
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jorge Antonio Custodio-Mendoza | -- | 3715 | 2023-10-23 10:04:09 | | | |
| 2 | Fanny Huang | Meta information modification | 3715 | 2023-10-26 07:34:15 | | |
Video Upload Options
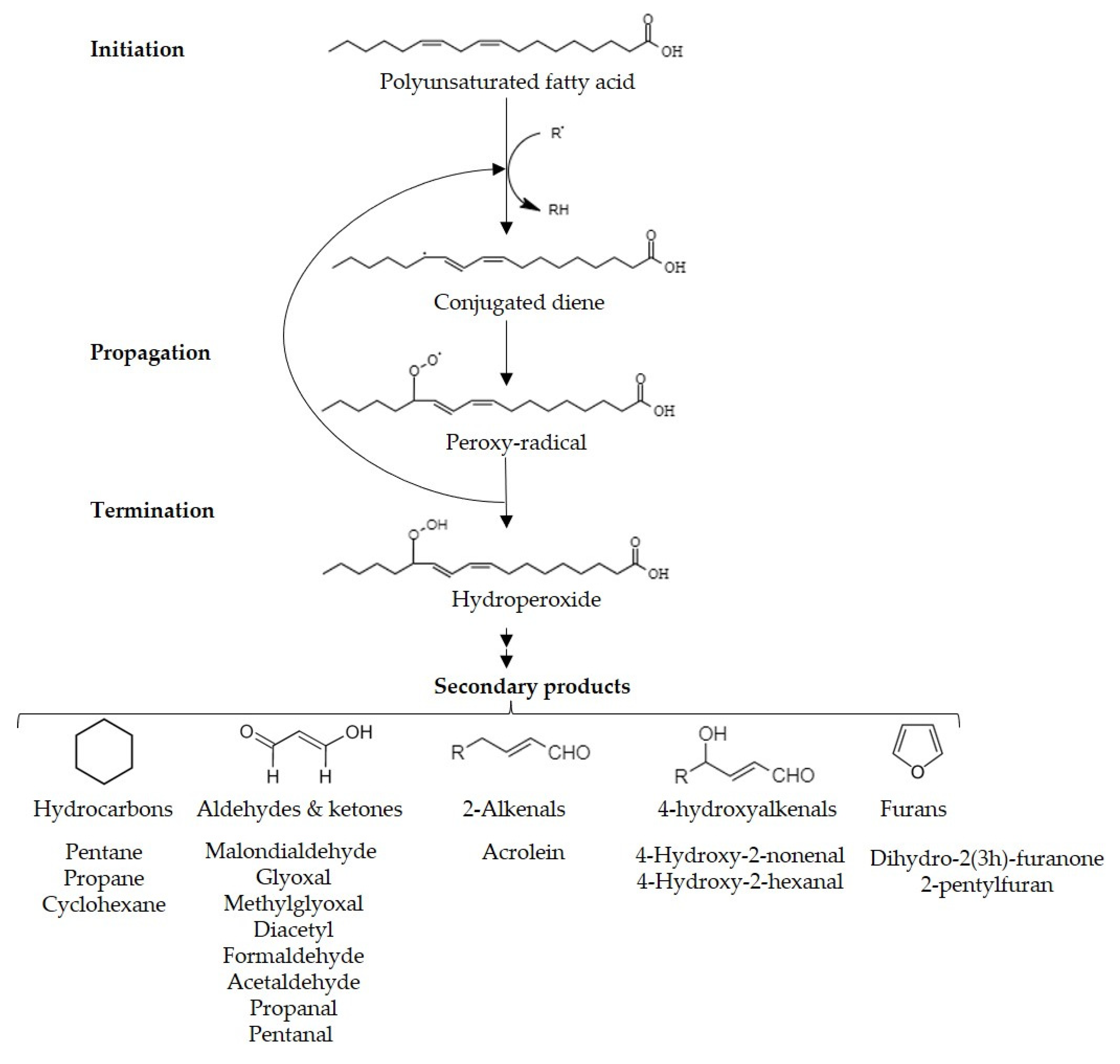
Lipid peroxidation, the most aggressive reaction in food, results in the formation of reactive organic compounds that detrimentally impact food sensory qualities and consumers’ health. While controlled lipid peroxidation can enhance flavors and appearance in certain foods, secondary peroxidation products lead to sensory deterioration in a variety of products, such as oils, alcoholic beverages, and meat. Dispersive liquid-liquid microextraction (DLLME), solid-phase microextraction (SPME), and gas-diffusion microextraction (GDME). These techniques offer efficient and sensitive approaches to extracting and quantifying lipid oxidation products and contribute to the understanding of oxidative deterioration in various food products.
1. Introduction
| Secondary Product | CAS Number | IARC Category | Tolerable Daily Intake µg/Kg bw/Day |
Reference | |
|---|---|---|---|---|---|
| Saturate Carbonyls | Formaldehyde | 50-00-0 | 1 | 150 | [22] |
| Acetaldehyde | 75-07-0 | 2B | 185 a | [23] | |
| Hexanal | 66-25-1 | - | 780 * | [24] | |
| α,β-Unsaturated Carbonyls | Acrolein | 107-02-8 | 2A | 7.5 | [25] |
| 4-hydroxy-2-nonenal | 75899-68-2 | 3 | 1.5 ** | [26] | |
| 4-hydroxy-2-hexenal | 17427-21-3 | 3 | 1.5 ** | [26] | |
| Acrylamide | 79-06-1 | 2A | NE | [27] | |
| Crotonaldehyde | 4170-30-3 | 2B | - | - | |
| Dicarbonyls | Malondialdehyde | 102-52-3 | 3 | 30 ** | [26] |
| Glyoxal | 107-22-2 | - | 200 | [28] | |
| Methylglyoxal | 78-98-8 | 3 | - | - | |
| Diacetyl | 431-03 | - | 900 * | [28] | |
| Furans | Dihydro-2(3H)-furanone | 96-48-0 | 3 | - | - |
| Furfural | 98-01-1 | 3 | 500 | [29] | |
2. Gas Diffusion Microextraction
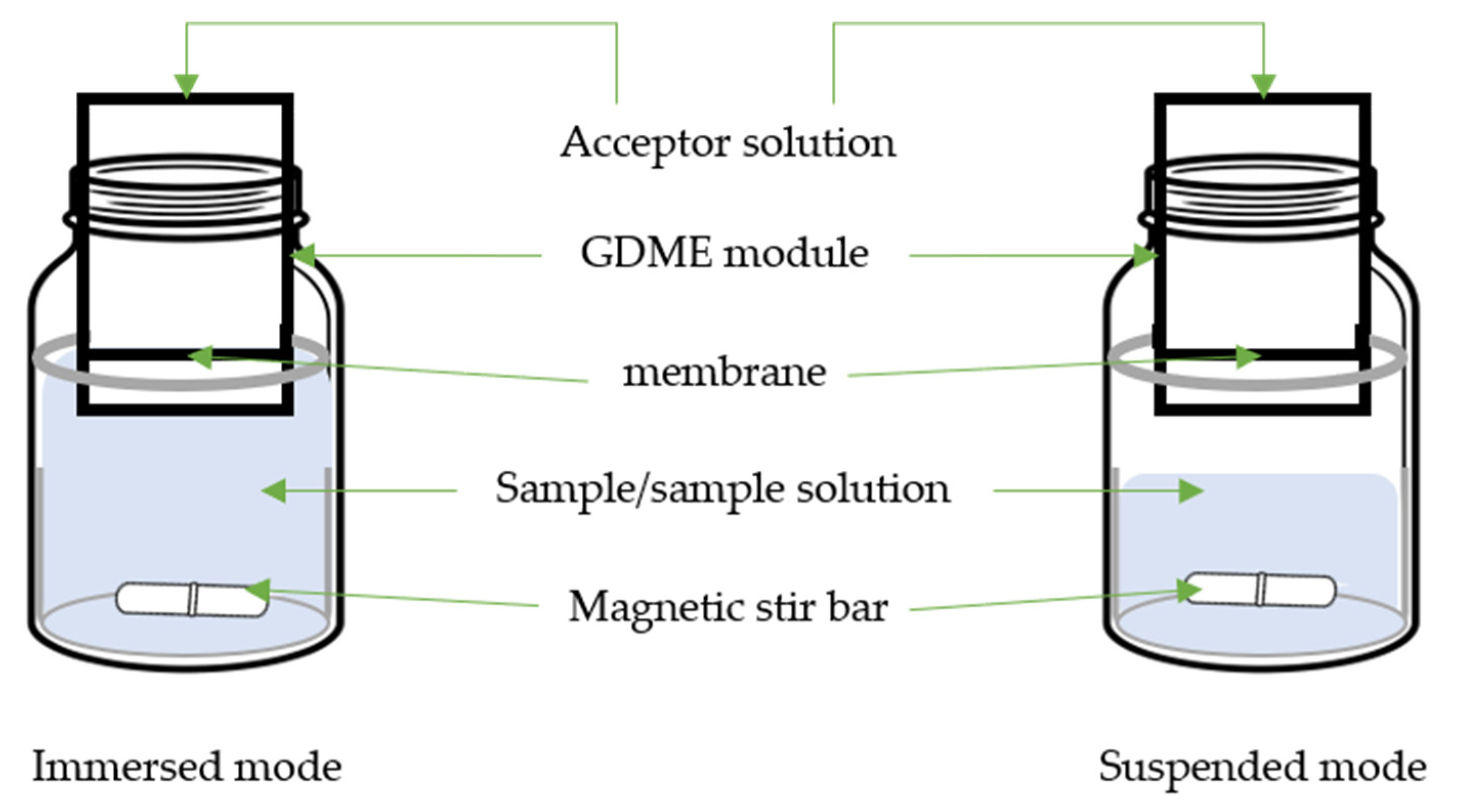
| Target Compound | Sample | GDME | Derivative Reagent | Determination | LOD µg/L or µg/Kg |
Recovery % |
Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mode | Vacceptor solution mL |
t min |
T °C |
|||||||
| 1,3-pentadione Diacetyl |
Beer | Immersed | 0.5 | 15 | 40 | O-PDA | HPLC-UV | 3.8–4.6 | - | [48] |
| 2 aldehydes & Furfural |
Beer | Immersed | 0.75 | 5 | 30 | DNPH | HPLC-UV | 1.5–12.3 | - | [54] |
| 5 aldehydes | Beer | Suspended | 0.5 | 20 | 40 | HBA | HPLC-DAD | 1.2–1857.7 | >96% | [55] |
| Diacetyl 1 | Wine | Immersed | 0.4 | 20 | 65 | O-PDA | HPLC-UV | 3.8 | - | [56] |
| Acetaldehyde 1 | Wine | Immersed | 1.0 | 15 | 50 | DNPH | HPLC-UV | 800–1100 | - | [57] |
| Diacetyl | Wine & beer | Suspended | 1.0 | 10 | 60 | O-PDA | DPV | 0.053 | - | [58] |
| α-DCC | Wine; black tea & soy sauce | Immersed 2 | 0.5 | 10 | 55 | O-PDA | HPLC-UV | 50–200 | - | [59] |
| MDA | Vegetable oil | Suspended | 0.5 | 30 | 65 | TBA | HPLC-UV/FLD | 250–350 | ≥82% | [60] |
| 4 aldehydes Acrolein & MDA | Vegetable oil | Suspended | 1.0 | 10 | 60 | DPNH | GC-MS | 50–100 | ≥95% | [10] |
| 2 ketones & diacetyl | Ground bread | Suspended | 0.5 | 15 | 65 | O-PDA | HPLC-UV | 6–12 | - | [61] |
| 27 carbonyl compounds 3 | Green & roast coffee beans | Suspended | 0.5 | 16 | 40 | O-PDA | HPLC-DAD | 50–200 | - | [62] |
3. Solid-Phase Microextraction
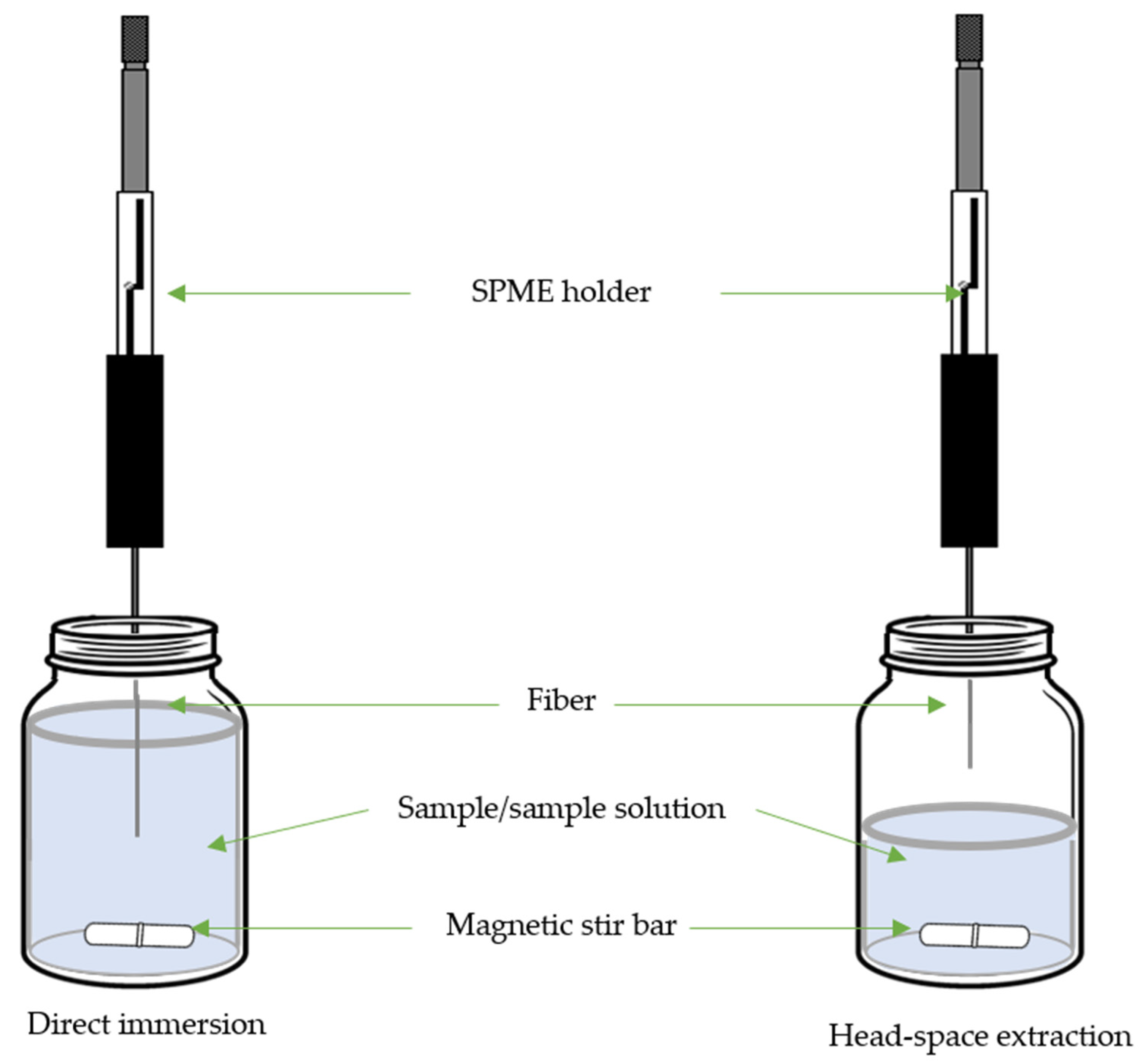
| Target Compound | Sample | SPME | Derivative Reagent | Determination | LOD µg/L or µg/Kg |
Recovery % |
Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode | t min |
T °C |
Fiber | Tdesorption °C |
|||||||
| 14 aldehydes & ketones | Vegetable oil | HS | 30 | 20 | DVB/CAR/PDMS | 270 | - | GC-FID & GC-MS | 0.04–2.24 | - | [64] |
| 4-HNE | Oils & porcine liver | DI | 15 | 40 | PDMS/DVB | DNPH | HPLC-SP | 0.001–1.42 | 66–87% | [65] | |
| MDA | Cod liver oil | HS | 10 | RT | PDMS/DVB | 200 | N-MH | GC-NPD | 0.74 | 91% | [66] |
| Hexanal | Hazelnut | HS | 10 | 60 | CAR/PDMS | 300 | - | GC-FID | 8.01 | - | [67] |
| 7 aldehydes | Peanut, soybean and olive oils | HS | 15 | 50 | CAR/PDMS | 250 | - | GC-FID | 4.6–10.2 | 85–110 | [68] |
| 3 α,β-UC | Sunflower oil digestion phases | HS | 60 | 50 | DVB/CAR/PDMS | 250 | - | GC-MS | - | - | [69] |
| 100 carbonyl compounds | Cod liver oil | HS | 60 | 50 | DVB/CAR/PDMS | 220 | - | GC-MS | - | - | [70] |
| 18 VOC | Sunflower oil emulsions | HS | 30 | 50 | DVB/CAR/PDMS | 250 | - | GC-MS | - | - | [71] |
| Aldehydes & 2-pentylfuran | Soybean oils | HS | 55 | 50 | DVB/CAR/PDMS | 250 | - | GC-MS | - | - | [72] |
| VOC | Peanut oil | HS | 40 | 50 | PDMS/DVB | 250 | - | GC-MS | - | - | [73] |
| 4 aldehydes & 1 ketone | Roast & boiled duck | HS | 40 | 45 | CAR/PDMS | 280 | - | GC-MS | - | - | [74] |
| 3 aldehydes | Chicken patties | HS | 10 | 60 | DVB/CAR/PDMS | 250 | - | GC-FID | - | - | [75] |
| Hexanal | Pig sausages | HS | 30 | 50 | DVB/CAR/PDMS | 220 | - | GC-MS | - | - | [76] |
| 2 aldehydes & 2 dialdehydes | Cod | HS | 30 | 50 | CAR/PDMS | 260 | - | GC-FID | - | - | [77] |
| 8 aldehydes | Fish | HS | 15 | 60 | PDMS/DVB | 260 | PFBHA | GC-MS | 1.4–6.1 | 79–102 | [78] |
| 6 aldehydes | Caviar | HS | 30 | 60 | DVB/CAR/PDMS | 250 | - | GC-MS | - | - | [79] |
| 198 VOCs | Dry cured meat | HS | 30 | 37 | 260 | - | GC-MS | - | - | [80] | |
| Aldehydes | Infant formula | HS | 10 | 25 | PDMS/DVB | 250 | - | GC-MS | - | - | [81] |
| 3 aldehydes & pentane | Infant formula | HS | 45 | 37 | CAR/PDMS | 250 | - | GC-FID | 0.02–1.05 | - | [82] |
| 13 Carbonyl compounds | Milk powder | HS | 45 | 43 | 250 | - | GC-MS | 2–6 | - | [83] | |
| VOC | Smoked cheese | HS | 45 | 50 | CAR/PDMS | 260 | - | GC-MS | - | - | [84] |
| VOC | Mozzarella | HS | 15 | 37 | 220 | - | GC-MS | - | - | [85] | |
| VOC | Portuguese cheese | HS | 45 | 50 | DVB/PDMS | 250 | - | GC-MS | - | - | [86] |
| 9 aldehydes | Beer | HS | 60 | 50 | PDMS/DVB | 250 | PFBHA * | GC-MS | - | 89–114 | [87] |
| 41 carbonyl compounds | Beer | HS | 40 | 60 | PDMS/DVB | 250 | PFBHA *,** | GC-MS | 0.003–20,000 | - | [88] |
| 250 carbonyl compounds | Beer | HS | 20 | 45 | PDMS/DVB | 250 | PFBAH ** | GC-ITMS | 0.003–0.510 | 88–114 | [89] |
| 6 carbonyl compound | Beer | HS | 60 | 55 | DVB/CAR/PDMS | 250 | TFEH ** | GC-MS | 0.03–0.5 | 90–105 | [16] |
| 6 carbonyl compound | Craft beer | HS | 60 | 55 | DVB/CAR/PDMS | 250 | TFEH ** | GC-MS | 0.03–0.5 | 90–105 | [90] |
| 18 carbonyl compound | Wine | HS | 45 | 40 | DVB/CAR/PDMS | 250 | - | GC-ITMS | 0.62–129.2 | 19–190 | [91] |
| 80 VOC | Wine | HS | 30 | 40 | DVB/CAR/PDMS | 240 | - | GC-MS | - | - | [92] |
| 6 carbonyl compound | Syrah wines | HS | 45 | 55 | DVB/CAR/PDMS | 250 | TFEH | GCxGC-TOFMS | 0.5–5.2 | 90–106 | [93] |
| 3 aldehydes | Must & wine | HS | 45 | 55 | DVB/CAR/PDMS | 250 | TFEH | GC-qMS | 0.1–0.8 | 90–102 | [94] |
| 38 carbonyl compound | Port wine | HS | 20 | 32 | PDMS/DVB | 250 | PFBHA | GC-MS | 0.006–0.089 | 88–119 | [95] |
| 45 carbonyl compound | Wine | HS | 20 | 40 | PDMS/DVB | 250 | PFBHA | GC-MS/MS | - | 71–146 | [96] |
| 9 aldehydes | Spirits and alcoholic beverages | DI | 15 | 20 | PDMS | 250 | PFBHA | GC-ECD | 0.05–0.5 | - | [97] |
| VOC & SVOC | Beer, wine & whisky | HS | 60 | 30 | PDMS CAR/PDMS DVB/CAR/PDMS |
250 260 260 |
- | GC-MS | - | - | [11] |
| 20 aldehydes | Green pomace distillates | HS | 40 | 55 | PDMS/DVB | 250 | PFBHA | GC-MS | 0.0007–0.02 | 76–110 | [98] |
| 107 VOC | Cider | HS | 30 | 50 | DVB/CAR/PDMS | 250 | - | GC-MS | - | - | [99] |
| 53 carbonyl compounds | Huangjiu (alcoholic beverage) | HS | 35 | 45 | DVB/CAR/PDMS | 250 | PFBHA | GC-MS/MS | - | 71–146 | [100] |
| 2 α-DCC | Soybean paste, red pepper past, soy sauce, wine, beer, distilled liquor | HS | 20 | 85 | DVB/CAR/PDMS | 240 | TFEH | GC-MS | 0.7–1.1 | 92–104 | [101] |
4. Dispersive Liquid-Liquid Microextraction
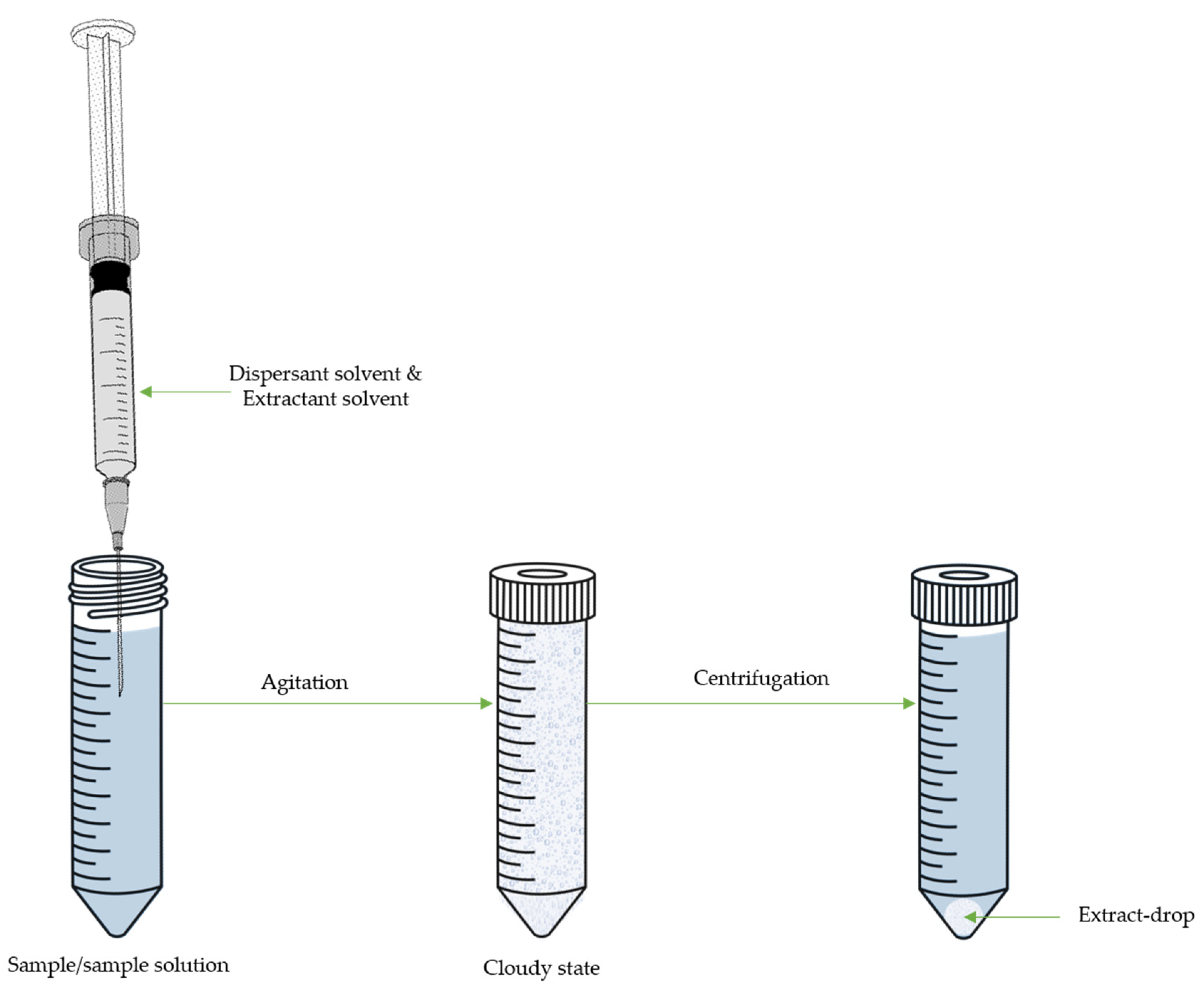
| Target Compound | Sample | DLLME | Derivative Reagent | Determ. | LOD µg/L or µg/Kg |
Rec. % |
Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode | Disperser | Extracting Solvent | T min |
T °C |
|||||||
| Formaldehyde | Beverages | MW-IL- | ACN | IL 3453W | 1.5 | - | DNPH | HPLC-UV | 0.12 | 85–95 | [108] |
| Acrylamide | Brewed coffee | - | ACN | DCM | - | - | - | UPLC-MS/MS | 900 | 97–106 | [109] |
| PCB and acrylamide | Milk/Coffee | IL | [HeOHMIM][Cl] | [BMIM][NTf2] | - | - | - | HS-GC-ECD-MS | - | - | [110] |
| MDA, acrolein, 4-HNE | Beverages | US | ACN | CH3Cl | 5 | 60 °C | DNPH | GC-MS | 50–200 | 94–102 | [111] |
| Formaldehyde | Milk | IL | MeOH | IL 3453W | 0.75 | 45 °C | ACAC | UV | 100 | 91–103 | [112] |
| Acrylamide | Coffee, chocolate, roasted nuts, French fries, cereals, biscuits, chips, bread, and caramelized fruit |
SSA | SUPRAS-2 (SDS/TBABr/AlCl3) |
2 | - | UV | 0.2 | 93–96 | [113] | ||
| Acrylamide | Nuts and seeds | - | PCE | EtOH | 3 | - | Xanthydrol | GC-MS | 0.6 | 95 | [114] |
| Acrylamide | Potato chips | UAE | PCE | EtOH | 2 | - | Xanthydrol | GC-MS | 0.6 | 97 | [115] |
| Acrylamide | Cereal products | - | PCE | EtOH | 1 | - | Xanthydrol | GC-MS | 0.6 | 95 | [116] |
| Acrylamide | Bread | UAE | PCE | MeOH | 1 | - | Xanthydrol | GC-MS | 0.54 | 98 | [117] |
| 4 aldehydes Acrolein & MDA | Vegetable oil | US | ACN | CH3Cl | 5 | 60 | DPNH | GC-MS | 50–100 | ≥95% | 10 |
5. Combined Microextraction Techniques
References
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972.
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124.
- Repetto, M.; Semprine, J.; Boveris, A. Lipid peroxidation: Chemical mechanism, biological implications and analytical determination. Lipid Peroxidation 2012, 1, 3–30.
- Sun, Y.E.; Wang, W.D.; Chen, H.W.; Li, C. Autoxidation of unsaturated lipids in food emulsion. Crit. Rev. Food Sci. Nutr. 2011, 51, 453–466.
- Pryoir, W.A.; Prier, D.G.; Lightsey, J.W. Kinetic and mechanism studies of peroxy, vitamin e and anti-oxidant free radicals by pulse radiolysis. In Autoxidation in Food and Biological Systems, 1st ed.; Simic, M.G., Karel, M., Eds.; Springer Science & Business Media: Washington, DC, USA, 2013; pp. 17–26.
- Zielinski, Z.A.; Pratt, D.A. Lipid Peroxidation: Kinetics, mechanisms, and products. J. Org. Chem. 2017, 82, 2817–2825.
- Islam, F.; Imran, A.; Nosheen, F.; Fatima, M.; Arshad, M.U.; Afzaal, M.; Ijaz, N.; Noreen, R.; Mehta, S.; Biswas, S.; et al. Functional roles and novel tools for improving-oxidative stability of polyunsaturated fatty acids: A comprehensive review. Food Sci Nutr. 2023, 11, 2471–2482.
- Rendón, M.Y.; Salva, T.D.J.G.; Bragagnolo, N. Impact of chemical changes on the sensory characteristics of coffee beans during storage. Food Chem. 2014, 147, 279–286.
- Pegg, R.B.; Shahidi, F. Off flavors and rancidity in foods. In Handbook of Meat, Poultry and Seafood Quality; Nollet, L.M.L., Ed.; Wiley-Blackwell: Oxford, UK, 2012; Part 2; pp. 127–139.
- Custodio-Mendoza, J.A.; Aja-Macaya, J.; Valente, I.M.; Rodrigues, J.A.; Almeida, P.J.; Lorenzo, R.A.; Carro, A.M. Determination of malondialdehyde, acrolein and four other products of lipid peroxidation in edible oils by Gas-Diffusion Microextraction combined with Dispersive Liquid-Liquid Microextraction. J. Chromatogr. A 2020, 1627, 461397.
- Rodrigues, F.; Caldeira, M.; Câmara, J.D.S. Development of a dynamic headspace solid-phase microextraction procedure coupled to GC–qMSD for evaluation the chemical profile in alcoholic beverages. Anal. Chim. Acta 2008, 609, 82–104.
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products-A review. Food Sci. Biotechnol. 2005, 14, 152–163.
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 1–13.
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernandez-Gutierrez, A. Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J. Agric. Food Chem. 2005, 53, 8918–8925.
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The analysis of raw spirits–a review of methodology. J. Inst. Brew. 2016, 122, 5–10.
- Hernandes, K.C.; Souza-Silva, É.A.; Assumpção, C.F.; Zini, C.A.; Welke, J.E. Carbonyl compounds and furan derivatives with toxic potential evaluated in the brewing stages of craft beer. Food Addit. Contam. Part A 2020, 37, 61–68.
- Perrone, G.G.; Tan, S.X.; Dawes, I.W. Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta Mol. Cell. Res. 2008, 1783, 1354–1368.
- Culleré, L.; Cacho, J.; Ferreira, V. An assessment of the role played by some oxidation-related aldehydes in wine aroma. J. Agric. Food. Chem. 2007, 55, 876–881.
- Amaral, A.B.; Silva, M.V.D.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Tech. 2018, 38, 1–15.
- Łuczaj, W.; Skrzydlewska, E. DNA damage caused by lipid peroxidation products. Cell Mol. Biol. Lett. 2003, 8, 391–413.
- World Health Organization. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. 2023. Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 25 August 2023).
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to use of formaldehyde as a preservative during the manufacture and preparation of food additives. EFSA J. 2007, 5, 415.
- Center for Drug Evaluation and Research M7(R2) Addendum: Application of the Principles of the ICH M7 Guideline, U.S. Food and Drug Administration. Available online: https://www.fda.gov/media/157451/download (accessed on 25 August 2023).
- Van Andel, I.; Sleijffers, A.; Schenk, E.; Rambali, B.; Wolterink, G.; Vleeming, W.; van Amsterdam, J.G.C. Adverse Health Effects of Cigarette Smoke: Aldehydes Crotonaldehyde, Butyraldehyde, Hexanal and Malonaldehyde; Dutch National Institute of Public Health and the Environment: Utrecht, The Netherland, 2006; Available online: http://hdl.handle.net/10029/7336 (accessed on 25 August 2023).
- Gomes, R.; Meek, M.K. Acrolein. Concise International Chemical Assessment Document 43. World Health Organization. 2002. Available online: https://www.who.int/ipcs/publications/cicad/en/cicad43.pdf (accessed on 25 August 2023).
- Papastergiadis, A.; Fatouh, A.; Jacxsens, L.; Lachat, C.; Shrestha, K.; Daelman, J.; Kolsteren, P.; Van Langenhove, H.; De Meulenaer, B. Exposure assessment of Malondialdehyde, 4-Hydroxy-2-(E)-Nonenal and 4-Hydroxy-2-(E)-Hexenal through specific foods available in Belgium. Food Chem. Tox. 2014, 73, 51–58.
- Guth, S.; Baum, M.; Cartus, A.T.; Diel, P.; Engel, K.H.; Engeli, B.; Epe, B.; Grune, T.; Haller, D.; Heinz, V.; et al. Evaluation of the genotoxic potential of acrylamide: Arguments for the derivation of a tolerable daily intake (TDI value). Food Chem. Toxicol. 2023, 173, 113632.
- Clark, S.; Winter, C.K. Diacetyl in foods: A review of safety and sensory characteristics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 634–643.
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Mennes, W. Scientific Opinion on Flavouring Group Evaluation 13 Revision 3 (FGE. 13Rev3): Furfuryl and furan derivatives with and without additional side-chain substituents and heteroatoms from chemical group 14. EFSA J. 2021, 19, 06386.
- Kielhorn, J.; Pohlenz-Michel, C.; Schmidt, S.; Mangelsdorf, I. Glyoxal. World Health Organization. 2004. Available online: https://apps.who.int/iris/handle/10665/42867 (accessed on 25 August 2023).
- Renwick, A.G. Structure-based thresholds of toxicological concern—Guidance for application to substances present at low levels in the diet. Toxicol. Appl. Pharmacol. 2005, 207, 585–591.
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649.
- Demirci-Cekic, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. 2022, 209, 114477.
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of secondary and tertiary volatile compounds resulting from the lipid oxidation of rapeseed oil. Foods 2021, 10, 2417.
- Laghrib, F.; Lahrich, S.; El Mhammedi, M.A. Recent Advances in Direct and Indirect Methods for Sensing Carbonyl Compounds Aldehydes in Environment and Foodstuffs. J. Electrochem. Soc. 2019, 166, B1543.
- Li, H.; Li, H.; Liu, X.; Chen, B. Analysis of volatile flavor compounds in top fermented wheat beer by headspace sampling-gas chromatography. Int. J. Agric. Biol. Eng. 2012, 5, 67–75.
- Zamani, A.; Fashi, A. Extraction and preconcentration of trace malondialdehyde from lipid-rich foods using ion pair–based solvent bar liquid-phase microextraction. Food Anal. Methods 2019, 12, 1625–1634.
- Zhang, D.; Zhang, J.; Li, M.; Li, W.; Aimaiti, G.; Tuersun, G.; Chu, Q. A novel miniaturised electrophoretic method for determining formaldehyde and acetaldehyde in food using 2-thiobarbituric acid derivatisation. Food Chem. 2011, 129, 206–212.
- Zeb, A.; Ullah, F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J. Anal. Methods Chem. 2016, 2016, 9412767.
- Mathew, S.; Grey, C.; Rumpunen, K.; Adlercreutz, P. Analysis of carbonyl compounds in sea buckthorn for the evaluation of triglyceride oxidation, by enzymatic hydrolysis and derivatisation methodology. Food Chem. 2011, 126, 1399–1405.
- Suh, J.H.; Ho, C.T.; Wang, Y. Evaluation of carbonyl species in fish oil: An improved LC–MS/MS method. Food Control 2017, 78, 463–468.
- Serrano, M.; Gallego, M.; Silva, M. Quantitative analysis of aldehydes in canned vegetables using static headspace–gas chromatography–mass spectrometry. J. Chromatogr. A 2017, 1524, 21–28.
- Zelzer, S.; Oberreither, R.; Bernecker, C.; Stelzer, I.; Truschnig-Wilders, M.; Fauler, G. Measurement of total and free malondialdehyde by gas–chromatography mass spectrometry–comparison with high-performance liquid chromatography methology. Free Radic. Res. 2013, 47, 651–656.
- Ghorbani, M.; Aghamohammadhassan, M.; Chamsaz, M.; Akhlaghi, H.; Pedramrad, T. Dispersive solid phase microextraction. TrAC Trends Anal. Chem. 2019, 118, 793–809.
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530.
- Atapattu, S.N.; Rosenfeld, J.M. Analytical derivatizations in environmental analysis. J. Chromatogr. 2022, 1678, 463348.
- Kokosa, J.M.; Przyjazny, A. Green microextraction methodologies for sample preparations. Green Anal. Chem. 2022, 3, 100023.
- Pacheco, J.G.; Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A.; Barros, A.A. Gas-diffusion microextraction. J. Sep. Sci. 2010, 33, 3207–3212.
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. A comprehensive look at solid-phase microextraction technique: A review of reviews. Microchem. J. 2020, 152, 104319.
- Sajid, M. Dispersive liquid-liquid microextraction: Evolution in design, application areas, and green aspects. TrAC Trends Anal. Chem. 2022, 152, 116636.
- More, V.N.; Mundhe, D.G. Microextraction techniques in analysis of drugs. Int. J. Res. Pharm.Chem. 2013, 3, 330–344.
- Risheed, C.M.; Fakhre, N.A.; Mohammed, M.I.; Ali, D.K. Hollow Fiber Nano Membrane as Liquid Phase Microextraction for Determination of Enrofloxacin in the Prsence of Florfenicol and Tylosin in Chicken Tissues. Egypt. J. Chem. 2022, 65, 133–141.
- Mirnaghi, F.S.; Goryński, K.; Rodriguez-Lafuente, A.; Boyacı, E.; Bojko, B.; Pawliszyn, J. Microextraction versus exhaustive extraction approaches for simultaneous analysis of compounds in wide range of polarity. J. Chromatogr. A 2013, 1316, 37–43.
- Gonçalves, L.M.; Magalhães, P.J.; Valente, I.M.; Pacheco, J.G.; Dostálek, P.; Sýkora, D.; Rodrigues, J.A.; Barros, A.A. Analysis of aldehydes in beer by gas-diffusion microextraction: Characterization by high-performance liquid chromatography–diode-array detection–atmospheric pressure chemical ionization–mass spectrometry. J. Chromatogr. A 2010, 1217, 3717–3722.
- Ferreira, I.M.; Carvalho, D.O.; da Silva, M.G.; Guido, L.F. Gas-Diffusion Microextraction (GDME) Combined with Derivatization for Assessing Beer Staling Aldehydes: Validation and Application. Foods 2021, 10, 1704.
- Ramos, R.M.; Pacheco, J.G.; Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A.; Barros, A.A. Determination of free and total diacetyl in wine by HPLC–UV using gas-diffusion microextraction and pre-column derivatization. Food Control 2012, 24, 220–224.
- Cruz, M.P.; Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A.; Barros, A.A. Application of gas-diffusion microextraction to the analysis of free and bound acetaldehyde in wines by HPLC–UV and characterization of the extracted compounds by MS/MS detection. Anal. Bioanal. Chem. 2012, 403, 1031–1037.
- Ramos, R.M.; Gonçalves, L.M.; Vyskočil, V.; Rodrigues, J.A. Voltammetric determination of trace amounts of diacetyl at a mercury meniscus modified silver solid amalgam electrode following gas-diffusion microextraction. Talanta 2017, 169, 203–208.
- Santos, C.M.; Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A. Chromatographic analysis of methylglyoxal and other α-dicarbonyls using gas-diffusion microextraction. Analyst 2013, 138, 7233–7237.
- Custodio-Mendoza, J.A.; Valente, I.M.; Ramos, R.M.; Lorenzo, R.A.; Carro, A.M.; Rodrigues, J.A. Analysis of free malondialdehyde in edible oils using gas-diffusion microextraction. J. Food Compos. Anal. 2019, 82, 103254.
- Ferreira, R.C.; Ramos, R.M.; Gonçalves, L.M.; Almeida, P.J.; Rodrigues, J.A. Application of gas-diffusion microextraction to solid samples using the chromatographic determination of α-diketones in bread as a case study. Analyst 2015, 140, 3648–3653.
- Cordeiro, L.; Valente, I.M.; Santos, J.R.; Rodrigues, J.A. Qualitative carbonyl profile in coffee beans through GDME-HPLC-DAD-MS/MS for coffee preliminary characterization. Food Res. Int. 2018, 107, 536–543.
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.; Gomes, T.M.; Olayanju, B.; Pereira, J.A. Green extraction techniques as advanced sample preparation approaches in biological, food, and environmental matrices: A review. Molecules 2022, 27, 2953.
- Jeleń, H.H.; Obuchowska, M.; Zawirska-Wojtasiak, R.; Wasowicz, E. Headspace solid-phase microextraction use for the characterization of volatile compounds in vegetable oils of different sensory quality. J. Agric. Food Chem. 2000, 48, 2360–2367.
- Uchida, T.; Gotoh, N.; Wada, S. Method for analysis of 4-hydroxy-2-(E)-nonenal with solid-phase microextraction. Lipids 2002, 37, 621–626.
- Fujioka, K.; Shibamoto, T. Improved malonaldehyde assay using headspace solid-phase microextraction and its application to the measurement of the antioxidant activity of phytochemicals. J. Agric. Food Chem. 2005, 53, 4708–4713.
- Pastorelli, S.; Valzacchi, S.; Rodriguez, A.; Simoneau, C. Solid-phase microextraction method for the determination of hexanal in hazelnuts as an indicator of the interaction of active packaging materials with food aroma compounds. Food Addit. Contam. 2006, 23, 1236–1241.
- Ma, C.; Ji, J.; Tan, C.; Chen, D.; Luo, F.; Wang, Y.; Chen, X. Headspace solid-phase microextraction coupled to gas chromatography for the analysis of aldehydes in edible oils. Talanta 2014, 120, 94–99.
- Goicoechea, E.; Van Twillert, K.; Duits, M.; Brandon, E.D.; Kootstra, P.R.; Blokland, M.H.; Guillén, M.D. Use of an in vitro digestion model to study the bioaccessibility of 4-hydroxy-2-nonenal and related aldehydes present in oxidized oils rich in omega-6 acyl groups. J. Agric. Food Chem. 2008, 56, 8475–8483.
- Guillén, M.D.; Carton, I.; Salmeron, J.; Casas, C. Headspace composition of cod liver oil and its evolution in storage after opening. First evidence of the presence of toxic aldehydes. Food chem. 2009, 114, 1291–1300.
- Damerau, A.; Kamlang-ek, P.; Moisio, T.; Lampi, A.M.; Piironen, V. Effect of SPME extraction conditions and humidity on the release of volatile lipid oxidation products from spray-dried emulsions. Food Chem. 2014, 157, 1–9.
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. The key role of ovalbumin in lipid bioaccessibility and oxidation product profile during the in vitro digestion of slightly oxidized soybean oil. Food Func. 2019, 10, 4440–4451.
- Liu, X.; Jin, Q.; Liu, Y.; Huang, J.; Wang, X.; Mao, W.; Wang, S. Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil. J. Food Sci. 2011, 76, C404–C412.
- Liu, Y.; Xu, X.L.; Ouyang, G.F.; Zhou, G.H. Changes in volatile compounds of traditional Chinese Nanjing water-boiled salted duck during processing. J. Food Sci. 2006, 71, S371–S377.
- Mariutti, L.R.; Nogueira, G.C.; Bragagnolo, N. Solid phase microextraction-gas chromatography for the evaluation of secondary lipid oxidation products in chicken patties during long-term storage. J. Braz. Chem. Soc. 2009, 20, 1849–1855.
- Estévez, M.; Ventanas, S.; Cava, R. Oxidation of lipids and proteins in frankfurters with different fatty acid compositions and tocopherol and phenolic contents. Food Chem. 2007, 100, 55–63.
- Chopin, C.; Kone, M.; Serot, T. Study of the interaction of fish myosin with the products of lipid oxidation: The case of aldehydes. Food Chem. 2007, 105, 126–132.
- Iglesias, J.; Gallardo, J.M.; Medina, I. Determination of carbonyl compounds in fish species samples with solid-phase microextraction with on-fibre derivatization. Food Chem. 2010, 123, 771–778.
- Lopez, A.; Bellagamba, F.; Tirloni, E.; Vasconi, M.; Stella, S.; Bernardi, C.; Pazzaglia, M.; Moretti, V.M. Evolution of food safety features and volatile profile in white sturgeon caviar treated with different formulations of salt and preservatives during a long-term storage time. Foods 2021, 10, 850.
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284.
- García-Llatas, G.; Lagarda, M.J.; Clemente, G.; Farré, R. Monitoring of headspace volatiles in milk-cereal-based liquid infant foods during storage. Eur. J. Lipid Sci. Technol. 2006, 108, 1028–1036.
- García-Llatas, G.; Lagarda, M.J.; Romero, F.; Abellán, P.; Farré, R. A headspace solid-phase microextraction method of use in monitoring hexanal and pentane during storage: Application to liquid infant foods and powdered infant formulas. Food Chem. 2007, 101, 1078–1086.
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a headspace solid-phase microextraction gas chromatography mass spectrometry method for the quantification of volatiles associated with lipid oxidation in whole milk powder using response surface methodology. Food Chem. 2019, 292, 75–80.
- Majcher, M.A.; Goderska, K.; Pikul, J.; Jeleń, H.H. Changes in volatile, sensory and microbial profiles during preparation of smoked ewe cheese. J. Sci. Food Agric. 2011, 91, 1416–1423.
- Natrella, G.; Faccia, M.; Lorenzo, J.M.; De Palo, P.; Gambacorta, G. Sensory characteristics and volatile organic compound profile of high-moisture mozzarella made by traditional and direct acidification technology. J. Dairy Sci. 2020, 103, 2089–2097.
- Cardinali, F.; Foligni, R.; Ferrocino, I.; Harasym, J.; Orkusz, A.; Milanović, V.; Franciosa, O.; Garofalo, C.; Mannozzi, C.; Mozzon, M.; et al. Microbiological, morpho-textural, and volatile characterization of Portuguese Queijo de Nisa PDO cheese. Food Res. Int. 2022, 162, 112011.
- Vesely, P.; Lusk, L.; Basarova, G.; Seabrooks, J.; Ryder, D. Analysis of aldehydes in beer using solid-phase microextraction with on-fiber derivatization and gas chromatography/mass spectrometry. J. Agric. Food Chem. 2003, 51, 6941–6944.
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Determination of carbonyl compounds in beer by derivatisation and headspace solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chromatogr. A 2009, 1216, 5061–5068.
- Moreira, N.; Meireles, S.; Brandão, T.; de Pinho, P.G. Optimization of the HS-SPME–GC–IT/MS method using a central composite design for volatile carbonyl compounds determination in beers. Talanta 2013, 117, 523–531.
- Hernandes, K.C.; Souza-Silva, É.A.; Assumpção, C.F.; Zini, C.A.; Welke, J.E. Validation of an analytical method using HS-SPME-GC/MS-SIM to assess the exposure risk to carbonyl compounds and furan derivatives through beer consumption. Food Addit. Contam. Part A 2019, 36, 1808–1821.
- Perez Olivero, S.J.; Perez Trujillo, J.P. A new method for the determination of carbonyl compounds in wines by headspace solid-phase microextraction coupled to gas chromatography−ion trap mass spectrometry. J. Agric. Food Chem. 2010, 58, 12976–12985.
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Chowtivannakul, S. HS-SPME-GC-MS analysis of volatile aromatic compounds in alcohol related beverages made with mulberry fruits. Food Sci. Biotechnol. 2011, 20, 1021–1032.
- Lago, L.O.; Nicolli, K.P.; Marques, A.B.; Zini, C.A.; Welke, J.E. Influence of ripeness and maceration of the grapes on levels of furan and carbonyl compounds in wine—Simultaneous quantitative determination and assessment of the exposure risk to these compounds. Food Chem. 2017, 230, 594–603.
- Ferreira, D.C.; Hernandes, K.C.; Nicolli, K.P.; Souza-Silva, É.A.; Manfroi, V.; Zini, C.A.; Welke, J.E. Development of a method for determination of target toxic carbonyl compounds in must and wine using HS-SPME-GC/MS-SIM after preliminary GC× GC/TOFMS analyses. Food Anal. Methods 2019, 12, 108–120.
- Moreira, N.; Araújo, A.M.; Rogerson, F.; Vasconcelos, I.; De Freitas, V.; de Pinho, P.G. Development and optimization of a HS-SPME-GC-MS methodology to quantify volatile carbonyl compounds in Port wines. Food Chem. 2019, 270, 518–526.
- Piergiovanni, M.; Carlin, S.; Lotti, C.; Vrhovsek, U.; Mattivi, F. Development of a Fully Automated Method HS-SPME-GC-MS/MS for the Determination of Odor-Active Carbonyls in Wines: A “Green” Approach to Improve Robustness and Productivity in the Oenological Analytical Chemistry. J. Agric. Food Chem. 2023.
- Wardencki, W.; Sowiński, P.; Curyło, J. Evaluation of headspace solid-phase microextraction for the analysis of volatile carbonyl compounds in spirits and alcoholic beverages. J. Chromatogr. A 2003, 984, 89–96.
- López-Vázquez, C.; Orriols, I.; Perelló, M.C.; De Revel, G. Determination of aldehydes as pentafluorobenzyl derivatives in grape pomace distillates by HS-SPME-GC/MS. Food Chem. 2012, 130, 1127–1133.
- Perestrelo, R.; Silva, C.L.; Silva, P.; Medina, S.; Pereira, R.; Câmara, J.S. Untargeted fingerprinting of cider volatiles from different geographical regions by HS-SPME/GC-MS. Microchem. J. 2019, 148, 643–651.
- Yu, J.; Zhou, Z.; Xu, X.; Ren, H.; Gong, M.; Ji, Z.; Liu, S.; Hu, Z.; Mao, J. Differentiating Huangjiu with Varying Sugar Contents from Different Regions Based on Targeted Metabolomics Analyses of Volatile Carbonyl Compounds. Foods 2023, 12, 1455.
- Lim, H.H.; Shin, H.S. In-solution derivatization and detection of glyoxal and methylglyoxal in alcoholic beverages and fermented foods by headspace solid-phase microextraction and gas chromatography–mass spectrometry. J. Food Compos. Anal. 2020, 92, 103584.
- Rezaee, M.; Assadi, Y.; Hosseini, M.R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9.
- Yan, H.; Wang, H. Recent development and applications of dispersive liquid-liquid microextraction. J. Chromatogr. A 2013, 1295, 1–15.
- Jain, R.; Singh, R. Applications of dispersive liquid–liquid micro-extraction in forensic toxicology. TrAC Trends Anal. Chem. 2016, 75, 227–237.
- El-Deen, A.K.; Elmansi, H.; Belal, F.; Magdy, G. Recent advances in dispersion strategies for dispersive liquid-liquid microextraction from green chemistry perspectives. Microchem. J. 2023, 191, 108807.
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339.
- Marcinkowska, R.; Konieczna, K.; Marcinkowski, Ł.; Namieśnik, J.; Kloskowski, A. Application of ionic liquids in microextraction techniques: Current trends and future perspectives. TrAC Trends Anal. Chem. 2019, 119, 115614.
- Xu, X.; Su, R.; Zhao, X.; Liu, Z.; Li, D.; Li, X.; Zhang, H.; Wang, Z. Determination of formaldehyde in beverages using microwave-assisted derivatization and ionic liquid-based dispersive liquid–liquid microextraction followed by high-performance liquid chromatography. Talanta 2011, 85, 2632–2638.
- Galuch, M.B.; Magon, T.F.S.; Silveira, R.; Nicácio, A.E.; Pizzo, J.S.; Bonafe, E.G.; Maldaner, L.; Santos, O.O.; Visentainer, J.V. Determination of acrylamide in brewed coffee by dispersive liquid–liquid microextraction (DLLME) and ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). Food Chem. 2019, 282, 120–126.
- Zhang, C.; Cagliero, C.; Pierson, S.A.; Anderson, J.L. Rapid and sensitive analysis of polychlorinated biphenyls and acrylamide in food samples using ionic liquid-based in situ dispersive liquid-liquid microextraction coupled to headspace gas chromatography. J. Chromatogr. A 2017, 1481, 1–11.
- Custodio-Mendoza, J.A.; Caamaño-Fernandez, C.; Lage, M.A.; Almeida, P.J.; Lorenzo, R.A.; Carro, A.M. GC–MS determination of malondialdehyde, acrolein, and 4-hydroxy-2-nonenal by ultrasound-assisted dispersive liquid-liquid microextraction in beverages. Food Chem. 2022, 384, 132530.
- Nascimento, C.F.; Brasil, M.A.; Costa, S.P.; Pinto, P.C.; Saraiva, M.L.M.; Rocha, F.R. Exploitation of pulsed flows for on-line dispersive liquid–liquid microextraction: Spectrophotometric determination of formaldehyde in milk. Talanta 2015, 144, 1189–1194.
- Altunay, N.; Elik, A.; Tuzen, M.; Lanjwani, M.F.; Mogaddam, M.R.A. Determination and extraction of acrylamide in processed food samples using alkanol-based supramolecular solvent-assisted dispersive liquid-liquid microextraction coupled with spectrophotometer: Optimization using factorial design. J. Food Compos. Anal. 2023, 115, 105023.
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Hadian, Z.; Ghasemi, J.; Mohammadi, A. Investigation and determination of acrylamide in 24 types of roasted nuts and seeds using microextraction method coupled with gas chromatography–mass spectrometry: Central composite design. J. Food Meas. Charact. 2020, 14, 1249–1260.
- Zokaei, M.; Abedi, A.S.; Kamankesh, M.; Shojaee-Aliababadi, S.; Mohammadi, A. Ultrasonic-assisted extraction and dispersive liquid-liquid microextraction combined with gas chromatography-mass spectrometry as an efficient and sensitive method for determining of acrylamide in potato chips samples. Food chem. 2017, 234, 55–61.
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A. Investigation and determination of acrylamide in the main group of cereal products using advanced microextraction method coupled with gas chromatography-mass spectrometry. J. Cereal Sci. 2019, 87, 157–164.
- Norouzi, E.; Kamankesh, M.; Mohammadi, A.; Attaran, A. Acrylamide in bread samples: Determining using ultrasonic-assisted extraction and microextraction method followed by gas chromatography-mass spectrometry. J. cereal sci. 2018, 79, 1–5.
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A. Recent advances in combining microextraction techniques for sample pre-treatment. TRAC Trends Anal. Chem. 2015, 71, 265–274.
- Sajid, M.; Płotka-Wasylka, J. Combined extraction and microextraction techniques: Recent trends and future perspectives. TRAC Trends Anal. Chem. 2018, 103, 74–86.





