas, M.M.; Domínguez, M.T.; Madejón, E.; Madejón, P.; Pastorelli, R.; Renella, G. Long-term effects of organic amendments on bacterial and fungal communities in a degraded Mediterranean soil. Geoderma 2018, 332, 20–28.
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542.
- Li, R.; Khafipour, E.; Krause, D.O.; Entz, M.H.; de Kievit, T.R.; Fernando, W.D. Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PLoS ONE 2012, 7, 51897.
- Memoli, V.; De Marco, A.; Baldantoni, D.; De Nicola, F.; Maisto, G. Short- and long-term effects of a single application of two organic amendments. Ecosphere 2017, 8, e02009.
- Ventorino, V.; de Marco, A.; Pepe, O.; Virzo de Santo, A.; Moschetti, G. Impact of Innovative Agricultural Practices of Carbon Sequestration on Soil Microbial Community. Carbon Sequestration in Agricultural Soils. A Multidisciplinary Approach to Innovative Methods; Piccolo, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–177.
- Abbott, L.K.; Macdonald, L.M.; Wong, M.T.F.; Webb, M.J.; Jenkins, S.N.; Farrell, M. Potential roles of biological amendments for profitable grain production—A review. Agric. Ecosyst. Environ. 2018, 256, 34–50.
- Edmeades, D.C. The long-term effects of manures and fertilizers on soil productivity and quality: A review. Nut. Cycl. Agroecosyst. 2003, 66, 165–180.
- Leroy, B.L.M.; Herath, H.M.S.K.; Sleutel, S.; De Neve, S.; Gabriels, D.; Reheul, D.; Moens, M. The quality of exogenous organic matter: Short-term effects on soil physical properties and soil organic matter fractions. Soil Use Manag. 2008, 24, 139–147.
- Scotti, R.; Conte, P.; Berns, A.E.; Alonzo, G.; Rao, M.A. Effect of organic amendments on the evolution of soil organic matter in soils stressed by intensive agricultural practices. Curr. Org. Chem. 2013, 17, 2998–3005.
- Six, J.; Paustian, K. Aggregate–associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9.
- Ingelmo-Sánchez, F.; Rubio-Delgado, J. Efecto de la aplicación del compost sobre las propiedades físicas y químicas del suelo. In Compostaje; Moreno-Casco, J., Moral-Herrero, R., Eds.; Mundi-Prensa: Madrid, Spain, 2008.
- Hernández, T.; García, E.; García, C. A strategy for marginal semiarid degraded soil restoration: A sole addition of compost at a high rate. A five-year field experiment. Soil Biol. Biochem. 2015, 89, 61–71.
- Zhang, H.; Luo, Y.; Wu, L.; Huang, Y.; Christie, P. Residues and potential ecological risks of veterinary antibiotics in manures and composts associated with protected vegetable farming. Environ. Sci. Pollut. Res. Int. 2015, 22, 5908–5918.
- Pan, M.; Chu, L.M. Leaching behavior of veterinary antibiotics in animal manure-applied soils. Sci. Total Environ. 2017, 579, 466–473.
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833.
- Tao, R.; Li, J.; Hu, B.; Chu, G. Ammonia-oxidizing bacteria are sensitive and not resilient to organic amendment and nitrapyrin disturbances, but ammonia-oxidizing archaea are resistant. Geoderma 2021, 384, 114814.
- EUROSTAT, Statistical Office of the European Communities. Waste Statistics. 2020. Available online: (accessed on 10 September 2020).
- EC, European Commission Decision 2015/2099. Review of Waste Policy and Legislation. 2020. Available online: (accessed on 10 September 2020).
- ECN, European Compost Network. Bio-Waste in Europe. 2020. Available online: (accessed on 10 September 2020).
- EEA, European Environment Agency. Waste Generation in Europe. 2020. Available online: (accessed on 10 September 2020).
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192.
- EC (European Commission). Impact Assessment of the Thematic Strategy on Soil SEC 620. 2006. Available online: (accessed on 10 September 2020).
- Martínez-Blanco, J.; Lazcano, C.; Christensen, T.H.; Muñoz, P.; Rieradevall, J.; Møller, J.; Antón, A.; Boldrin, A. Compost benefits for agriculture evaluated by life cycle assessment. A review. Agron. Sustain. Dev. 2013, 33, 721–732.
- Schreuder, R.; De Visser, C. EIP AGRI Focus Group Protein Crops: Final Report. European Innovation Partnership for Agricultural Productivity and Sustainability (EIP AGRI). Brussels. 2014. Available online: (accessed on 10 September 2020).
- Saveyn, H.; Eder, P. End of Waste Criteria for Biodegradable Waste Subjected to Biological Treatment (Compost and Digestate): Technical Proposal; © European Commission in 2014, EUR 26425; Joint Research Centre—Institute for Prospective Technological Studies: Luxembourg, 2014; p. 310.
- Büyüksönmez, F.; Rink, R.; Hess, T.; Bechinski, E. Occurrence, degradation and fate of pesticides during composting. Part I. Composting, pesticides, and pesticides degradation. Compost Sci. Util. 1999, 7, 66–82.
- Büyüksönmez, F.; Rynk, R.; Hess, T.; Bechinski, E. Occurrence, degradation and fate of pesticides during composting. Part II. Occurrence and fate de pesticides in compost and composting systems. Compost Sci. Util. 2000, 8, 61–81.
- Briceño, G.; Palma, G.; Durán, N. Influence of organic amendment on the biodegradation and movement of pesticides. Crit. Rev. Environ. Sci. Technol. 2007, 37, 233–271.
- Marín-Benito, J.M.; Rodríguez-Cruz, M.S.; Andrades, M.S.; Sánchez-Martín, M.J. Assessment of spent mushroom substrate as sorbent of fungicides: Influence of sorbent and sorbate properties. J. Environ. Qual. 2012, 41, 814–822.
- Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. J. Agric. Food Chem. 2012, 60, 6936–6945.
- Marín-Benito, J.M.; Brown, C.D.; Herrero-Hernández, E.; Arienzo, M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Use of raw or incubated organic wastes as amendments in reducing pesticide leaching through soil columns. Sci. Total Environ. 2013, 463–464, 589–599.
- Marín-Benito, J.M.; Herrero-Hernández, E.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M. Effect of different organic amendments on the dissipation of linuron, diazinon and myclobutanil in an agricultural soil incubated for different time periods. Sci. Total Environ. 2014, 476–477, 611–621.
- Zolgharnein, J.; Shahmoradi, A.; Ghasemi, J. Pesticides removal using conventional and low–cost adsorbents: A review. Clean-Soil Air Water 2011, 39, 1105–1119.
- Garrido, I.; Vela, N.; Fenoll, J.; Navarro, G.; Pérez–Lucas, G.; Navarro, S. Testing of leachability and persistence of sixteen pesticides in three agricultural soils of a semiarid Mediterranean region. Span. J. Agric. Res. 2015, 13, 1104.
- Zhang, G.; Liu, X.; Sun, K.; Zhao, Y.; Lin, C. Sorption of Tetracycline to Sediments and Soils: Assessing the roles of pH, the presence of cadmium and properties of sediments and soils. FESE 2010, 4, 421–429.
- Gavrilescu, M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005, 5, 497–525.
- Jiang, L.; Lin, J.L.; Jia, L.X.; Liu, Y.; Pan, B.; Yang, Y.; Lin, Y. Effects of two different organic amendments addition to soil on sorption-desorption, leaching, bioavailability of penconazole and the growth of wheat (Triticum. aestivum L.). J. Environ. Manag. 2016, 167, 130–138.
- Loffredo, E.; Parlavecchia, M.; Perri, G.; Gattullo, R. Comparative assessment of metribuzin sorption efficiency of biochar, hydrochar and vermicompost. J. Environ. Sci. Health B 2019, 54, 728–735.
- Petter, F.A.; Ferreira, T.S.; Sinhorin, A.P.; Lima, L.B.; Almeida, F.A.; Pacheco, L.P.; Silva, A.F. Biochar increases diuron sorption and reduces the potential contamination of subsurface water with diuron in a sandy soil. Pedosphere 2019, 29, 801–809.
- García-Jaramillo, M.; Trippe, K.M.; Helmus, R.; Knicker, H.E.; Cox, L.; Hermosín, M.C.; Kalbitz, K. An examination of the role of biochar and biochar water-extractable substances on the sorption of ionizable herbicides in rice paddy soils. Sci. Total Environ. 2020, 706, 135682.
- Khan, S.U. Pesticides in the Soil Environment; Elsevier: New, York, NY, USA, 2016.
- Wu, C.; Liu, X.; Wu, X.; Dong, F.; Xu, J.; Zheng, Y. Sorption, degradation and bioavailability of oxyfluorfen in biochar-amended soils. Sci. Total Environ. 2019, 658, 87–94.
- Cox, L.; Cecchi, A.; Celis, R.; Hermosín, M.; Koskinen, W.; Cornejo, J. Effect of exogenous carbon on movement of simazine and 2,4–D in soils. Soil Sci. Soc. Am. J. 2001, 65, 1688–1695.
- Plaza, C.; Polo, A.; Brunetti, G.; Garcia-Gil, J.; D’Orazio, V. Soil fulvic acid properties as a means to assess the use of pig amendment. Soil Till. Res. 2003, 74, 179–190.
- Thorstensen, C.; Lode, O.; Eklo, O.; Christianse, A. Sorption of bentazone, dichlorprop, MCPA, and propiconazole in references soils from Norway. J. Environ. Qual. 2001, 30, 2046–2052.
- Cambier, P.; Pot, V.; Mercier, V.; Michaud, A.; Benoit, P.; Revallier, A.; Houot, S. Impact of long–term organic residue recycling in agriculture on soil solution composition and trace metal leaching in soils. Sci. Total Environ. 2014, 499, 560–573.
- Barriuso, E.; Andrades, M.S.; Benoit, P.; Houot, S. Pesticide desorption from soils facilitated by dissolved organic matter coming from composts: Experimental data and modelling approach. Biogeochemistry 2011, 106, 117–133.
- Huang, X.; Lee, S. Effects of dissolved organic matter from animal waste effluent on chlorpyrifos sorption by soils. J. Environ. Qual. 2001, 30, 1258–1265.
- Álvarez-Martín, A.; Sánchez-Martín, M.J.; Ordax, J.M.; Marín-Benito, J.M.; Rodríguez-Cruz, M.S. Leaching of two fungicides in spent mushroom substrate amended soil: Influence of amendment rate; fungicide ageing and flow condition. Sci. Total Environ. 2017, 584–585, 828–837.
- Rodríguez-Liébana, J.A.; Peña, A. Adsorption-desorption of dimethenamid and fenarimol onto three agricultural soils as affected by treated wastewater and fresh sewage sludge-derived dissolved organic carbon. J. Environ. Manag. 2018, 217, 592–599.
- Wanner, U.; Führ, F.; Burauel, P. Influence of the amendment of corn straw on the degradation behaviour of the fungicide dithianon in soil. Environ. Pollut. 2005, 133, 63–70.
- Wang, H.; Lin, K.; Hou, Z.; Richardson, B.; Gan, J. Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J. Soils Sediments 2010, 10, 283–289.
- Thevenot, M.; Dousset, S. Compost effect on diuron retention and transport in structured vineyard soils. Pedosphere 2015, 25, 25–36.
- Li, K.; Xing, B.S.; William, A.T. Effect of organic fertilizers derived dissolved organic matter on they sorption and leaching. Environ. Pollut. 2005, 134, 187–194.
- Spark, K.M.; Swift, R.S. Effect of soil composition and dissolved organic matter on pesticide sorption. Sci. Total Environ. 2002, 298, 147–161.
- Parlavecchia, M.; Orazio, V.D.; Loffredo, E. Wood biochars and vermicomposts from digestate modulate the extent of adsorption-desorption of the fungicide metalaxyl-m in a silty soil. Environ. Sci. Pollut. Res. Int. 2019, 26, 35924–35934.
- Deng, H.; Feng, D.; He, J.; Li, F.; Yu, H.; Ge, C. Influence of biochar amendments to soil on the mobility of atrazine using sorption-desorption and soil thin-layer chromatography. Ecol. Eng. 2017, 99, 381–390.
- Mendes, K.F.; de Sousa, R.N.; Takeshita, V.; Alonso, F.G.; Régo, A.P.J.; Tornisielo, V.L. Cow bone char as a sorbent to increase sorption and decrease mobility of hexazinone, metribuzin, and quinclorac in soil. Geoderma 2019, 343, 40–49.
- Mendes, K.F.; Hall, K.E.; Takeshita, V.; Rossi, M.L.; Tornisielo, V.L. Animal bonechar increases sorption and decreases leaching potential of aminocyclopyrachlor and mesotrione in a tropical soil. Geoderma 2018, 316, 11–18.
- Marín-Benito, J.M.; Herrero-Hernández, E.; Rodríguez-Cruz, M.S.; Arienzo, M.; Sánchez-Martín, M.J. Study of processes influencing bioavailability of pesticides in wood-soil systems: Effect of different factors. Ecotoxicol. Environ. Saf. 2017, 139, 454–462.
- Mendes, K.F.; Alonso, F.G.; Mertens, T.B.; Inoue, M.; Oliveira, M.G.D.; Tornisielo, V.L. Aminocyclopyrachlor and mesotrione sorption-desorption in municipal sewage sludge-amended soil. Soil Plant Nutr. 2019, 78, 131–140.
- Duhan, A.; Oliver, D.P.; Rashti, M.R.; Du, J.; Kookana, R.S. Organic waste from sugar mills as a potential soil ameliorant to minimize herbicide runoff to the Great Barrier Reef. Sci. Total Environ. 2020, 713, 136640.
- Di Marsico, A.; Scrano, L.; Amato, M.; Gàmiz, B.; Real, M.; Cox, L. Mucilage from seeds of chia (Salvia hispanica L.) used as soil conditioner, effects on the sorption-desorption of four herbicides in three different soils. Sci. Total Environ. 2018, 625, 531–538.
- Gaonkar, O.D.; Nambi, I.M.; Govindarajan, S.K. Soil organic amendments: Impacts on sorption of organophosphate pesticides on an alluvial soil. J. Soils Sediments 2019, 19, 566–578.
- Xing, B. Sorption of naphthalene and phenanthrene by soil humic acids. Environ. Pollut. 2001, 111, 303–309.
- Wang, T.; Zhang, Z.; Zhang, H.; Zhong, X.; Liu, Y.; Liao, S.; Yuea, X.; Zhouc, G. Sorption of carbendazim on activated carbons derived from rape straw and its mechanism. RSC Adv. 2019, 9, 41745–41754.
- García-Delgado, C.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Organic carbon nature determines the capacity of organic amendments to adsorb pesticides in soil. J. Hazard. Mater. 2020, 390, 122162.





