
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kevin Yang Wu | -- | 1492 | 2023-09-16 21:50:09 | | | |
| 2 | Catherine Yang | + 1 word(s) | 1493 | 2023-09-18 08:11:56 | | |
Video Upload Options
Retinal prostheses depend on specific outcomes and engineering constraints. These include effective electrical stimulation by direct contact with the retina, achieving high-resolution images through miniaturization, selective targeting of retinal cells, and customization with bidirectional functionality. Clinical results of retinal prostheses have shown limited visual resolution, posing challenges for object recognition and visual acuity. The pixel size of microelectrodes in prostheses limits acuity, indicating smaller pixels may improve resolution. Physiologically, smaller, densely packed electrodes are desired to stimulate individual retinal neurons. However, miniaturization increases charge density, necessitating material innovations to maintain safe stimulation. Considerations of biocompatibility and manufacturability are crucial. Retinal prostheses' success hinges on meeting key outcomes: effective stimulation, high-resolution images, selective targeting, and customization. These design considerations shape their manufacturing and market adoption, explored here for a comprehensive understanding.
1. Introduction
Retinal prostheses rely on meeting specific outcomes to ensure their effectiveness and acceptance. These outcomes are closely tied to engineering design challenges. This research explores the four main outcomes and their corresponding constraints (Figure 1).
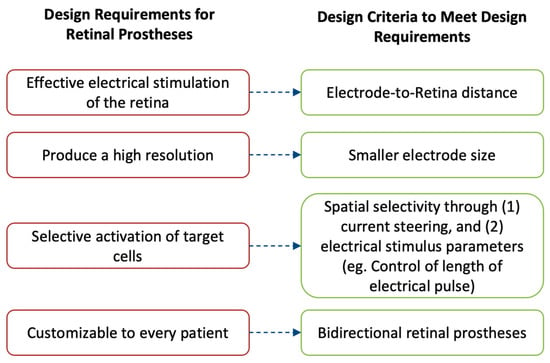
Figure 1. The performance of retinal prostheses is dependent on a few outcomes. Fulfilling these outcomes is important to drive their adoption in the market and by patients. These outcomes are (1) to provide effective electrical stimulation of the retina; (2) to produce a high-resolution image; (3) to selectively activate desired retinal cells, thereby avoiding image distortion; and (4) to be customizable for different patients. To achieve these requirements, retinal prostheses are designed with four main design criteria: (1) the electrode-to-retina distance, (2) having smaller electrode size, (3) implementing techniques to produce spatial selectivity, and (4) implementing bidirectional systems.
Firstly, retinal prostheses must deliver effective electrical stimulation to the retina, enabling the perception of light. Direct contact between the microelectrode array and the retina is crucial for successful stimulation. Even minor gaps can diminish the prosthetic's efficacy. This review addresses the challenges and proposed solutions to ensure optimal design considerations are met.
Secondly, achieving a high-resolution image that can be interpreted by the recipient is essential. Minimizing the size of individual microelectrodes on the array allows for stimulation of single retinal cells, maximizing resolution. However, miniaturization poses technical challenges, which are discussed along with potential solutions.
Thirdly, selective targeting of desired retinal cells is a key consideration. Different retinal cells produce distinct perceptions that directly impact patient outcomes. This review explores two techniques for targeted stimulation: current steering utilizing return electrodes and manipulation of electric stimulation parameters.
Lastly, customization is vital to meet each recipient's unique needs. Prostheses should convey electrical pulses while recording the effects of stimulation on the retina. The incorporation of bidirectional retinal prostheses is described as a means to achieve this outcome.
These design criteria play a crucial role in guiding the manufacturing of retinal prostheses and shaping their future market adoption. A detailed exploration of each criterion helps readers understand the challenges, recent advancements, and the potential of retinal prostheses.
2. Electrode–Retina (ER) Topographical Alignment
Microelectrode arrays (MEAs) play a critical role in retinal prostheses by delivering electrical stimuli to the retina. The signals generated by MEAs are received by retinal ganglion cells (RGCs), either directly or indirectly. In epiretinal prostheses, the electrical signals are directly received by RGCs since the prostheses are in direct contact with them. Conversely, in subretinal/suprachoroidal prostheses, the signals are first received by cells in the posterior retina, which then transmit them to the RGCs through physiological pathways. The signals are then conveyed via the optic nerve to the brain, where they are interpreted as images[1].
To ensure effective transmission of electric stimuli to retinal neurons, a close topographical fit between the MEA and retinal tissue is necessary[2]. Gaps between the implant and the retina, even if several hundred micrometers wide, can impair or even eliminate signals in those areas, thereby reducing the device's effectiveness (Figure 2, A)[3]. Researchers have proposed various solutions to overcome this topographical misalignment.
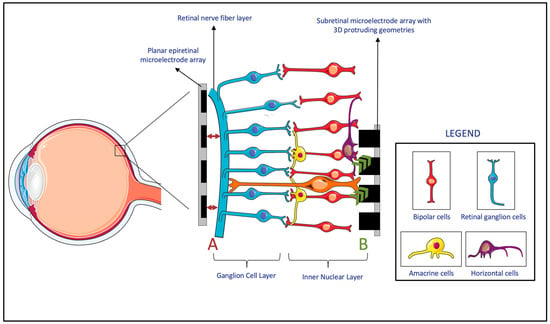
Figure 2. (A) The lack of the topographical alignment between a planar epiretinal microelectrode array and the retinal ganglion cells. (B) Migration and integration of the cells in the Inner Nuclear Layer to 3D protruding geometries in subretinal microelectrode array. (Figure 2 was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.)
Firstly, researchers have developed MEAs with three-dimensional (3D) geometries. Experiments have shown that cells from the inner neuron layer (INL) can migrate and integrate with 3D MEAs within the retina (Figure 2, B)[4]. Subretinal prostheses, located in the INL, benefit the most from this integration, as it reduces the separation distance between the INL and the implanted electrodes[4][5][6][7][8]. However, epiretinal and suprachoroidal implants have been observed to experience an increasing electrode-retina (ER) distance over time due to fibrosis and inflammatory responses[9]. While retinal migration may decrease the ER distance in subretinal implants, achieving close proximity to the MEA in epiretinal implants requires mechanical pressure[8]. As a result, these 3D geometries have been primarily considered for subretinal devices, while alternative methods are used for epiretinal and suprachoroidal implants.
Secondly, researchers have explored the integration of pneumatic cavities to dynamically control the electrode position and reduce the electrode-retina distance. Pneumatic cavities can be placed beneath the electrodes in MEAs, allowing adjustment of the electrode position by varying the pneumatic pressure[2]. Similarly, hydraulic systems can be tested for incorporation, particularly in epiretinal devices, to improve topographical alignment.
Thirdly, flexible MEA substrates have been developed to achieve better topographical alignment. Initially used for brain neuron stimulation, flexible probes have been suggested for epiretinal electrode arrays[10][11]. These flexible designs conform better to surface topographies, enabling the creation of larger and higher-density devices that can extend over the curvature of the retina. Flexible MEAs have demonstrated safety and efficacy[12][13], and their flexibility is beneficial in reducing acute insertion footprint and preserving retinal cell viability after implantation[14]. However, filling smaller gaps and sharp corners remains challenging for these microelectrode arrays[2].
In summary, several solutions have been proposed to decrease the electrode-retina distance in different types of retinal prostheses. Each solution has its advantages and disadvantages, and the appropriate features should be incorporated based on the location of the implant. Careful consideration of these engineering approaches is crucial to optimize the performance and effectiveness of retinal prostheses.
3. Electrode Size and Material, Charge Density, and Resolution Limit
Despite the positive outcomes observed in clinical trials of retinal prostheses, the achieved visual resolution remains limited, posing challenges for tasks such as object recognition and facial identification[15]. The restoration of visual acuity in patients with retinal prostheses has also been limited, with recent trials reporting visual acuities in the range of 20/460–20/565[16]. These acuities closely match the fundamental sampling limit set by the pixel size of the prosthetic microelectrodes, indicating that smaller pixels may provide higher resolution (Figure 3)[17]. Physiologically, to restore natural vision, it is ideal to stimulate individual retinal neurons[1]. Given these clinical and physiological observations, it is logical to develop MEAs with smaller and more densely packed electrodes.
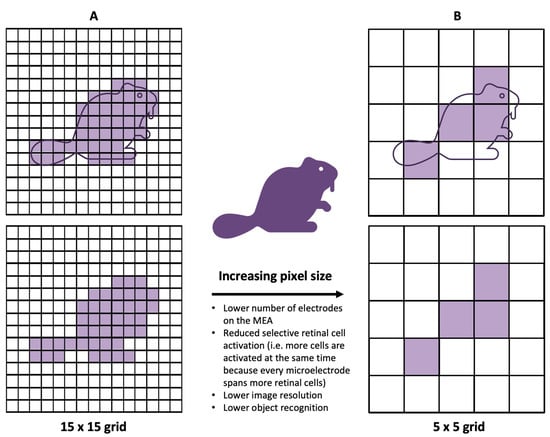
Figure 3. Demonstrating the impact of smaller electrodes. In comparison to the 15 × 15 grid (A), the 5 × 5 grid (B) produces a lower image resolution and enables less object recognition. Additionally, since each square in the grid represents the size of an electrode, having larger electrodes correlates to larger activation of retinal cells—i.e., less selective activation of desired, target retinal cells.
However, miniaturizing MEAs and reducing pixel size presents engineering challenges, particularly in terms of charge density[18]. Smaller pixel electrodes have less surface area available for charge transfer to the surrounding tissue, while retinal cells require a minimum amount of charge to reach the activation threshold for relaying visual information[19]. To maintain a similar electric field penetration depth, higher charge densities per surface area are necessary with smaller electrodes[20]. Meeting this increased charge-density requirement is challenging due to material limitations and safety considerations[21][22]. Conventional platinum electrodes commonly used in visual prostheses exceed the safe charge injection limits when pixel size is reduced[23].
To address this challenge, researchers have explored modifications to MEAs using innovative materials. For example, nanocone-shaped platinum–iridium oxide neural microelectrodes have been manufactured to increase the electrode surface area[24]. Other approaches include incorporating high-conductivity materials like graphene, using materials with superior charge injection properties such as polymer/carbon nanotubes, and utilizing carbon nanotube-modified gold[23][25][26]. These material innovations enable efficient charge injection and offer the potential to decrease electrode size on MEAs, ultimately improving the resolution limit of retinal prostheses[27]. However, the biocompatibility, manufacturability, and batch-to-batch consistency of these proposed materials must be thoroughly studied to maximize benefits while minimizing risks and variability.
Even thought, the field of material science has made significant contributions to advancing retinal prostheses, it is nonetheless crucial to carefully consider these advancements to overcome the limitations of current MEAs, enhance visual resolution, and improve the effectiveness of retinal prostheses. By developing smaller and more densely packed electrodes while ensuring biocompatibility and charge injection efficiency, researchers aim to bring retinal prostheses closer to emulating natural vision and providing meaningful visual experiences for individuals with visual impairments.
References
- Tong, W.; Stamp, M.; Apollo, N.V.; Ganesan, K.; Meffin, H.; Prawer, S.; Garrett, D.J.; Ibbotson, M.R. Improved Visual Acuity Using a Retinal Implant and an Optimized Stimulation Strategy. J. Neural Eng. 2019, 17, 016018.
- Xu, Y.; Pang, S. Microelectrode Array With Integrated Pneumatic Channels for Dynamic Control of Electrode Position in Retinal Implants. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2292–2298.
- Avraham, D.; Yitzhaky, Y. Simulating the Perceptual Effects of Electrode–Retina Distance in Prosthetic Vision. J. Neural Eng. 2022, 19, 035001.
- Flores, T.; Huang, T.; Bhuckory, M.; Ho, E.; Chen, Z.; Dalal, R.; Galambos, L.; Kamins, T.; Mathieson, K.; Palanker, D. Honeycomb-Shaped Electro-Neural Interface Enables Cellular-Scale Pixels in Subretinal Prosthesis. Sci. Rep. 2019, 9, 10657.
- Seo, H.W.; Kim, N.; Ahn, J.; Cha, S.; Goo, Y.S.; Kim, S. A 3D Flexible Microelectrode Array for Subretinal Stimulation. J. Neural Eng. 2019, 16, 056016.
- Vu, Q.A.; Seo, H.W.; Choi, K.-E.; Kim, N.; Kang, Y.N.; Lee, J.; Park, S.-H.; Kim, J.T.; Kim, S.; Kim, S.-W. Structural Changes in the Retina after Implantation of Subretinal Three-Dimensional Implants in Mini Pigs. Front. Neurosci. 2022, 16, 1010445.
- Seo, H.W.; Kim, N.; Kim, S. Fabrication of Subretinal 3D Microelectrodes with Hexagonal Arrangement. Micromachines 2020, 11, 467.
- Flores, T.; Lei, X.; Huang, T.; Lorach, H.; Dalal, R.; Galambos, L.; Kamins, T.; Mathieson, K.; Palanker, D. Optimization of Pillar Electrodes in Subretinal Prosthesis for Enhanced Proximity to Target Neurons. J. Neural Eng. 2018, 15, 036011.
- Abbott, C.J.; Baglin, E.K.; Kolic, M.; McGuinness, M.B.; Titchener, S.A.; Young, K.A.; Yeoh, J.; Luu, C.D.; Ayton, L.N.; Petoe, M.A.; et al. Interobserver Agreement of Electrode to Retina Distance Measurements in a Second-Generation (44-Channel) Suprachoroidal Retinal Prosthesis. Transl. Vis. Sci. Technol. 2022, 11, 4.
- Sharafkhani, N.; Kouzani, A.Z.; Adams, S.D.; Long, J.M.; Lissorgues, G.; Rousseau, L.; Orwa, J.O. Neural Tissue-Microelectrode Interaction: Brain Micromotion, Electrical Impedance, and Flexible Microelectrode Insertion. J. Neurosci. Methods 2022, 365, 109388.
- Zhou, M.; Kang, D.H.; Kim, J.; Weiland, J.D. Shape Morphable Hydrogel/Elastomer Bilayer for Implanted Retinal Electronics. Micromachines 2020, 11, 392.
- Ferlauto, L.; Airaghi Leccardi, M.J.I.; Chenais, N.A.L.; Gilliéron, S.C.A.; Vagni, P.; Bevilacqua, M.; Wolfensberger, T.J.; Sivula, K.; Ghezzi, D. Design and Validation of a Foldable and Photovoltaic Wide-Field Epiretinal Prosthesis. Nat. Commun. 2018, 9, 992.
- Vagni, P.; Airaghi Leccardi, M.J.I.; Vila, C.-H.; Zollinger, E.G.; Sherafatipour, G.; Wolfensberger, T.J.; Ghezzi, D. POLYRETINA Restores Light Responses in Vivo in Blind Göttingen Minipigs. Nat. Commun. 2022, 13, 3678.
- Rincón Montes, V.; Gehlen, J.; Ingebrandt, S.; Mokwa, W.; Walter, P.; Müller, F.; Offenhäusser, A. Development and in Vitro Validation of Flexible Intraretinal Probes. Sci. Rep. 2020, 10, 19836.
- Tong, W.; Meffin, H.; Garrett, D.J.; Ibbotson, M.R. Stimulation Strategies for Improving the Resolution of Retinal Prostheses. Front. Neurosci. 2020, 14, 262.
- Palanker, D.; Le Mer, Y.; Mohand-Said, S.; Sahel, J.A. Simultaneous Perception of Prosthetic and Natural Vision in AMD Patients. Nat. Commun. 2022, 13, 513.
- Chen, Z.C.; Wang, B.-Y.; Goldstein, A.K.; Butt, E.; Mathieson, K.; Palanker, D. Photovoltaic Implant Simulator Reveals Resolution Limits in Subretinal Prosthesis. J. Neural Eng. 2022, 19, 055008.
- Zheng, X.S.; Yang, Q.; Vazquez, A.L.; Tracy Cui, X. Imaging the Efficiency of Poly(3,4-Ethylenedioxythiophene) Doped with Acid-Functionalized Carbon Nanotube and Iridium Oxide Electrode Coatings for Microstimulation. Adv. NanoBiomed Res. 2021, 1, 2000092.
- Xu, Z.; Lu, Y.; Qin, S.; Wu, T.; Qin, B. Electrical Stimulation Scheme Optimization for Retinal Prosthesis: Considerations from Biological Perspective. Ann. Eye Sci. 2020, 5, 13.
- Weiland, J.D.; Fink, W.; Humayun, M.; Liu, W.; Rodger, D.C.; Tai, Y.-C.; Tarbell, M. Progress Towards A High-Resolution Retinal Prosthesis. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2005; pp. 7373–7375.
- Cui, H.; Xie, X.; Xu, S.; Chan, L.L.H.; Hu, Y. Electrochemical Characteristics of Microelectrode Designed for Electrical Stimulation. BioMed. Eng. OnLine 2019, 18, 86.
- Wang, B.-Y.; Chen, Z.C.; Bhuckory, M.; Kochnev Goldstein, A.; Palanker, D. Pixel Size Limit of the PRIMA Implants: From Humans to Rodents and Back. J. Neural Eng. 2022, 19, 055003.
- Zheng, X.S.; Yang, Q.; Vazquez, A.L.; Tracy Cui, X. Imaging the Efficiency of Poly(3,4-Ethylenedioxythiophene) Doped with Acid-Functionalized Carbon Nanotube and Iridium Oxide Electrode Coatings for Microstimulation. Adv. NanoBiomed Res. 2021, 1, 2000092.
- Zeng, Q.; Yu, S.; Fan, Z.; Huang, Y.; Song, B.; Zhou, T. Nanocone-Array-Based Platinum-Iridium Oxide Neural Microelectrodes: Structure, Electrochemistry, Durability and Biocompatibility Study. Nanomaterials 2022, 12, 3445.
- Nguyen, D.; Valet, M.; Dégardin, J.; Boucherit, L.; Illa, X.; de la Cruz, J.; Del Corro, E.; Bousquet, J.; Garrido, J.A.; Hébert, C.; et al. Novel Graphene Electrode for Retinal Implants: An in Vivo Biocompatibility Study. Front. Neurosci. 2021, 15, 615256.
- Vafaiee, M.; Mohammadpour, R.; Vossoughi, M.; Asadian, E.; Janahmadi, M.; Sasanpour, P. Carbon Nanotube Modified Microelectrode Array for Neural Interface. Front. Bioeng. Biotechnol. 2020, 8, 582713.
- Tang, J.; Qin, N.; Chong, Y.; Diao, Y.; Yiliguma; Wang, Z.; Xue, T.; Jiang, M.; Zhang, J.; Zheng, G. Nanowire Arrays Restore Vision in Blind Mice. Nat. Commun. 2018, 9, 786.




