
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlos M. Villalón | -- | 2352 | 2023-07-12 10:06:40 | | | |
| 2 | Wendy Huang | -26 word(s) | 2326 | 2023-07-13 07:43:27 | | | | |
| 3 | Wendy Huang | -3 word(s) | 2323 | 2023-07-14 13:46:48 | | |
Video Upload Options
5-Hydroxytryptamine (5-HT), or serotonin, plays a crucial role as a neuromodulator and/or neurotransmitter of several nervous system functions. Its actions are complex, and depend on multiple factors, including the type of effector or receptor activated. Briefly, 5-HT can activate: (i) metabotropic (G-protein-coupled) receptors to promote inhibition (5-HT1, 5-HT5) or activation (5-HT4, 5-HT6, 5-HT7) of adenylate cyclase, as well as activation (5-HT2) of phospholipase C; and (ii) ionotropic receptor (5-HT3), a ligand-gated Na+/K+ channel. Regarding blood pressure regulation (and beyond the intricacy of central 5-HT effects), this monoamine also exerts direct postjunctional (on vascular smooth muscle and endothelium) or indirect prejunctional (on autonomic and sensory perivascular nerves) effects. At the prejunctional level, 5-HT can facilitate or preclude the release of autonomic (e.g., noradrenaline and acetylcholine) or sensory (e.g., calcitonin gene-related peptide) neurotransmitters facilitating hypertensive or hypotensive effects. Hence, we cannot formulate a specific impact of 5-HT on blood pressure level, since an increase or decrease in neurotransmitter release would be favoured, depending on the type of prejunctional receptor involved.
1. 5-HT Receptors
| 5-HT Receptor |
Receptor Subtype | Agonists | Antagonists | Some Functions | Canonical Transduction System |
|---|---|---|---|---|---|
| 5-HT1 | 5-HT1A | 8-OH-DPAT | WAY 100635 | Central hypotension | G-protein coupled receptor (Gi)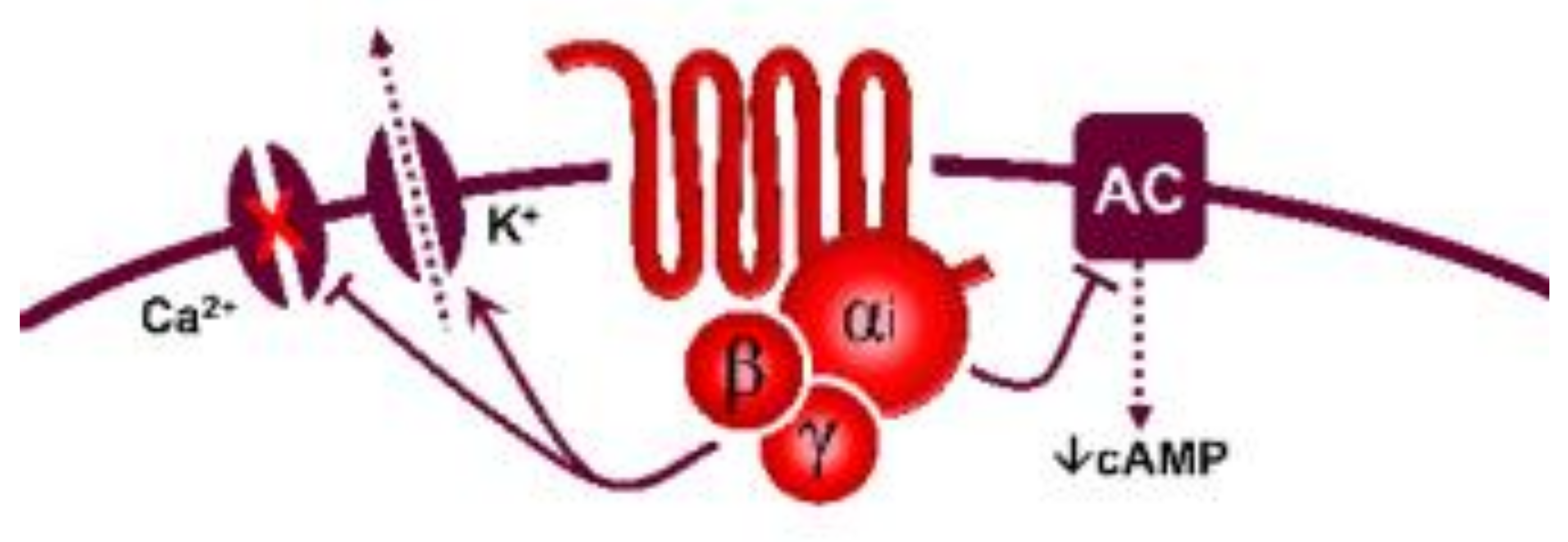 |
| 5-HT1B | Sumatriptan CP-93,129 (rodents) |
SB224289 | Vasoconstriction, sympatho-inhibition | ||
| 5-HT1D | PNU-109291 PNU-142633 |
BRL15572 | Autoreceptor, sympatho-inhibition | ||
| 5-HT1e * | 5-HT >> 5-CT LY334370 | Methiothepin (non-selective) |
Unknown | ||
| 5-HTF | LY344864, lasmiditan, LY334370 | Methysergide (non-selective) |
(−) Trigeminal system | ||
| 5-HT5 | 5-HT5A | 5-HT, ergotamine | SB699551 | Cardiac sympatho-inhibition in rats | |
| 5-HT5b * | 5-CT (non-selective) | Unknown | Unknown | ||
| 5-HT4 | - | Renzapride, BIMU8, ML10302, SC53116 | GR 113808 SB204070 | (+) Neuronal activity, vasodilatation, tachycardiain pigs and humans |
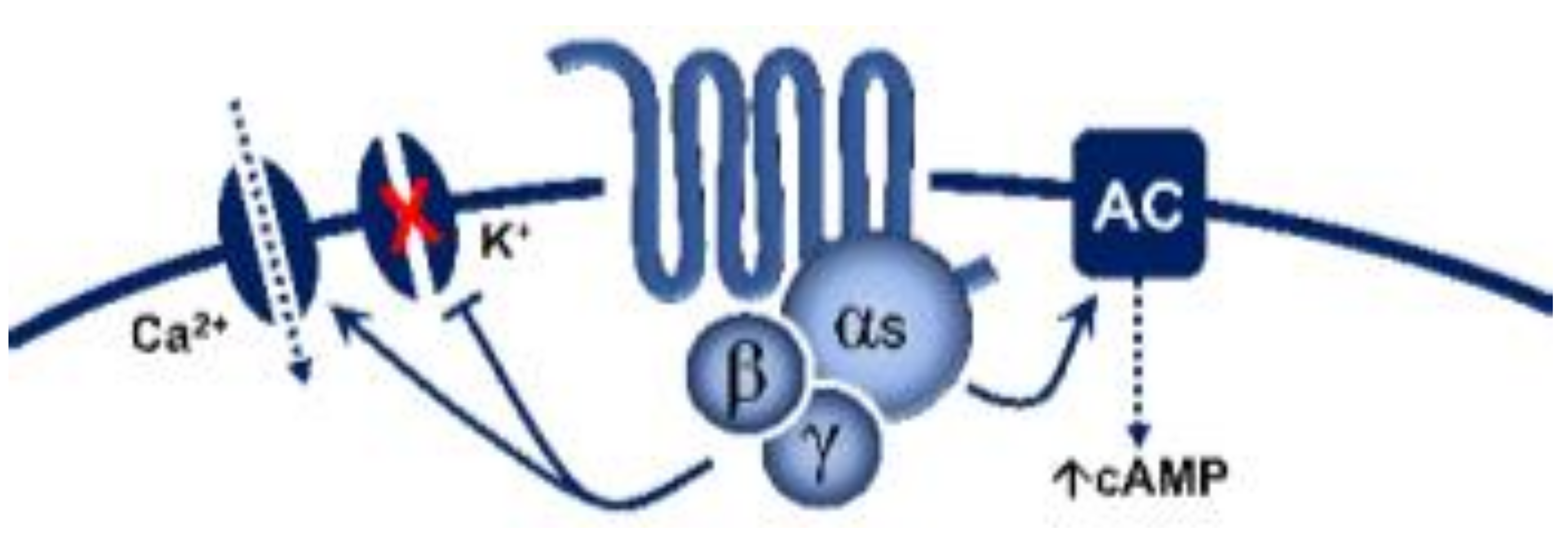 G-protein coupled receptor (Gs) |
| 5-HT6 | - | 5-MeO-T ≥ 5-HT SB357134 SB271046 |
Ro 630563 | Memory, not involved in cardiovascular regulation |
|
| 5-HT7 | - | 5-CT>>5-HT AS-19 |
SB269970 SB258719 | Circadian rhythm, vasodila- tation, tachycardia in cats |
|
| 5-HT2 | 5-HT2A | DOI, DOB α-methyl-5-HT |
MDL100907 Ketanserin |
Vasoconstriction, plateletaggregation | 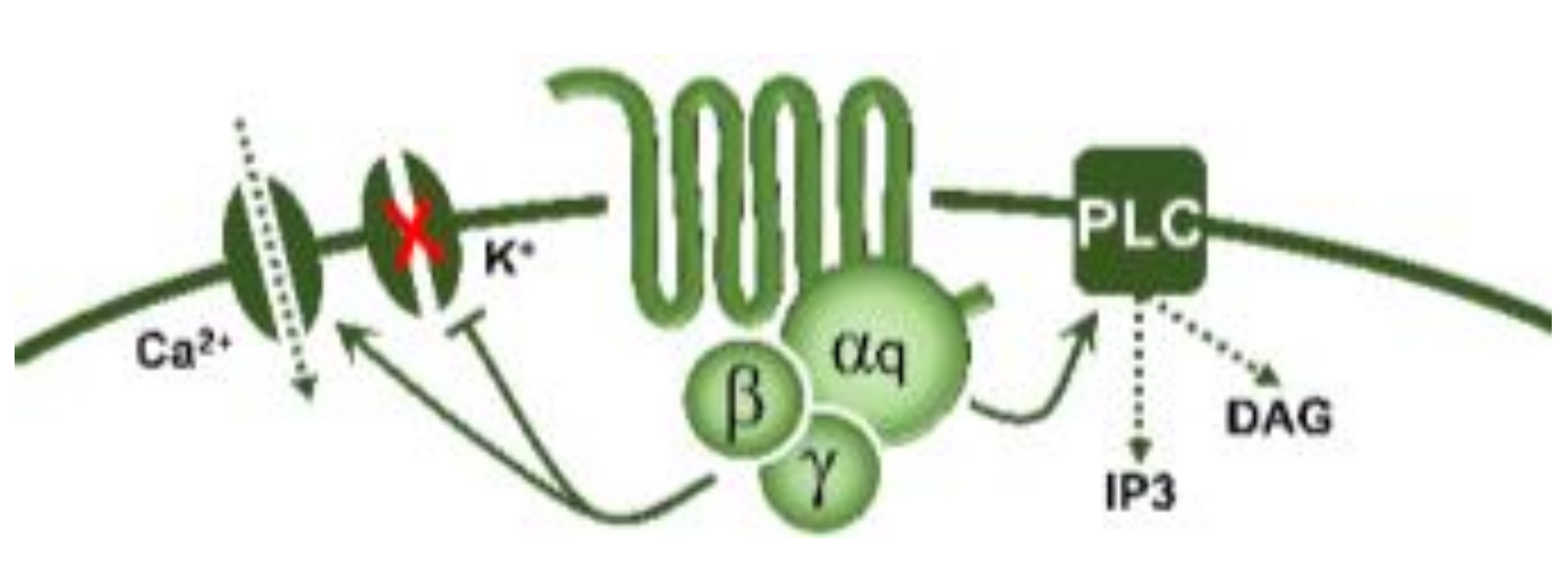 G-protein coupled receptor (Gq) |
| 5-HT2B | DOI, BW723C86 α-methyl-5-HT |
SB204741 RS-127445 |
Vasoconstriction, release of NO | ||
| 5-HT2C | DOI, Ro 60-0175 α-methyl-5-HT |
SB242084 RS-102221 |
CSF production | ||
| 5-HT3 | Pentameric ion channel ** | Phenylbiguanide 2-methyl-5-HT |
Tropisetron Granisetron MDL-72222 |
(+) Neuronal activity, reflex bradycardia |
Ligand-gated ion channel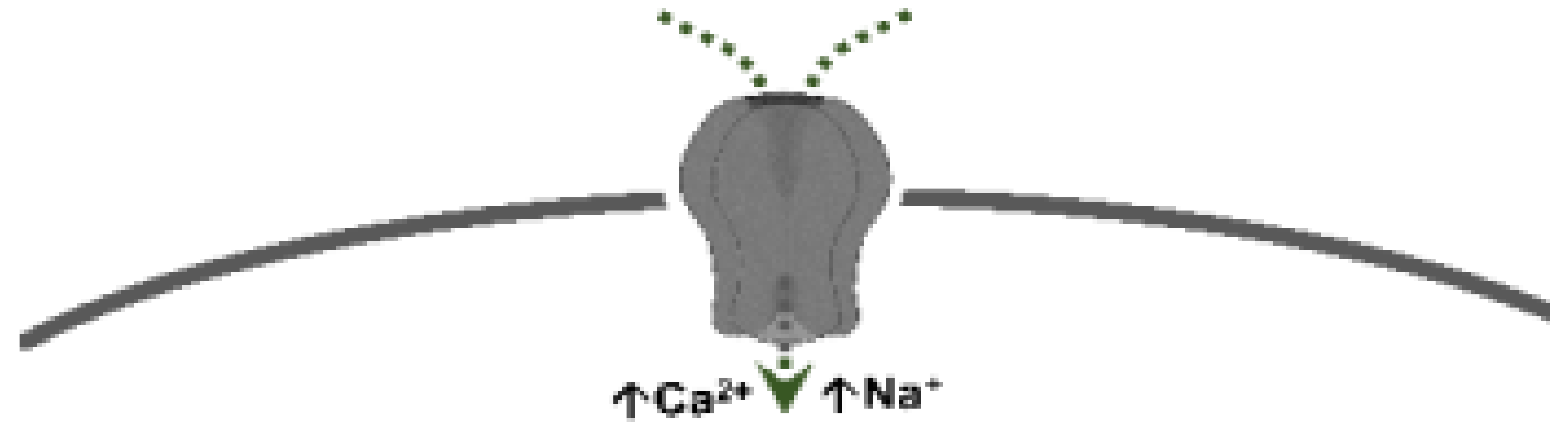 |
2. An Overview of the Effects of 5-HT on the Cardiovascular System
3. The Specific Interactions of 5-HT at Peripheral and Central Levels to Induce Cardiovascular Effects
3.1. Sensory Afferents
3.2. Sympathetic Ganglia
3.3. Cardiac Effects of 5-HT
3.3.1. Bradycardia
3.3.2. Tachycardia
3.4. Vascular and Blood Pressure Effects of 5-HT
3.4.1. Initial Transient Vasodepressor Effect
3.4.2. Vasopressor Effect
3.4.3. Late Long-Lasting Vasodepressor Effect
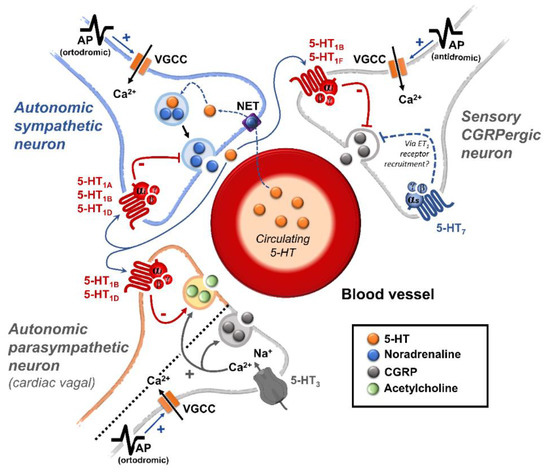
3.5. Receptor-Independent Actions of 5-HT
References
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520.
- Villalón, C.M. The role of serotonin receptors in the control of cardiovascular function. In The Serotonin System; Tricklebank, M.D., Daly, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 3; pp. 45–61.
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203.
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554.
- Kaumann, A.J.; Levy, F.O. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 2006, 111, 674–706.
- Watts, S.W.; Davis, R.P. 5-hydroxtryptamine receptors in systemic hypertension: An arterial focus. Cardiovasc. Ther. 2011, 29, 54–67.
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and blood pressure regulation. Pharmacol. Rev. 2012, 64, 359–388.
- González-Hernández, A.; Marichal-Cancino, B.A.; Lozano-Cuenca, J.; López-Canales, J.S.; Muñoz-Islas, E.; Ramírez-Rosas, M.B.; Villalón, C.M. Heteroreceptors Modulating CGRP Release at Neurovascular Junction: Potential Therapeutic Implications on Some Vascular-Related Diseases. Biomed. Res. Int. 2016, 2016, 2056786.
- Ramage, A.G. Influence of 5-HT1A receptor agonists on sympathetic and parasympathetic nerve activity. J. Cardiovasc. Pharmacol. 1990, 15 (Suppl. S7), S75–S85.
- Ramage, A.G. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res. Bull. 2001, 56, 425–439.
- Sánchez-Lopez, A.; Centurión, D.; Vázquez, E.; Arulmani, U.; Saxena, P.R.; Villalón, C.M. Pharmacological profile of the 5-HT-induced inhibition of cardioaccelerator sympathetic outflow in pithed rats: Correlation with 5-HT1 and putative 5-ht5A/5B receptors. Br. J. Pharmacol. 2003, 140, 725–735.
- García-Pedraza, J.; Hernández-Abreu, O.; García, M.; Morán, A.; Villalón, C.M. Chronic 5-HT(2) receptor blockade unmasks the role of 5-HT(1F) receptors in the inhibition of rat cardioaccelerator sympathetic outflow. Can. J. Physiol. Pharmacol. 2018, 96, 328–336.
- García-Pedraza, J.; García, M.; Martín, M.L.; Gómez-Escudero, J.; Rodríguez-Barbero, A.; Román, L.S.; Morán, A. Peripheral 5-HT₁D and 5-HT₇ serotonergic receptors modulate sympathetic neurotransmission in chronic sarpogrelate treated rats. Eur. J. Pharmacol. 2013, 714, 65–73.
- Dabiré, H. Central 5-hydroxytryptamine (5-HT) receptors in blood pressure regulation. Therapie 1991, 46, 421–429.
- Bedi, U.S.; Arora, R. Cardiovascular manifestations of posttraumatic stress disorder. J. Natl. Med. Assoc. 2007, 99, 642–649.
- Tania, V.; Catherine, V. Roles of the Serotoninergic System in Coping with Traumatic Stress. In Serotonin and the CNS; Berend, O., Ed.; IntechOpen: Rijeka, Croatia, 2021; pp. 1–5.
- Paine, N.J.; Watkins, L.L.; Blumenthal, J.A.; Kuhn, C.M.; Sherwood, A. Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure. Psychosom. Med. 2015, 77, 136–144.
- Brindley, R.L.; Bauer, M.B.; Blakely, R.D.; Currie, K.P.M. An interplay between the serotonin transporter (SERT) and 5-HT receptors controls stimulus-secretion coupling in sympathoadrenal chromaffin cells. Neuropharmacology 2016, 110, 438–448.
- Nakatani, Y.; Sato-Suzuki, I.; Tsujino, N.; Nakasato, A.; Seki, Y.; Fumoto, M.; Arita, H. Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur. J. Neurosci. 2008, 27, 2466–2472.
- Wang, H.M.; Wang, Y.; Liu, M.; Bai, Y.; Zhang, X.H.; Sun, Y.X.; Wang, H.L. Fluoxetine inhibits monocrotaline-induced pulmonary arterial remodeling involved in inhibition of RhoA-Rho kinase and Akt signalling pathways in rats. Can. J. Physiol. Pharmacol. 2012, 90, 1506–1515.
- Lin, J.C.; Chou, C.C.; Tu, Z.; Yeh, L.F.; Wu, S.C.; Khoo, K.H.; Lin, C.H. Characterization of protein serotonylation via bioorthogonal labeling and enrichment. J. Proteome Res. 2014, 13, 3523–3529.
- Penumatsa, K.C.; Fanburg, B.L. Transglutaminase 2-mediated serotonylation in pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L309–L315.




