The management of large volumes of organic residues generated in different livestock, urban, agricultural and industrial activities is a topic of environmental and social interest. The high organic matter content of these residues means that their application as soil organic amendments in agriculture is considered one of the more sustainable options, as it could solve the problem of the accumulation of uncontrolled wastes while improving soil quality and avoiding its irreversible degradation. However, the behavior of pesticides applied to increase crop yields could be modified in the presence of these amendments in the soil.
- soil amendment
- organic matter
1. Introduction
2. Organic Residues as Soil Amendments
2.1. Origin, Characteristics and Impact on Soil Properties
2.2. European Legislation on the Use of Organic Residues as Soil Amendments
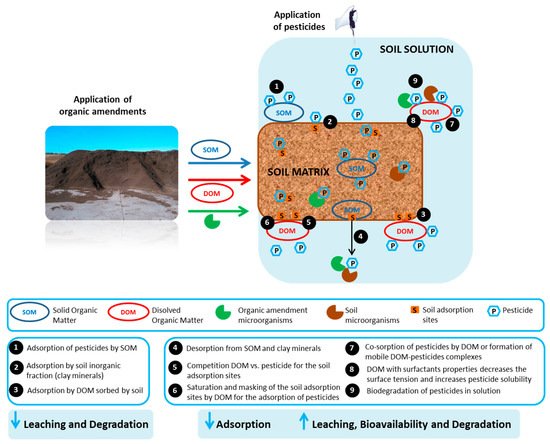
3. Effect of Organic Residues on the Fate of Pesticides in Soil
3.1. Effect of Organic Residues on the Adsorption-Desorption of Pesticides
| Pesticide | Soil Characteristics | Organic Amendment/Dose | Experimental Design | Results | Reference |
|---|---|---|---|---|---|
| Metalaxyl-M | Silt loam soil (pH 6.70, OC 2.90%) |
Biochar from grape vine pruning residues (BC–G) (pH 9.9, OC 75.1%) and spruce wood (BC–S) (pH 9.1, OC 83.8%). Vermicomposts (VC) from manure and olive mill wastewater (VC–M) (pH 7.9, OC 31.6%) and buffalo manure (VC–B) (pH 7.8, OC 36.6%) Biochar/soil: 2% (w w–1) |
Sorbent/Solution: 25 mg biochar/5 mL or 3 g soil/8 mL water solution Herbicide concentration: 1–20 mg L−1 Shaken: 24 h, T: 20 °C Analytical determination: HPLC |
Metalaxyl sorption order: non–amended soil < soil–VC–M ≤ soil–VC–B < soil–BC–S < soil–BC–G Much higher sorption efficiency by BC than by VC and a lower extent of metalaxyl desorption due to composition and structural differences of the organic matter of BC. |
Parlavecchia et al. [103] |
| Oxyfluorfen | Loamy clay soil (pH 4.85, OC 0.84%) Sandy loam soil (pH 7.55, OC 0.98%) Clay loam soil (pH 6.59, OC 2.23%) |
Biochar from peanut (BCP) (pH, 7.05, C 49.17%), chestnut (BCC) (pH 6.08, C 58.07%), bamboo (BCB) (pH 7.45, C 63.25%), maize straw (BCM) (pH 6.83, C 43.36%), rice hull (BCR) (pH 6.96, C 33.60%) BCR/soil: 0.5%, 1%, or 2% (w w–1) |
Sorbent/Solution: 0.1 g biochar/40 mL or 2 g soil/200 mL 0.01 M CaCl2 Herbicide concentration: 0.05–10 mg L−1 Shaken: 6 days, T: 25 °C Aging time of BCR-soil: 1, 3, 6 months Analytical determination: GC/MS |
BC sorption capacities followed the order: BCR > BCB > BCM > BCC > BCP owing to differences in physicochemical properties. BCR sorption capacity decreased with aging time. |
Wu et al. [89] |
| Atrazine | Krasnozem soil (pH 7.05, OC 0.89%, clay 28.2%, silt 37.8%) |
Biochar from cassava wastes (pH 9.55, C 62.38%) obtained at 750 °C (MS750). SSA: 430.4 m2/g, MP: 0.144 m3/g Biochar/soil: 0%, 0.1%, 0.5%, 1%, 3% and 5% (w w–1) |
Sorbent/Solution: 0.2–2 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.5–20 mg L−1 Shaken: 24 h T: 15, 25, 35 °C, pH: 3,5, 7, 9 Analytical determination: HPLC |
Great sorption capacity for atrazine of MS750 in soil due to high surface area and micropore volume. High degrees of aromaticity and hydrophobicity (H/C: 0.02, N + O/C: 0.09) of MS750 supplied numerous sorption sites. | Deng et al. [104] |
| Hexazinone Metribuzin Quinclorac |
Sandy loam soil (pH 6.9, OC 0.52%, clay 15.1%, silt 3.3%) |
Bone char (BC) (pH 9.72, C 11%) BC/soil: 5% (w w–1) or 60 t ha−1 |
Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.63–3.13 mgL−1, 1.60–8 mgL−1, 0.31–1.56 mgL−1 Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation |
High sorption of herbicides by BC, regardless of the application form of the material (topsoil or incorporated in the surface layer in leaching columns). | Mendes et al. [105] |
| Aminocyclopyrachlor Mesotrione |
Clay soil (pH 6.44, OC 2.73%), clay 50.9%, silt 19.6%) |
Bone Char (BC) (pH 9.72, C 11%) BC/soil: 0%, 1%, 5%, 10%, and 100% (w w−1) or 0, 12, 60, 120, and 1200 t ha−1 BC particle size groups: 0.3–0.6 and 0.15–0.3 mm |
Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.051 mg L−1 (0.32 Bq L−1) aminocyclopyrachlor 5.0 mg L−1 (1.13 Bq L−1) mesotrione Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation |
Higher BC rates (regardless of the particle size) increased both herbicides adsorption and decreased their desorption. | Mendes et al. [106] |
| Linuron Alachlor Metalaxyl |
Sandy loam soil (pH 6.3, OC 0.51%, clay 11.8, silt 13.6%), Sandy clay soil (pH 6.9, OC 1.04%, clay 38.1%, silt 5.8%) |
Pine Wood (OC 41.6%, DOM 1.62%, lignin 24.4%), oak wood (OC 38.5%, DOM 6.86%, lignin 18.2%) Wood/soil: 5% and 50% (w w–1) (40 and 400 t C ha–1) |
Sorbent/Solution: 5 g/10 mL water solution Herbicide concentration: 1–25 mg L−1 (100 kBq L−1) Shaken: 24 h, T: 20 °C Incubation times: 0, 5 and 12 months Analytical determination: Liquid scintillation |
Pesticide adsorption increased with high wood dose but OC nature was not relevant. Adsorption did not change after incubation times. The adsorption irreversibility decreased in presence of wood for alachlor and increased that of linuron and metalaxyl. | Marín–Benito et al. [107] |
| Aminocyclopyrachlor Mesotrione |
Clay soil (pH 6.0, OC 2.21%, clay 60.5%, silt 11.3%) |
Sewage sludge (SS) (pH 6.8, OC 16.64%) SS/soil: 0.1%, 1%, and 10% (w∙w–1) or 1.2, 12, and 120 t∙ha–1 |
Sorbent/Solution: 10 g/10 mL 0.01 M CaCl2 Herbicide concentration: 0.08–0.64 Bq·L−1 (aminocyclopyrachlor) 0.28–2.27 Bq·L−1 (mesotrione) Shaken: 24 h, T: 20 °C Analytical determination: Liquid scintillation |
SS slightly affected sorption–desorption of both herbicides (lowest Kd at soil-SS1%). Kd for mesotrione was ~3.5–fold higher than for aminocyclopyrachlor (higher water solubility). | Mendes et al. [108] |
| Imazapic Atrazine Hexazinone Diuron Metribuzin |
Red Ferrusol (pH 7.1, OC 2.1%, clay 41%, silt 23%), Grey Dermosol (pH 5.7, OC 0.9%, clay 30%, silt 22%), Red Kandosol (pH 6.5, OC 3.5%, clay 22%, silt 8%) |
Eleven mill muds/ash from different sugar mills (pH 6.04–7.26, OC 27.7–37.8%) Mill muds/soil: 5–25% (w w–1) |
Sorbent/Solution: 1 g/5 mL 0.01 M CaCl2 Herbicide concentration: 0.5 mg L−1 Shaken: 24 h, T: 25 °C Analytical determination: Q-TOF |
Sorption order: diuron > atrazine = metribuzin > hexazinone = imazapic (consistent with herbicide properties). Mill muds at 5% dose increased herbicide retention up to tenfold. Amendments reduced desorption of mobile herbicides in low OC soils. | Duhan et al. [109] |
| MCPA Diuron Clomazone Terbuthylazine |
Sandy loam soil (pH 7.93, OC 0. 54%, clay 6.7%, silt 16.8%) Loam soil (pH 6.77, OC 1.77%, clay 22.1%, silt 34.2%) Clay loam soil (pH 8.14, OC 1.38%, clay 31.1%, silt 26.8%) |
Mucilage extracted from chia seeds (Salvia hispanica L.) Organic residue/soil: 10% (w w–1) |
Sorbent/Solution: 0.5 g unamended or amended soil/8 mL water solution Herbicide concentration: 1 mg L−1 Shaken: 24 h, T: 20 °C Analytical determination: HPLC |
Soil porosity decreased by mucilage amendment. Sorption of herbicides increased in amended soils (sandy–loam < loam < clay–loam). Diuron recorded the highest Kd value and desorption was observed only for terbuthylazine. | Marsico et al. [110] |
| Dichlorvos Chlorpyrifos |
Sandy soil (pH 8.52, OC 0.7%, clay + silt 9.3%) | Compost (C) from mixed wastes (pH 6.61, OC 29.5%, DOM 354 mg L−1), and dried goat organic manure (OM) (pH 8.67, OC 14.4%, DOM 620 mg L−1) Organic residues/soil: 2.5 and 5% (w w–1) |
Sorbent/Solution: 5 g soil/100 mL in C-DOM or 0.01 M CaCl2 Herbicide concentration: 0.1–10 mg L−1 (chlorpyrifos) 0.25–100 mg L−1 (dichlorvos) Shaken: 24 h, T: 25 °C Analytical determination: GC |
C–and OM–DOM increased dichlorvos sorption (S < S–OM–DOM< S–C–DOM) and decreased chlorpyrifos sorption (S > S–C–DOM> S–OM–DOM). Humified and aromatic nature of DOM determines the interactions with pesticides with different hydrophobic character. | Gaonkar et al. [112] |
| Triasulfuron Prosulfocarb Chlorotoluron Flufenacet |
Sandy loam soil (pH 7.36, OC 1.20%, clay 17%, silt 25%) Loamy sand soil (pH 7.61, OC 0.9%, clay 13%, silt 6%) |
Spent mushroom substrate (pH 7.9, C 26.4%, DOM 1.29%), green compost (pH 7.2, C 23.6%, DOM 0.69%), manure (C 18.5%, DOM 1.32%), sewage sludge (pH 7.6, C 28.9%, DOM 1.18%) Organic residues/soils: 10% (w w–1) |
Sorbent/Solution: 5 g soil or 0.1 g organic residues/10 mL 0.01 M CaCl2 Herbicide concentration: 1–25 mg L−1 (TSF, CTL, FNC) 0.25–10 mg L−1 (100 Bq mL−1) (PSC) Shaken: 24 h, T: 20 °C Analytical determination: HPLC/MS and Liquid scintillation |
Highest adsorption for prosulfocarb (lowest water solubility and highest Kow) in all materials. Aliphatic and aromatic structures optimize adsorption and O-alkyl and N-alkyl groups enhance desorption hysteresis. |
This entry is adapted from the peer-reviewed paper 10.3390/environments8040032
