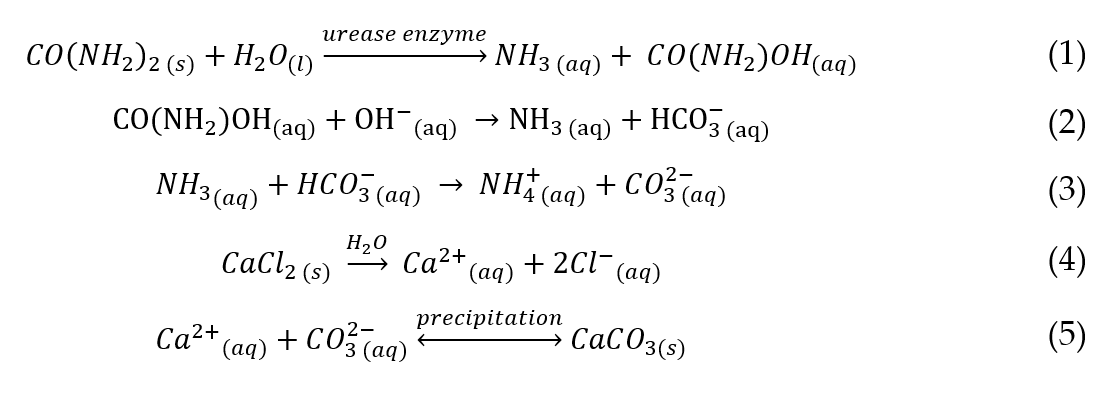| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Isaac Ahenkorah | + 2954 word(s) | 2954 | 2021-03-10 10:25:37 | | | |
| 2 | Lily Guo | -6 word(s) | 2948 | 2021-03-24 04:52:17 | | | | |
| 3 | Lily Guo | -6 word(s) | 2948 | 2021-03-24 05:13:57 | | |
Video Upload Options
Enzyme-induced carbonate precipitation (EICP) is a relatively new bio-cementation technique for ground improvement. In EICP, calcium carbonate (CaCO3) precipitation occurs via urea hydrolysis catalysed by the urease enzyme sourced from plants. EICP offers significant potential for innovative and sustainable engineering applications, including strengthening of soils, remediation of contaminants, enhancement of oil recovery through bio-plugging and other in situ field applications. Given the numerous potential applications of EICP, theoretical understanding of the rate and quantity of precipitation via the ureolytic chemical reaction is vital for optimising the process.
1. Introduction
Enzyme induced carbonate precipitation (EICP) is an innovative ground improvement technique that involves calcium carbonate (CaCO3) precipitation via the hydrolysis of urea (CO(NH2)2) into ammonium (NH4+) and carbonate (CO32) ions catalysed by the urease enzyme. The EICP process has the potential to be applied as bio-cementation and bio-remediation solutions in many environmental, construction, geotechnical and civil engineering problems, such as improving soil strength, reducing soil liquefaction potential, surface erosion control, reducing permeability, heavy metal contaminant remediation and so forth [1][2][3][4][5]. One advantage of EICP is the smaller size of the urease enzyme crystals (typically 12 nm or 120 Å), rendering the process effective for a wider range of soils, including fine-grained soils [6]. However, the cost of EICP treatment can be high. Pure urease enzyme is the most expensive component (~70% to 80% of the total cost) of the chemical ingredients used. Although some studies have used crude urease extract as a cost-effective source of enzyme, some extraction techniques may require additional processes or chemicals and may sometimes yield only a small quantity of urease enzyme. Other drawbacks of the EICP process can be the lack of nucleation sites, meaning that a portion of CaCO3 is precipitated in the pore spaces, which may remain ineffective in binding soil particles. Hence, a sustainable adaptation of EICP as a bio-cementation technique depends on the optimisation of chemical ingredients and curing time (reaction/precipitation time) to reduce construction cost and time.
The catalytic actions of enzymes speed up the process of urea hydrolysis by a factor of millions compared to the rate of an uncatalyzed reaction [7][8][9]. The concentration and activity of the urease enzyme dictate the catalytic mechanism and thus the reaction/precipitation rate. Accordingly, the theoretical understanding of enzyme kinetics is important for controlling and predicting the rate of CaCO3 precipitation. Hence, the catalytic mechanism, structure, function and kinetic properties of the urease enzyme has been a subject of extensive research. Thus, a thorough understanding of enzyme kinetics, which dictates the urea hydrolysis rate that is proportional to the rate of CaCO3 precipitation in ideal conditions is required for developing an effective framework for CaCO3 precipitation in EICP.
2. Biogeochemical Reactions in EICP
2.1. Molecular Structure of Urease Enzyme
Urease enzyme is a nickel-containing metalloenzyme synthesized by some plants, bacteria and fungi [10]. Ureases belong to the superfamily of amidohydrolases and phosphotriesterases, which display catalytic mechanisms in their active sites. In general, ureases contain two Ni2+ ions in their active sites. It has been well-established in the literature that the overall protein scaffold is conserved among ureases from different sources [11]. Urease enzymes in plants and fungi generally consist of homo-oligomeric proteins with identical sub-units compared to the multimeric proteins found in bacterial ureases which are formed from a complex of two (αβ) or three (αβγ) subunits [12]. These proteins appear to act as urease-specific chaperones required for assembling an active urease [13][14][15].
From the literature, the most extensively studied urease enzymes are sourced from Jack bean [16][17]. Other plant species rich in urease include Weeping bottlebrush (Callistemon viminalis), Mulberry (Morus alba), Palo verde (Parkinsonia florida), Pigweed (Chenopodium album), Pigeonpea (Cajanus cajan), Bitter melon seeds (Momordica charantia), Squash seeds (Cucurbitaceae), Soybean (Glycine max), Sword beans (Canavalia gladiata), Watermelon seeds (Citrullus lanatus), Cabbage leaves and Soy pulp [6][18].
In this study, the crystal and molecular structure of urease sourced from Jack bean are investigated. The Jack bean was selected as it is one of the most common sources of urease used in many different studies of the EICP process. Jack bean urease complex with phosphate (PDB: 3LA4) was extracted from the Protein Data Bank (PDB) using the University of California San Francisco (UCSF) Chimera software [19][20]. Figure 1 shows the structural components of the Jack bean urease enzyme, which consists of the N-terminal, C-terminal and α-β domains. The C-terminal (αβ) triose-phosphate isomerase (TIM) barrel domain contains the active site which controls the activity of the enzyme. This activity is largely controlled by the presence of a binuclear Ni complex active site in the β-sheet structure and the dynamic opening and closing of the mobile flap located adjacent to the active site [20][21][22][23][24]. Higher availability of the active site can be achieved during the wider opening of the mobile flap and can result in higher activity [23][25]. The functional unit of ureases from plants is made of six identical subunits, called α subunits, each of which are reported to have a molecular weight of around 90 kDa, making the total molecular weight of a subunit approximately 540 kDa [26][27].
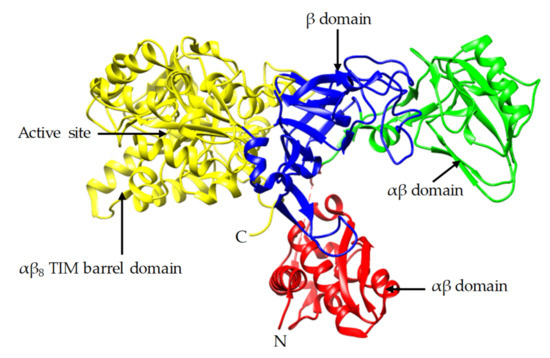
Figure 1. Crystal structure of Jack bean urease (PDB: 3LA4).
2.2. Urease Catalysed Chemical Reactions
The major chemical constituents involved in the EICP process are urease enzyme, urea (CO(NH2)2) and calcium chloride (CaCl2). The chemical reactions involved are presented in Equations (1)–(5) [28].
The chemical reaction which occurs during urea hydrolysis brings about primary geochemical changes, such as an increase in pH and inorganic carbon (Equations (1)–(3)). The geochemical changes in the EICP process are dynamic and dependent on the ratios and concentration of the chemical constituents used. For example, the precipitation of occurs in the presence of which shifts the carbonate equilibrium reaction from to to in a suitable alkaline environment (pH 8.0 to 9.0) [5].
The use of non-equimolar quantities of urea and may result in an increase or decrease in ammonia ( ) release which affects the alkalinity (pH) of the chemical environment [5]. For example, a high urea concentration may result in an increase in alkalinity due to the abundance of in the absence of . Hence, becomes the limiting reagent in the reaction[5]. In contrast, there may be a reduction in alkalinity with high concentration and there may be an excess of available in the system with a limited amount of carbonate ions[5].
It has been reported in the literature that the presence of the urease enzyme accelerates the urea hydrolysis and reaction speed up to 1014 times compared to the rate of the uncatalyzed reaction [5][29]. The activity of the urease enzyme is usually expressed in Units (U), defined as the amount of enzyme required to hydrolyse 1 µmol urea per minute at a pH of 7.0 and at a temperature of 25 ºC to produce and [6].
3. Applications of Enzyme Kinetic Models in EICP
In relation to the Michaelis-Menten Equation [10], a similar enzymatic reaction occurs during urea hydrolysis in EICP where urea interacts with the enzyme at a constant rate. The concentration and activity of urease enzyme can be used to develop a relation to determine the catalytic rate during urea hydrolysis for a given urea concentration. However, the reaction products (i.e., NH4+ and CO32-) can exist in different forms in an aqueous solution, which may affect the reaction rate. In EICP, the addition of calcium salt, changes in the geochemical environment (pH, temperature, heavy metals etc.) of soils/concrete may significantly affect the catalytic rate/kinetic parameters [30]. However, the influence of urease enzyme concentration and activity, geochemical environment and so forth on the catalytic rate and efficiency of CaCO3 precipitation during the EICP process has not been investigated. Therefore, a simple but reliable kinetic expression for evaluating the catalytic reaction in EICP is required. This study, through a comprehensive meta-analysis of data from literature, attempts to correlate the initial ratios, concentration and the catalytic effect of chemical constituent (urease enzyme, urea and CaCl2) with product (CaCO3) formation rate.
3.1. Factors Affecting the Kinetic Parameters
3.1.1. pH
A range of standard kinetic parameters (i.e., Km and Vmax) for urea hydrolysis catalysed by Jack bean urease enzyme have been reported in the literature [31][32]. However, Km and Vmax are largely influenced by pH which consequently affects the kinetic reaction through perturbation of the distribution of enzyme[33][34][35]. Barth and Michel [36] investigated the activity of urease enzyme in the pH range of 49 and indicated that both Km and Vmax depend on pH. The results from their study show a minimum value of Km at pH 7, whereas Vmax was maximum at the same pH. Similarly, Fidaleo and Lavecchia[33] evaluated the dependency of Km and Vmax on pH by assuming the enzymatic urea hydrolysis described by Tipton and Dixon [33].
3.1.2. Temperature
In a kinetic reaction, kcat is the only temperature-dependent parameter and has often been adjusted to capture the influence of temperature variations. Fidaleo and Lavecchia[34] studied the influence of temperature (25 and 37 °C) on the reaction rate. A parametric approach was recently used by Krajewska, van Eldik[35] to elucidate the influence of temperature (T) on the steady-state kinetic parameter, i.e., Km and kcat. Note, kcat is a direct measure of Vmax. Their study indicated that Km controls the formation of the ES complex: E+S=ES, during the binding of the substrate, whereas kcat controls the activation process of the ES complex: ES=ES-EP, when bond reorganization leading to the formation of the products occurs. The authors found that Km and kcat increased with increasing temperature which may directly affect the kinetic rate reaction during urea hydrolysis.
3.1.3. Product Inhibition
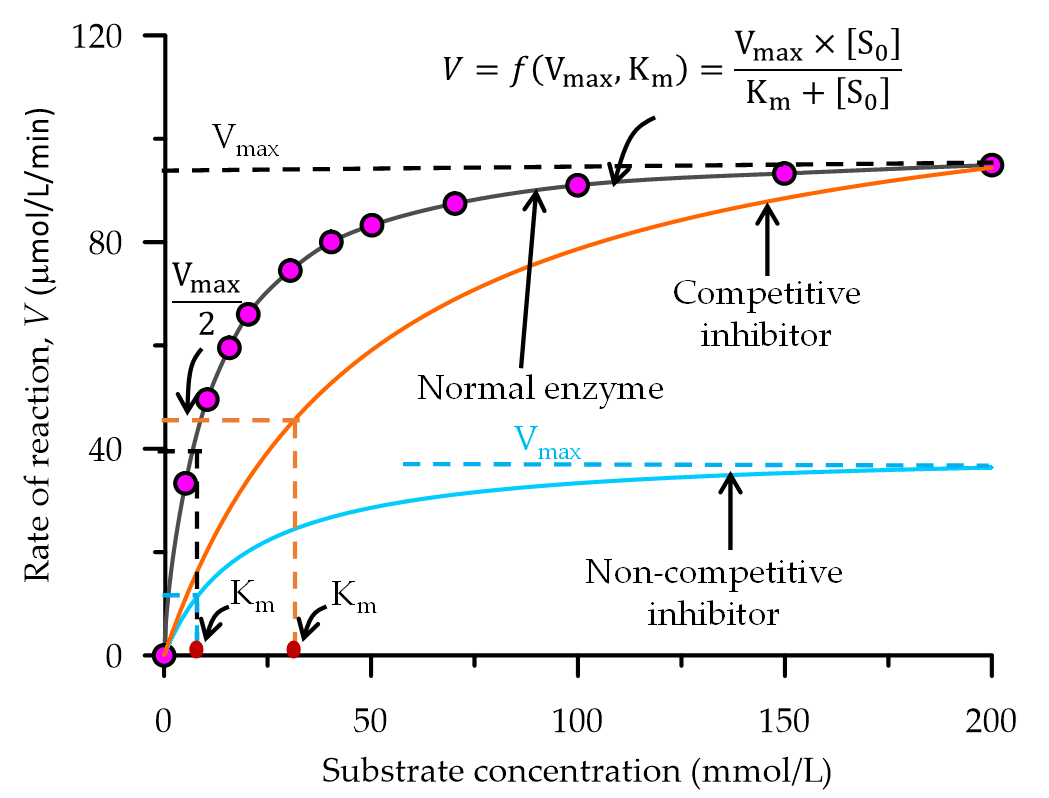
The rate of urea hydrolysis is mostly affected by the presence of in-vitro inhibitors, soil inhibitors and plant-soil inhibitors. From an enzyme kinetic point of view, these inhibitors can be classified into two groups i.e., competitive inhibitors where remains the same but changes with the concentration of inhibitor and non-competitive inhibitor where changes with the concentration of inhibitor but is not affected as shown in Figure 2.
Figure 2. A graphical representation of the influence of inhibitors in an enzyme catalysed reaction.
Competitive inhibitors bind to the active site of the urease enzyme and prevent the substrate from binding. At low substrate concentrations, the presence of competitive inhibitors in an enzyme-catalysed reaction can significantly decrease the rate of the reaction. In EICP, the presence of competitive inhibitors such as heavy metal ions may affect the effectiveness of CaCO3 precipitation. However, this effect can be significantly reduced by increasing the substrate (urea-CaCl2) concentration. Some studies have indicated that the presence of magnesium[37][38] or anionic compounds such as polyelectrolytes [39][40][41] delays the rate of CaCO3 precipitation in EICP, which consequently alters the structure, size (approximately 10 µm) and quantity of the precipitated crystals. This may result in the precipitation of dolomite/magnesium carbonate (MgCO3) or other polymorphs of CaCO3, which may affect the strength properties of EICP treated soil [42][37][38][43].
Non-competitive inhibitors allosterically bind the enzyme at a site other than the active site, thereby reducing the ability of the enzyme to perform its function. Hence, the velocity rate of the reaction usually asymptotes at lower than the maximum velocity. On the other hand, uncompetitive inhibitors bind the enzyme-substrate complex thereby resulting in an inactive enzyme-substrate complex. Uncompetitive and non-competitive inhibitors usually occur in a multiple-substrate system, such as in the case of EICP, and therefore this requires further investigation.
One way of controlling the action of urease is by immobilizing the enzyme[44]. Immobilization occurs when urease enzymes are physically confined or localised in a defined region of space with retention of their catalytic activities, which can be used repeatedly and continuously[45]. Even though upon immobilization the kinetic properties of enzymes may be degraded, their stabilities, operational lifetimes and sensitivities to inhibition[46][47][48][49] are improved, thus providing robust and reliable enzyme preparations. Knowledge of urease inhibition and immobilization is significant for enhancing and controlling the rate of precipitation, as well as for the removal of contaminated ions or chemicals.
4. Engineering Applications of Urease Aided- Precipitation
4.1. Improvement of the Strength and Stiffness of Soils
In biocemented soils, the precipitated CaCO3 within the soil matrix provides bridges/bonds between the grains of the soil particles, thereby restricting their movement and hence improving the strength and stiffness of the soil [18][50][51][52]. Application of EICP in soil strengthening and stabilisation can include crack remediation in concrete[53][54][55][56][57] strengthening of granular soil [12][50][58][59] and liquefaction mitigation [54][55].
Despite past works on the application of EICP for soil improvement, the overall controllability of the technique requires significant research. In EICP, it is often difficult to accurately predict and/or control the rate and amount of CaCO3 precipitation. Also, the distribution and morphology of the precipitated CaCO3 in an EICP process is often difficult to control, especially, under field conditions. In general, precipitated CaCO3 from the EICP process can appear in six different crystal forms (polymorphs) which include calcite, aragonite, vaterite, CaCO3 hexahydrate, CaCO3 monohydrate and amorphous CaCO3, in decreasing order of stability [1]. Rhombohedral calcite crystals exhibit well developed and distinct consolidation and these have been identified as the most desirable CaCO3 polymorph for geotechnical applications due to its thermodynamic stability[19][60]. The least thermodynamically stable polymorphs of CaCO3 appear during rapid precipitation at high supersaturation levels and these change rapidly into a more stable anhydrous phase. Almajed, Khodadadi Tirkolaei [19] stated that, rapid precipitation of CaCO3 in EICP, compared to MICP, can be disadvantageous because it can sometimes result in the formation of unstable vaterite and other amorphous CaCO3. The influence of urease enzyme activity on CaCO3 polymorphs in EICP was studied by Ahenkorah, Rahman [17] via SEM images using high-activity (U/g) and low-activity (3,500 U/g) purified enzyme. The authors found that the low-activity enzyme produced anhedral calcite crystals while the high-activity enzyme produced mostly euhedral calcite crystals. The differences in the observed morphology may be attributed to the degree of purity of both enzymes, as also suggested by Khodadadi, Javadi[57].
It is well understood that the chemical reactions in EICP may be influenced by numerous factors including enzyme inhibitors, which cannot be captured via CaCO3 quantification in test-tubes, as used in previous studies[19][20][50]]. Therefore, an effective bonding via soil improvement in EICP can be achieved by taking into account the kinetic mechanisms of the reaction.
4.2. Erosion and Dust Control
In addition to the improvement of soil strength, the precipitated CaCO3 in EICP can fill the voids within the soil matrix thereby reducing porosity and permeability [18][61][62][63][64][65][66][67][68][69][70][71][72][73]. Knorr [61] applied the EICP technique to control the impacts of water and wind erosion using different soil types such as Ottawa F60 sand, silty-sand and mine tailings. The results showed that EICP can be a potential technique to prevent erosion caused by both wind and runoff of surface water. Hamdan and Kavazanjian [74] tested the effectiveness of EICP in stabilising soils against fugitive dust emissions in a wind tunnel. In their experiment, different types of sand were prepared in a pan and sprayed in a series with an EICP solution. They concluded that the EICP technique gives a promising outcome for mitigating fugitive dust emissions.
Cuccurullo, Gallipoli[62]applied the EICP technique to mitigate the effects of water erosion on a silty clay. They found that EICP-treated samples exhibited a three-fold improvement in terms of the mass of soil lost compared to untreated specimens. Many other researchers have evaluated the effectiveness of the EICP technique for dust control [63][64][65][66]and surface water erosion mitigation [67][68][69][70], and have found the results to be satisfactory.
4.3. Removal of Heavy Metals
Heavy metals and other contaminants generated as industrial by-products can lead to a significant impact on our environment. The use of conventional treatment methods to remove heavy metals from contaminated environments can be expensive and consumes high amounts of chemicals and energy. Therefore, EICP can be an environmentally friendly alternative for the removal of heavy metals and other waste contaminants.
Nam, Roh[75] applied the EICP technique to immobilize and remove heavy metals and metalloids in contaminated mine wastes. The results from their study indicated that the concentrations of As, Mn, Zn, Pb, Cr and Cu were reduced by 31.7%, 65.8%, 50.6%, 51.6%, 45.1% and 49.7%, respectively. Moghal, Lateef[76] and Moghal, Lateef [77] investigated the efficacy of the EICP method on adsorption and desorption of soils mixed with different combinations of heavy metals. Their results indicated that EICP could immobilize to a significant level the heavy metals in selected soils.
5. Conclusions and Future Perspectives
Studies on optimisation of the EICP process have often been conducted by using the discontinuous approach, which involves mixing the substrate and enzyme and measuring the product formed after a set period. However, this approach cannot easily capture the catalytic properties, such as the influence of urease activity and product inhibition on the enzyme-catalysed reaction. Therefore, the continuous enzyme kinetic assay, which involves mixing the enzyme with the substrate and continuously measuring the product formed or the dissociation of the substrate over time, should be considered in future studies. It is understood from this study that the reaction velocity of an enzyme catalysed reaction is mainly influenced by pH, temperature and inhibitors (ammonium ion). A meta-analysis of data from a previous study indicate that pH and ammonium ions greatly affect compared to , whereas was greatly influenced by temperature. A modified form of the Michaelis–Menten equation was proposed in this study, which can be used to capture the kinetic reaction in EICP under various conditions. The findings from this study indicate that ignoring the influence of product inhibition in an enzyme-catalysed reaction may result in a poor prediction of the kinetic parameters. Hence, various sources of urease inhibitors including amides and esters of phosphoric acid, thiols, hydroxamic acids, phosphinic and thiophosphinic acids, boric acid, phosphate, heavy metal ions, bismuth compounds, quinones and fluoride have been studied. Although the kinetic equations analysed and proposed in this study are useful for the EICP process, future studies on the influence of enzyme kinetic reactions in different soil environments are highly recommended. The development of kinetic models that capture the effects of using an enzyme from different plant sources should also be considered for future studies.
References
- Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 2017, 13, 59–67.
- Putra, H.; Yasuhara, H.; Kinoshita, N. Applicability of natural zeolite for nh-forms removal in enzyme-mediated calcite precipitation technique. Geosciences 2017, 7, 61.
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Teasdale, P.R. A comparison of mechanical responses for microbial and enzyme-induced cemented sand. Géotechnique Lett. 2020, 10, 1–26.
- Neupane, D.; Yasuhara, H.; Kinoshita, N.; Ando, Y. Distribution of mineralized carbonate and its quantification method in enzyme mediated calcite precipitation technique. Soils Found. 2015, 55, 447–457.
- Hamdan, N.M. Applications of Enzyme Induced Carbonate Precipitation (Eicp) for Soil Improvement. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2015.
- Dilrukshi, R.; Kawasaki, S. Effective use of plant-derived urease in the field of geoenvironmental. Geotech. Eng. J. Civ. Environ. Eng. 2016, 6, 2.
- Suárez, D.; Díaz, N.; Merz, K.M. Ureases: Quantum chemical calculations on cluster models. J. Am. Chem. Soc. 2003, 125, 15324–15337.
- Zimmer, M. Are classical molecular mechanics calculations still useful in bioinorganic simulations? Coord. Chem. Rev. 2009, 253, 817–826.
- Smyj, R.P. A conformational analysis study of a nickel (ii) enzyme: Urease. J. Mol. Struct. THEOCHEM 1997, 391, 207–223.
- Sumner, J.B. The isolation and crystallization of the enzyme urease preliminary paper. J. Biol. Chem. 1926, 69, 435–441.
- Mazzei, L.; Musiani, F.; Ciurli, S. The structure-based reaction mechanism of urease, a nickel dependent enzyme: Tale of a long debate. J. Biol. Inorg. Chem. 2020, 25, 829–845.
- Kafarski, P.; Talma, M. Recent advances in design of new urease inhibitors: A review. J. Adv. Res. 2018, 13, 101–112.
- Follmer, C. Insights into the role and structure of plant ureases. Phytochemistry 2008, 69, 18–28.
- Mobley, H.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480.
- Sirko, A.; Brodzik, R. Plant ureases: Roles and regulation. Acta Biochim. Pol. 2000, 47, 1189–1195.
- Das, N.; Kayastha, A.M.; Srivastava, P.K. Purification and characterization of urease from dehusked pigeonpea (cajanus cajan l.) seeds. Phytochemistry 2002, 61, 513–521.
- Nam, I.-H.; Chon, C.-M.; Jung, K.-Y.; Choi, S.-G.; Choi, H.; Park, S.-S. Calcite precipitation by ureolytic plant (canavalia ensiformis) extracts as effective biomaterials. KSCE J. Civ. Eng. 2015, 19, 1620–1625.
- Takatsu, M.; Nadeeka, R.; Kawasaki, S. Development of biogrouting using plant-derived urease and calcium phosphate compound. In Proceedings of the 50th US Rock Mechanics/Geomechanics Symposium, Huston, TX, USA, 26–29 June 2016.
- Balasubramanian, A.; Ponnuraj, K. Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. J. Mol. Biol. 2010, 400, 274–283.
- Kunduru, K.R.; Kutcherlapati, S.R.; Arunbabu, D.; Jana, T. Armored urease: Enzyme-bioconjugated poly (acrylamide) hydrogel as a storage and sensing platform. Methods Enzymol. 2017, 590, 143–167.
- Jabri, E.; Carr, M.B.; Hausinger, R.P.; Karplus, P.A. The crystal structure of urease from klebsiella aerogenes. Science 1995, 268, 998–1004.
- Krajewska, B.; Zaborska, W. Jack bean urease: The effect of active-site binding inhibitors on the reactivity of enzyme thiol groups. Bioorg. Chem. 2007, 35, 355–365.
- Roberts, B.P.; Miller, B.R., III; Roitberg, A.E.; Merz, K.M., Jr. Wide-open flaps are key to urease activity. J. Am. Chem. Soc. 2012, 134, 9934–9937.
- Sharma, B.; Mandani, S.; Sarma, T.K. Biogenic growth of alloys and core-shell nanostructures using urease as a nanoreactor at ambient conditions. Sci. Rep. 2013, 3, 2601.
- Kucharski, E.S.; Cord-Ruwisch, R.; Whiffin, V.; Al-thawadi, S.M. Microbial Biocementation; Google Patents: San Francisco, CA, USA, 2012.
- Kappaun, K.; Piovesan, A.R.; Carlini, C.R.; Ligabue-Braun, R. Ureases: Historical aspects, catalytic, and non-catalytic properties–A review. J. Adv. Res. 2018, 13, 3–17.
- Dixon, N.E.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (ec 3.5. 1.5). Metalloenzyme. Simple biological role for nickel. J. Am. Chem. Soc. 1975, 97, 4131–4133.
- Zimmer, M. Molecular mechanics evaluation of the proposed mechanisms for the degradation of urea by urease. J. Biomol. Struct. Dyn. 2000, 17, 787–797.
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular biology of the cell 4th edn (new york: Garland science). Ann. Bot. 2002, 91, 401
- Wen, K.J.; Li, Y.; Amini, F.; Li, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotech. 2020, 15, 17–27.
- Cesareo, S.D.; Langton, S.R. Kinetic properties of helicobacter pylori urease compared with jack bean urease. FEMS Microbiol. Lett. 1992, 99, 15–21.
- Dixon, N.E.; Riddles, P.W.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (ec 3.5. 1.5). V. On the mechanism of action of urease on urea, formamide, acetamide, n-methylurea, and related compounds. Can. J. Biochem. 1980, 58, 1335–1344.
- Tipton, K.F.; Dixon, H.B. Effects of ph on enzymes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1979; Volume 63, pp. 183–234.
- Fidaleo, M.; Lavecchia, R. Kinetic study of enzymatic urea hydrolysis in the ph range 4–9. Chem. Biochem. Eng. Q. 2003, 17, 311–318.
- Krajewska, B.; van Eldik, R.; Brindell, M. Temperature-and pressure-dependent stopped-flow kinetic studies of jack bean urease. Implications for the catalytic mechanism. J. Biol. Inorg. Chem. 2012, 17, 1123–1134.
- Barth, A.; Michel, H. A contribution on the mechanism of the enzymatic cleavage of urea. Biochem. Physiol. Pflanzen 1972, 163, 103–109.
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Hirata, A. Application of magnesium to improve uniform distribution of precipitated minerals in 1-m column specimens. Geomech. Eng. 2017, 12, 803–813.
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Neupane, D.; Lu, C. Effect of magnesium as substitute material in enzyme-mediated calcite precipitation for soil-improvement technique. Front. Bioeng. Biotechnol. 2016, 4, 37.
- Jada, A.; Jradi, K. Role of polyelectrolytes in crystallogenesis of calcium carbonate. In Macromolecular Symposia; Wiley Online Library: Weinheim, Germany, 2006; pp. 147–151.
- Yashchenok, A.; Parakhonskiy, B.; Donatan, S.; Kohler, D.; Skirtach, A.; Möhwald, H. Polyelectrolyte multilayer microcapsules templated on spherical, elliptical and square calcium carbonate particles. J. Mater. Chem. B 2013, 1, 1223–1228.
- Williams, F.V.; Ruehrwein, R.A. Effect of polyelectrolytes on the precipitation of calcium carbonate. J. Am. Chem. Soc. 1957, 79, 4898–4900.
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Hirata, A. Optimization of enzyme-mediated calcite precipitation as a soil-improvement technique: The effect of aragonite and gypsum on the mechanical properties of treated sand. Crystals 2017, 7, 59.
- Putra, H.; Yasuhara, H.; Kinoshita, N. Optimum condition for the application of enzyme-mediated calcite precipitation technique as soil improvement technique. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 2145–2151.
- Beal, S.L. On the solution to the michaelis-menten equation. J. Pharmacokinet. Biopharm. 1982, 10, 109–119.
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307.
- Krajewska, B. Ureases i. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B Enzym. 2009, 59, 9–21.
- Krajewska, B. Ureases. Ii. Properties and their customizing by enzyme immobilizations: A review. J. Mol. Catal. B Enzym. 2009, 59, 22–40.
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Miletti, S.; Ciurli, S.; Mangani, S. The complex of bacillus pasteurii urease with acetohydroxamate anion from x-ray data at 1.55 å resolution. JBIC J. Biol. Inorg. Chem. 2000, 5, 110–118.
- Krajewska, B. Urease immobilized on chitosan membrane. Inactivation by heavy metal ions. J. Chem. Technol. Biotechnol. 1991, 52, 157–162.
- Yasuhara, H.; Neupane, D.; Hayashi, K.; Okamura, M. Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found. 2012, 52, 539–549.
- Kavazanjian, E.; Hamdan, N. Enzyme induced carbonate precipitation (eicp) columns for ground improvement. In IFCEE 2015; American Society of Civil Engineers: Reston, VA, USA, 2015; pp. 2252–2261.
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 1–43.
- Dakhane, A.; Das, S.; Hansen, H.; O’Donnell, S.; Hanoon, F.; Rushton, A.; Perla, C.; Neithalath, N. Crack healing in cementitious mortars using enzyme-induced carbonate precipitation: Quantification based on fracture response. J. Mater. Civil Eng. 2018, 30, 04018035.
- Simatupang, M.; Okamura, M. Liquefaction resistance of sand remediated with carbonate precipitation at different degrees of saturation during curing. Soils Found. 2017, 57, 619–631.
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Fauzan, M. Promoting precipitation technique using bio-chemical grouting for soil liquefaction prevention. Civil Eng. Dimens. 2020, 22, 1–5.
- Dilrukshia, R.; Kawasakib, S. Plant-derived urease induced sand cementation used in geotechnical engineering applications. In Proceedings of the International Conference on Geomechanics, Geo-Energy and Geo-Resources: Melbourne, Australia, 28–29 September 2016.
- Khodadadi, T.H.; Javadi, N.; Krishnan, V.; Hamdan, N.; Kavazanjian, E.J. Crude urease extract for biocementation. J. Mater. Civil Eng. 2020, 32, 04020374.
- Hamdan, N.; Kavazanjian, E., Jr.; O’Donnell, S. Carbonate Cementation via Plant Derived Urease. In Proceedings of the 18th International Conference on Soil Mechanics and Geotechnical Engineering, Paris, France, 2–6 September 2013.
- Dilrukshi, R.; Nakashima, K.; Kawasaki, S. Soil improvement using plant-derived urease-induced calcium carbonate precipitation. Soils Found. 2018, 58, 894–910.
- Dilrukshia, R.; Kawasakib, S. Plant-derived urease induced sand cementation used in geotechnical engineering applications. In Proceedings of the International Conference on Geomechanics, Geo-Energy and Geo-Resources: Melbourne, Australia, 28–29 September 2016
- Knorr, B. Enzyme-Induced Carbonate Precipitation for the Mitigation of Fugitive Dust. PhD thesis, Arizona State University, Tempe, USA, 2014.
- Cuccurullo, A.; Gallipoli, D.; Bruno, A.W.; Augarde, C.; Hughes, P.; La Borderie, C. Soil stabilization against water erosion via calcite precipitation by plant-derived urease. In Proceedings of the National Conference of the Researchers of Geotechnical Engineering: Lecco, Italy, 3–5 July 2019; Springer: pp. 753–762.
- Bang, S.C.; Min, S.H.; Bang, S.S. Kgs awards lectures: Application of microbiologically induced soil stabilization technique for dust suppression. Int. J. Geo-Eng. 2011, 3, 27–37.
- Bang, S.S.; Bang, S.; Frutiger, S.; Nehl, L.M.; Comes, B.L. Application of novel biological technique in dust suppression. In Proceedings of the 88th Transportation Research Board Annual Meeting, Washington, DC, USA, 11–15 January 2009.
- Lo, C.-Y.; Tirkolaei, H.K.; Hua, M.; De Rosa, I.M.; Carlson, L.; Kavazanjian, E., Jr.; He, X. Durable and ductile double-network material for dust control. Geoderma 2020, 361, 114090.
- Woolley, M.A.; Van Paassen, L.; Kavazanjian, E., Jr. Impact on surface hydraulic conductivity of eicp treatment for fugitive dust mitigation. In Geo-Congress 2020: Biogeotechnics; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 132–140.
- Almajed, A.; Lemboye, K.; Arab, M.G.; Alnuaim, A. Mitigating wind erosion of sand using biopolymer-assisted eicp technique. Soils Found. 2020, 60, 356–371.
- Liu, K.-W.; Jiang, N.-J.; Qin, J.-D.; Wang, Y.-J.; Tang, C.-S.; Han, X.-L. An experimental study of mitigating coastal sand dune erosion by microbial-and enzymatic-induced carbonate precipitation. Acta Geotech. 2021, 16, 467–480.
- Miao, L.; Wu, L.; Sun, X. Enzyme-catalysed mineralisation experiment study to solidify desert sands. Sci. Rep. 2020, 10, 1–12.
- Ossai, R.; Rivera, L.; Bandini, P. Experimental study to determine an eicp application method feasible for field treatment for soil erosion control. In Geo-Congress 2020: Biogeotechnics; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 205–213.
- Nemati, M.; Greene, E.; Voordouw, G. Permeability profile modification using bacterially formed calcium carbonate: Comparison with enzymic option. Process Biochem. 2005, 40, 925–933.
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzym. Microb. Technol. 2003, 33, 635–642.
- Song, J.Y.; Sim, Y.; Jang, J.; Hong, W.T.; Yun, T.S. Near-surface soil stabilization by enzyme-induced carbonate precipitation for fugitive dust suppression. Acta Geotech. 2020, 15, 1967–1980.
- Hamdan, N.; Kavazanjian, E. Enzyme-induced carbonate mineral precipitation for fugitive dust control. Geotechnique 2016, 66, 546–555.
- Nam, I.-H.; Roh, S.-B.; Park, M.-J.; Chon, C.-M.; Kim, J.-G.; Jeong, S.-W.; Song, H.; Yoon, M.-H. Immobilization of heavy metal contaminated mine wastes using canavalia ensiformis extract. Catena 2015, 136, 53–58.
- Moghal, A.A.B.; Lateef, M.A.; Mohammed, S.A.S.; Ahmad, M.; Usman, A.R.; Almajed, A. Heavy metal immobilization studies and enhancement in geotechnical properties of cohesive soils by eicp technique. Appl. Sci. 2020, 10, 7568.
- Moghal, B.A.A.; Lateef, M.A.; Mohammed, S.A.S.; Lemboye, K.K.; CS Chittoori, B.; Almajed, A. Efficacy of enzymatically induced calcium carbonate precipitation in the retention of heavy metal ions. Sustainability 2020, 12, 7019.