5-Hydroxytryptamine (5-HT), or serotonin, plays a crucial role as a neuromodulator and/or neurotransmitter of several nervous system functions. Its actions are complex, and depend on multiple factors, including the type of effector or receptor activated. Briefly, 5-HT can activate: (i) metabotropic (G-protein-coupled) receptors to promote inhibition (5-HT1, 5-HT5) or activation (5-HT4, 5-HT6, 5-HT7) of adenylate cyclase, as well as activation (5-HT2) of phospholipase C; and (ii) ionotropic receptor (5-HT3), a ligand-gated Na+/K+ channel.
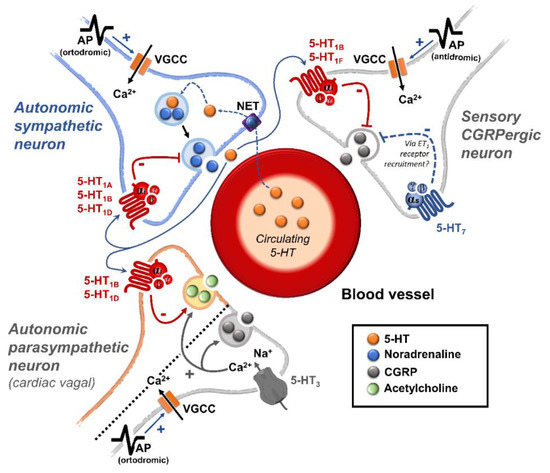
Regarding blood pressure regulation (and beyond the intricacy of central 5-HT effects), this monoamine also exerts direct postjunctional (on vascular smooth muscle and endothelium) or indirect prejunctional (on autonomic and sensory perivascular nerves) effects. At the prejunctional level, 5-HT can facilitate or preclude the release of autonomic (e.g., noradrenaline and acetylcholine) or sensory (e.g., calcitonin gene-related peptide) neurotransmitters facilitating hypertensive or hypotensive effects. Hence, we cannot formulate a specific impact of 5-HT on blood pressure level, since an increase or decrease in neurotransmitter release would be favoured, depending on the type of prejunctional receptor involved.
This review summarizes and discusses the current knowledge on the prejunctional mechanisms involved in blood pressure regulation by 5-HT and its impact on some vascular-related diseases.
- 5-hydroxytryptamine
- serotonin
- CGRP
- blood pressure
- hypertension
1. A Summary on 5-HT Receptors
| 5-HT Receptor |
Receptor Subtype | Agonists | Antagonists | Some Functions | Canonical Transduction System |
|---|---|---|---|---|---|
| 5-HT1 | 5-HT1A | 8-OH-DPAT | WAY 100635 | Central hypotension | G-protein coupled receptor (Gi)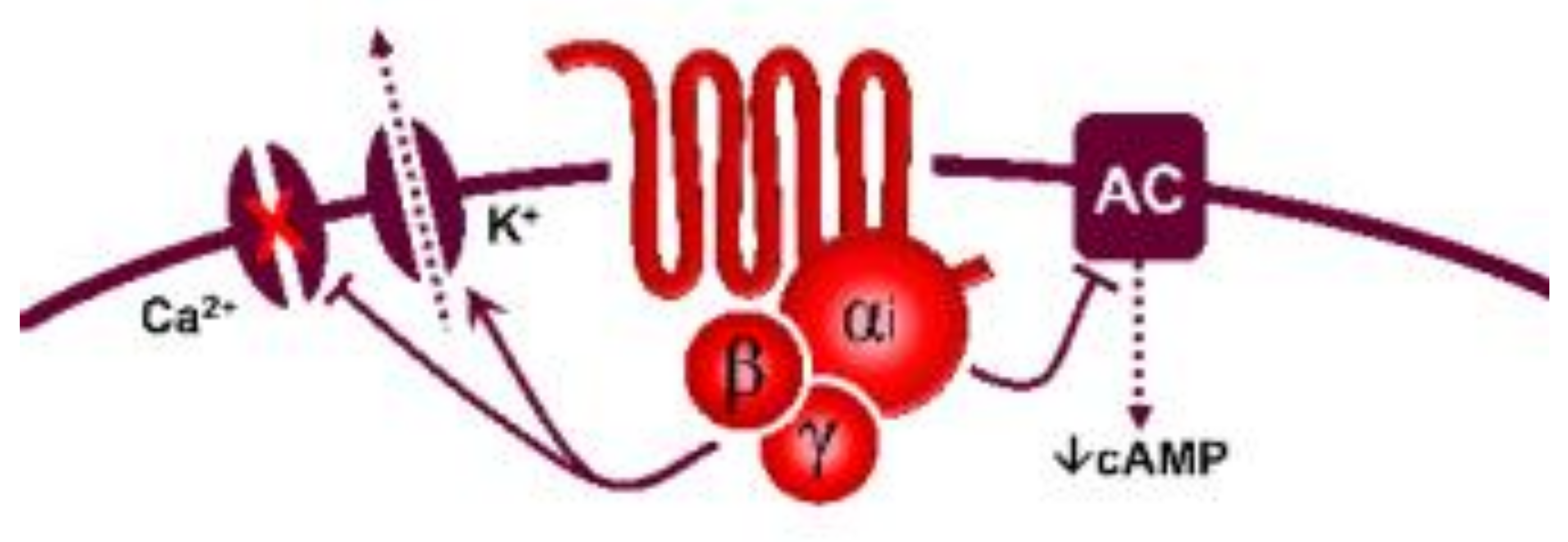 |
| 5-HT1B | Sumatriptan CP-93,129 (rodents) |
SB224289 | Vasoconstriction, sympatho-inhibition | ||
| 5-HT1D | PNU-109291 PNU-142633 |
BRL15572 | Autoreceptor, sympatho-inhibition | ||
| 5-HT1e * | 5-HT >> 5-CT LY334370 | Methiothepin (non-selective) |
Unknown | ||
| 5-HTF | LY344864, lasmiditan, LY334370 | Methysergide (non-selective) |
(−) Trigeminal system | ||
| 5-HT5 | 5-HT5A | 5-HT, ergotamine | SB699551 | Cardiac sympatho-inhibition in rats | |
| 5-HT5b * | 5-CT (non-selective) | Unknown | Unknown | ||
| 5-HT4 | - | Renzapride, BIMU8, ML10302, SC53116 | GR 113808 SB204070 | (+) Neuronal activity, vasodilatation, tachycardiain pigs and humans |
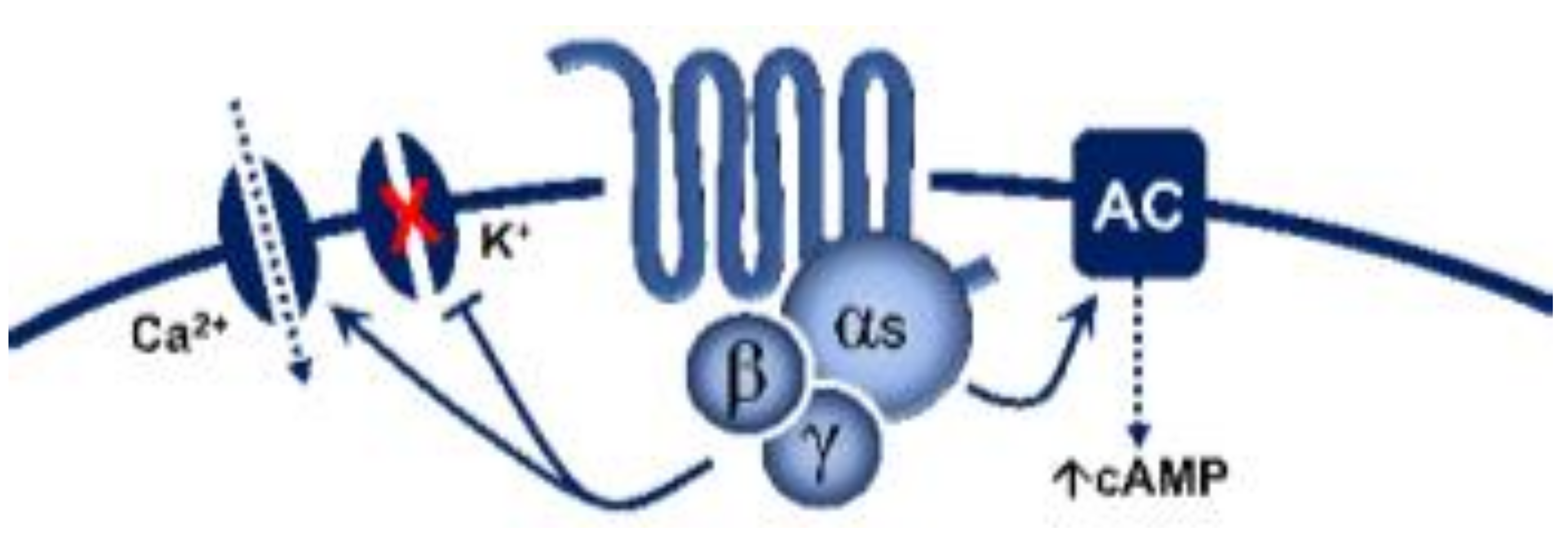 G-protein coupled receptor (Gs) |
| 5-HT6 | - | 5-MeO-T ≥ 5-HT SB357134 SB271046 |
Ro 630563 | Memory, not involved in cardiovascular regulation |
|
| 5-HT7 | - | 5-CT>>5-HT AS-19 |
SB269970 SB258719 | Circadian rhythm, vasodila- tation, tachycardia in cats |
|
| 5-HT2 | 5-HT2A | DOI, DOB α-methyl-5-HT |
MDL100907 Ketanserin |
Vasoconstriction, plateletaggregation | 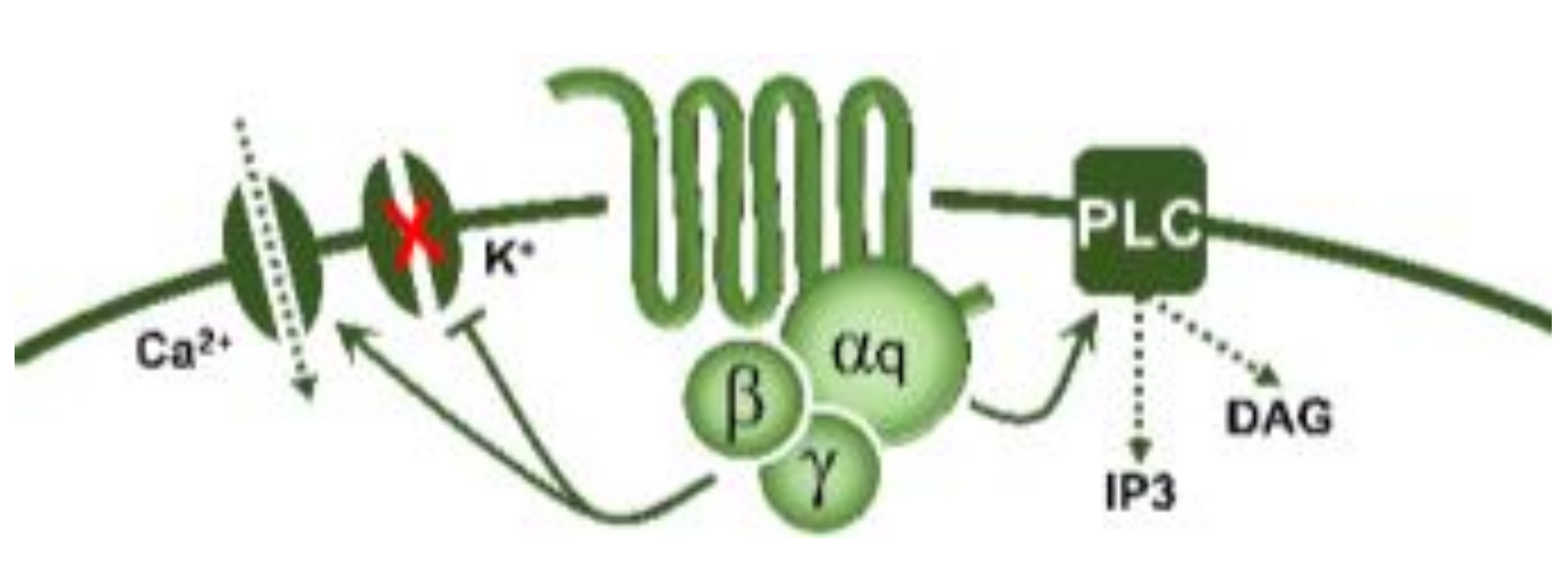 G-protein coupled receptor (Gq) |
| 5-HT2B | DOI, BW723C86 α-methyl-5-HT |
SB204741 RS-127445 |
Vasoconstriction, release of NO | ||
| 5-HT2C | DOI, Ro 60-0175 α-methyl-5-HT |
SB242084 RS-102221 |
CSF production | ||
| 5-HT3 | Pentameric ion channel ** | Phenylbiguanide 2-methyl-5-HT |
Tropisetron Granisetron MDL-72222 |
(+) Neuronal activity, reflex bradycardia |
Ligand-gated ion channel |
2. An Overview of the Effects of 5-HT on the Cardiovascular System
The cardiovascular effects of 5-HT are complex and include bradycardia/tachycardia, hypotension/hypertension, and vasodilatation/vasoconstriction. This complexity of effects is due to (i) the capability of 5-HT to interact at various levels, including the heart and blood vessels, as well as the central and peripheral (autonomic and sensory) nervous systems; and (ii) the involvement of serotonin 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5A, and 5-HT7 receptors, as well as a tyramine-like action or unidentified mechanisms, depending on the species and the experimental conditions [2][5][6][7][8][3,6,7,8,9]. Interestingly, the 5-HT6 receptor is not involved in the cardiovascular effects of 5-HT [2][7][3,8].3. The Specific Interactions of 5-HT at Peripheral and Central Levels to Induce Cardiovascular Effects
3.1. Sensory Afferents
Overall, an intravenous (i.v.) bolus injection of 5-HT in anaesthetised animals results in a reflex bradycardia and hypotension by stimulating 5-HT3 receptors on vagal sensory afferents [2][3]. These neuronal 5-HT3 receptors were identified using selective agonists and antagonists (see Table 1).3.2. Sympathetic Ganglia
It has been shown that i.v. 5-HT can stimulate and/or inhibit the sympathetic ganglia producing stimulation or inhibition of the sympathetic drive, and this results in changes in blood pressure and heart rate [2][3]. Moreover, the hyperpolarization of sympathetic ganglia produced by 5-HT is caused by the activation of 5-HT1A receptors in rats; these 5-HT1A receptors were identified by using selective agonists and antagonists (see Table 1).3.3. Cardiac Effects of 5-HT
Central or i.v. administration of 5-HT may produce bradycardia and/or tachycardia, and the 5-HT receptors involved in these effects have been identified by using some of the agonists and antagonists shown in Table 1 [2][5][3,6]. Overall, two central 5-HT receptors regulate cardiovascular function: 5-HT1A receptors (generally inhibiting the sympathetic drive) and 5-HT2 receptors (largely stimulating the sympathetic drive) [2][9][10][3,10,11]; some of the agonists and antagonists used to identify these receptors are shown in Table 1. Admittedly, central administration of 5-HT elicits complex and contradictory cardiac effects which depend on, among other factors, the species, the exact site of central application, the drug used, and the dose employed [2][9][10][3,10,11]. In contrast, the bradycardia or tachycardia produced by i.v. administration of 5-HT is more controllable and consistent (see below) in view of the implied simplicity of the procedure.3.3.1. Bradycardia
I.v. administration of 5-HT in intact animals results in a pronounced and transient bradycardia that is abolished after ganglion blockade, vagotomy, atropine, spinal section, or 5-HT3 receptor antagonists [2][5][3,6]. This response involves the Bezold–Jarisch reflex, originating from the depolarization of afferent cardiac sensory neurons via activation of 5-HT3 receptors [2][5][3,6]. Furthermore, 5-HT can also produce bradycardia by (i) a cardiac sympatho-inhibition via activation of prejunctional 5-HT1B, 5-HT1D and 5-HT5A receptors in pithed rats [2][11][12][3,12,13]; or (ii) a cardiac vagal stimulation via activation of 5-HT3 receptors on parasympathetic ganglia and postganglionic vagal nerves in rabbits [2][5][3,6] (see Table 1 for pharmacological tools).3.3.2. Tachycardia
I.v. administration of 5-HT in vagotomised animals induces a tachycardic effect that may be mediated by a wide variety of receptors/mechanisms, depending on the species and the experimental conditions [2][5][3,6]. These receptors/mechanisms include: (i) a tyramine-like action in spinal guinea pigs; (ii) direct stimulation of 5-HT2A receptors on the cardiac pacemaker in reserpinized pithed rats; (iii) activation of 5-HT3 receptors on cardiac sympathetic neurons in the perfused heart of a rabbit, resulting in noradrenaline release and cardiac stimulation; (iv) activation of 5-HT3 receptors on a calcitonin gene-related peptide (CGRP)-containing sensory neurons in an isolated guinea pig atrium, resulting in CGRP release and cardiac stimulation; (v) direct stimulation of 5-HT3 receptors on a cardiac pacemaker in conscious dogs; (vi) direct stimulation of 5-HT4 receptors on a cardiac pacemaker in healthy anaesthetized pigs (which is also involved in the positive inotropic effects of 5-HT in isolated human atria and in rats with chronic heart failure); (vii) direct stimulation of 5-HT7 receptors on a cardiac pacemaker in spinal cats; and (viii) unidentified mechanisms in the isolated hearts of certain lamellibranch and gastropod species (including Mercenaria mercenaria, Patella vulgata, Tapes watlingi, Helix aspersa, Aplysia, etc.). These receptors were pharmacologically identified using selective agonists and antagonists for each type (see Table 1).3.4. Vascular and Blood Pressure Effects of 5-HT
As explained in other reviews [2][6][7][3,7,8], i.v. administration of 5-HT results in a triphasic effect on arterial blood pressure, consisting of an initial transient vasodepressor effect followed by a vasopressor effect, and then a late long-lasting vasodepressor effect.3.4.1. Initial Transient Vasodepressor Effect
This response results from an abrupt bradycardia (and the consequent decrease in cardiac output) following stimulation of 5-HT3 receptors on afferent cardiac vagal afferents (i.e., the Bezold–Jarisch reflex; see above and Table 1).3.4.2. Vasopressor Effect
This effect (which varies quantitatively, depending on the species and the experimental conditions) involves the activation of vascular 5-HT2 receptors in resistance blood vessels (resulting in peripheral vasoconstriction). It is worth noting that a release of catecholamines by activation of 5-HT2 receptors in the adrenal medulla also plays a role in dogs, whereas activation of 5-HT1B receptors produces vasoconstriction in cranial and carotid arteries in humans, pigs and dogs [2][3]. Interestingly, 5-HT1B and 5-HT2 receptors elicit vasoconstriction in the internal carotid bed of anaesthetised dogs, while 5-HT directly activates, in vitro, α-adrenoceptors in rabbit ears and external carotid arteries [2][3]. Some of the agonists and antagonists used to identify these receptors are shown in Table 1.3.4.3. Late Long-Lasting Vasodepressor Effect
This effect predominantly involves the activation of musculotropic 5-HT7 receptors [2][6][7][3,7,8], although several receptors/mechanisms may play a role, depending on the experimental conditions. These receptors/mechanisms may include: (i) Direct vasodilatation. The direct vasodilatation to 5-HT involves 5-HT7 receptors in a wide variety of blood vessels under different experimental conditions [2][5][6][7][3,6,7,8]. Some of the agonists and antagonists used to identify these receptors (applying the aforementioned inclusion and exclusion criteria) are shown in Table 1. Moreover, in the blood vessels where 5-HT7 receptors produce vasodilatation and 5-HT2/5-HT1B receptors produce vasoconstriction, the final effect of 5-HT would depend on the pre-existing vascular tone, the dose employed, and the proportions in which these receptors are distributed [2][3]. (ii) Prejunctional inhibition of perivascular sympathetic neurons. The prejunctional inhibition induced by 5-HT and related agonists on perivascular sympathetic neurons has been confirmed in vitro and in vivo in many blood vessels [2][3]. This vascular sympatho-inhibition, generally mediated by 5-HT1 receptors, may involve the 5-HT1A, 5-HT1B and/or 5-HT1D receptor subtypes, depending on the vascular bed under study, the species, and the experimental conditions [2][3]. Interestingly, sympatho-inhibitory 5-HT7 receptors could also be involved when rats are chronically pretreated with the 5-HT2 receptor antagonist sarpogrelate [2][13][3,14]. These receptors were pharmacologically identified by applying the inclusion and exclusion criteria (see Table 1 and Figure 1).