
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xijuan Jiang | -- | 1519 | 2023-06-30 04:27:57 | | | |
| 2 | Xijuan Jiang | + 1520 word(s) | 3039 | 2023-07-04 08:49:55 | | | | |
| 3 | Sirius Huang | + 604 word(s) | 3643 | 2023-07-31 09:17:12 | | |
Video Upload Options
Drug development for Alzheimer’s disease, the leading cause of dementia, has been a long-standing challenge. Saponins, which are steroid or triterpenoid glycosides with various pharmacological activities, have displayed therapeutic potential in treating Alzheimer’s disease.
1. Introduction
Alzheimer’s disease (AD), the leading cause of dementia, is a progressive neurodegenerative disease [1] that is clinically characterized by memory loss, cognitive impairment, and behavioral disturbances [2]. Since its discovery in 1906, AD has emerged as one of the most costly, fatal, and burdensome diseases of this century [3]. The pathogenesis of AD involves various biological processes [4] involving the abnormal deposition of amyloid beta peptide (Aβ) [5], the accumulation of neurofibrillary tangles (NFTs) [6], neuroinflammation [7], neuronal apoptosis [8], neurotransmitter abnormities [9], and oxidative stress [10]. Despite considerable efforts, drug discovery for the treatment of AD has been slow, with only acetylcholinesterase (AChE)/butyrylcholinesterase (BChE) inhibitors [11] such as galantamine, donepezil, tacrine, and rivastigmine currently available as therapies [12]. However, these treatments only delay the onset of symptoms and cannot halt disease progression and are often associated with significant side effects [13]. Therefore, the development of new therapeutic drugs is urgently needed. Saponins, a type of natural compound, have been extensively studied for their various pharmacological properties [14]. Of particular interest is their potential to enhance learning and memory in individuals with AD [15].
Saponins are naturally occurring compounds that are widely distributed in various plants [16], and they can be divided into two major groups based on their chemical structure: triterpenoid saponins and steroidal saponins [17]. Triterpenoid saponins are further subdivided into tetracyclic triterpenes and pentacyclic triterpenes and are mainly found in plants such as Pentaaceae, Leguminosae, Poria, and Platycodonaceae [18]. The main saponin skeletons of the triterpenoid saponins include dammarane, oleanane, ursane, and lupane (Figure 1A–D) [19]. Steroidal saponins, on the other hand, are mainly found in plants such as Dioscoreaceae, Liliaceae, and Scrophulariaceae [20]. The main saponin metaskeletons of steroidal saponins include spirostane, furostane, cholestane, and cardenolide (Figure 1E–H) [21]. Saponins possess multiple bioactivities, such as reduction of amyloid beta (Aβ) deposition [22], inhibition of tau protein phosphorylation [23], antioxidation [24], antiapoptosis [25], and anti-inflammation [26]. These properties make saponins promising therapeutic candidates for AD and other neurological disorders [27]. Meanwhile, the diversity of saponins found in different plants [28] makes them a valuable source of potential drugs for the treatment of AD.
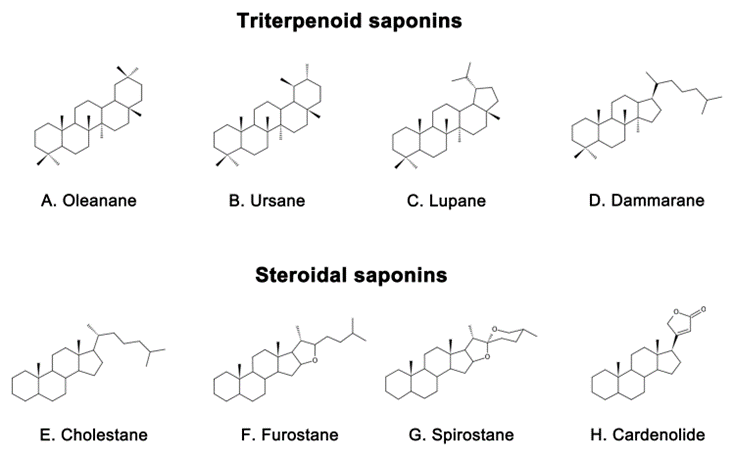
Figure 1. Representative triterpenoid saponins include (A) Oleanane, (B) Ursane, (C) Lupane, (D) Dammarane and steroidal saponins include (E) Cholestane, (F) Furostane, (G) Spirostane, (H) Cardenolide.
2. Mechanism of Saponins in Treating Alzheimer’s Disease
2.1. Inhibition of Aβ Deposition and Neurotoxicity
2.2. Inhibiting Aberrant Tau Protein Phosphorylation
2.3. Anti-Inflammatory Effect
2.4. Improvement in Mitochondrial Function and Antioxidative Stress
Oxidative stress refers to an imbalance between oxidation and antioxidation in the body [117], which is mainly characterized by the increased production of reactive oxygen species and reduced ability of antioxidants to combat them [118]. The brain is susceptible to oxidative damage due to its high lipid content and lacks effective antioxidant defense mechanisms, despite high oxygen consumption [119][120]. It is now well established that the level of oxidation in the brain also increases with age [121]. Interestingly, extensive oxidative damage can be observed in the stage of mild cognitive impairment that precedes the typical clinical manifestations of AD, suggesting that oxidative stress may be a central mechanism in driving the disease [122].
2.5. Antiapoptotic Effect
References
- Vossel, K.; Ranasinghe, K.G.; Beagle, A.J.; La, A.; Ah, P.K.; Castro, M.; Mizuiri, D.; Honma, S.M.; Venkateswaran, N.; Koestler, M.; et al. Effect of Levetiracetam on Cognition in Patients with Alzheimer Disease With and Without Epileptiform Activity: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 1345–1354.
- Balupuri, A.; Choi, K.E.; Kang, N.S. Aggregation Mechanism of Alzheimer’s Amyloid β-Peptide Mediated by α-Strand/α-Sheet Structure. Int. J. Mol. Sci. 2020, 21, 1094.
- Grimm, A.; Biliouris, E.E.; Lang, U.E.; Götz, J.; Mensah-Nyagan, A.G.; Eckert, A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein. Cell. Mol. Life Sci. 2016, 73, 201–215.
- Zheng, H.; Fridkin, M.; Youdim, M. From single target to multitarget/network therapeutics in Alzheimer’s therapy. Pharmaceuticals 2014, 7, 113–135.
- Tamayev, R.; Matsuda, S.; Arancio, O.; D’Adamio, L. β- but not γ-secretase proteolysis of APP causes synaptic and memory deficits in a mouse model of dementia. EMBO Mol. Med. 2012, 4, 171–179.
- Chandra, S.; Roy, A.; Jana, M.; Pahan, K. Cinnamic acid activates PPARα to stimulate Lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol. Dis. 2019, 124, 379–395.
- Polis, B.; Srikanth, K.D.; Elliott, E.; Gil-Henn, H.; Samson, A.O. L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer’s Disease. Neurotherapeutics 2018, 15, 1036–1054.
- Steffen, J.; Krohn, M.; Schwitlick, C.; Brüning, T.; Paarmann, K.; Pietrzik, C.U.; Biverstål, H.; Jansone, B.; Langer, O.; Pahnke, J. Expression of endogenous mouse APP modulates β-amyloid deposition in hAPP-transgenic mice. Acta Neuropathol. Commun. 2017, 20, 49.
- Govindpani, K.; Turner, C.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Impaired Expression of GABA Signaling Components in the Alzheimer’s Disease Middle Temporal Gyrus. Int. J. Mol. Sci. 2020, 21, 8704.
- Ruthirakuhan, M.; Herrmann, N.; Vieira, D.; Gallagher, D.; Lanctot, K.L. The Roles of Apathy and Depression in Predicting Alzheimer Disease: A Longitudinal Analysis in Older Adults With Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry 2019, 27, 873–882.
- Mphahlele, M.J.; Gildenhuys, S.; Agbo, E.N. In Vitro Evaluation and Docking Studies of 5-oxo-5H-furo chromene-6-carbaldehyde Derivatives as Potential Anti-Alzheimer’s Agents. Int. J. Mol. Sci. 2019, 20, 5451.
- Saleem, F.; Mehmood, R.; Mehar, S.; Khan, M.T.J.; Khan, Z.U.; Ashraf, M.; Ali, M.S.; Abdullah, I.; Froeyen, M.; Mirza, M.U.; et al. Bioassay Directed Isolation, Biological Evaluation and in Silico Studies of New Isolates from Pteris cretica L. Antioxidants 2019, 8, 231.
- Lee, B.D.; Yoo, J.M.; Baek, S.Y.; Li, F.Y.; Sok, D.E.; Kim, M.R. 3,3’-Diindolylmethane Promotes BDNF and Antioxidant Enzyme Formation via TrkB/Akt Pathway Activation for Neuroprotection against Oxidative Stress-Induced Apoptosis in Hippocampal Neuronal Cells. Antioxidants 2019, 9, 3.
- Chen, C.; Zhu, H.; Kang, J.; Warusawitharana, H.K.; Chen, S.; Wang, K.; Yu, F.; Wu, Y.; He, P.; Tu, Y.; et al. Comparative Transcriptome and Phytochemical Analysis Provides Insight into Triterpene Saponin Biosynthesis in Seeds and Flowers of the Tea Plant (Camellia sinensis). Metabolites 2022, 12, 204.
- Yoon, S.; Iqbal, H.; Kim, S.M.; Jin, M. Phytochemicals That Act on Synaptic Plasticity as Potential Prophylaxis against Stress-Induced Depressive Disorder. Biomol. Ther. 2023, 31, 148–160.
- Guclu-Ustundag, O.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258.
- Ramos-Morales, E.; de la Fuente, G.; Duval, S.; Wehrli, C.; Bouillon, M.; Lahmann, M.; Preskett, D.; Braganca, R.; Newbold, C.J. Antiprotozoal Effect of Saponins in the Rumen Can Be Enhanced by Chemical Modifications in Their Structure. Front. Microbiol. 2017, 8, 399.
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules. 2020, 25, 5557.
- Dashbaldan, S.; Pączkowski, C.; Szakiel, A. Variations in Triterpenoid Deposition in Cuticular Waxes during Development and Maturation of Selected Fruits of Rosaceae Family. Int. J. Mol. Sci. 2020, 21, 9762.
- Massana-Codina, J.; Schnee, S.; Allard, P.M.; Rutz, A.; Boccard, J.; Michellod, E.; Cleroux, M.; Schurch, S.; Gindro, K.; Wolfender, J.L. Insights on the Structural and Metabolic Resistance of Potato (Solanum tuberosum) Cultivars to Tuber Black Dot (Colletotrichum coccodes). Front. Plant Sci. 2020, 11, 1287.
- Sy, L.K.; Lok, C.N.; Wang, J.Y.; Liu, Y.; Cheng, L.; Wan, P.K.; Leung, C.T.; Cao, B.; Kwong, W.L.; Chang, R.C.; et al. Identification of “sarsasapogenin-aglyconed” timosaponins as novel Aβ-lowering modulators of amyloid precursor protein processing. Chem. Sci. 2016, 7, 3206–3214.
- Liu, Y.W.; Zhu, X.; Lu, Q.; Wang, J.Y.; Li, W.; Wei, Y.Q.; Yin, X.X. Total saponins from Rhizoma Anemarrhenae ameliorate diabetes-associated cognitive decline in rats: Involvement of amyloid-beta decrease in brain. J. Ethnopharmacol. 2012, 139, 194–200.
- Abduljawad, A.A.; Elawad, M.A.; Elkhalifa, M.; Ahmed, A.; Hamdoon, A.; Salim, L.; Ashraf, M.; Ayaz, M.; Hassan, S.; Bungau, S. Alzheimer’s Disease as a Major Public Health Concern: Role of Dietary Saponins in Mitigating Neurodegenerative Disorders and Their Underlying Mechanisms. Molecules 2022, 27, 6804.
- Rao, A.V.; Gurfinkel, D.M. The bioactivity of saponins: Triterpenoid and steroidal glycosides. Drug Metabol. Drug Interact. 2000, 17, 211–235.
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384.
- Dandawate, P.R.; Subramaniam, D.; Padhye, S.B.; Anant, S. Bitter melon: A panacea for inflammation and cancer. Chin. J. Nat. Med. 2016, 14, 81–100.
- Shalini, V.T.; Neelakanta, S.J.; Sriranjini, J.S. Neuroprotection with Bacopa monnieri—A review of experimental evidence. Mol. Biol. Rep. 2021, 48, 2653–2668.
- Bahrami, Y.; Zhang, W. MMFC Distribution of Saponins in the Sea Cucumber Holothuria lessoni; the Body Wall Versus the Viscera, and Their Biological Activities. Mar. Drugs 2018, 16, 423.
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Protective Effects of 6-(Methylsulfinyl) hexyl Isothiocyanate on Abeta (1-42)-Induced Cognitive Deficit, Oxidative Stress, Inflammation, and Apoptosis in Mice. Int. J. Mol. Sci. 2018, 19, 2083.
- Mucke, L.; Selkoe, D.J. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012, 2, a006338.
- Lesne, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature 2006, 440, 352–357.
- Mumford, P.; Tosh, J.; Anderle, S.; Gkanatsiou, W.E.; Lau, G.; Noy, S.; Cleverley, K.; Saito, T.; Saido, T.C.; Yu, E.; et al. Genetic Mapping of APP and Amyloid-beta Biology Modulation by Trisomy 21. J. Neurosci. 2022, 42, 6453–6468.
- Zhao, P.; Xu, Y.; Jiang, L.L.; Fan, X.; Ku, Z.; Li, L.; Liu, X.; Deng, M.; Arase, H.; Zhu, J.J.; et al. LILRB2-mediated TREM2 signaling inhibition suppresses microglia functions. Mol. Neurodegener. 2022, 17, 44.
- Lee, E.K.; Kim, H.H.; Kuwano, Y.; Abdelmohsen, K.; Srikantan, S.; Subaran, S.S.; Gleichmann, M.; Mughal, M.R.; Martindale, J.L.; Yang, X.; et al. hnRNP C promotes APP translation by competing with FMRP for APP mRNA recruitment to P bodies. Nat. Struct. Mol. Biol. 2010, 17, 732–739.
- Sachdev, R.; Kappes-Horn, K.; Paulsen, L.; Duernberger, Y.; Pleschka, C.; Denner, P.; Kundu, B.; Reimann, J.; Vorberg, I. Endoplasmic Reticulum Stress Induces Myostatin High Molecular Weight Aggregates and Impairs Mature Myostatin Secretion. Mol. Neurobiol. 2018, 55, 8355–8373.
- Mockett, B.G.; Guévremont, D.; Elder, M.K.; Parfitt, K.D.; Peppercorn, K.; Morrissey, J.; Singh, A.; Hintz, T.J.; Kochen, L.; Tom Dieck, S.; et al. Glutamate Receptor Trafficking and Protein Synthesis Mediate the Facilitation of LTP by Secreted Amyloid Precursor Protein-α. J. Neurosci. 2019, 39, 3188–3203.
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. R. Soc. B. Biol. Sci. 2016, 283, 20152317.
- Samaey, C.; Schreurs, A.; Stroobants, S.; Balschun, D. Early Cognitive and Behavioral Deficits in Mouse Models for Tauopathy and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 335.
- Koon, N.A.; Itokazu, Y.; Yu, R.K. Ganglioside-Dependent Neural Stem Cell Proliferation in Alzheimer’s Disease Model Mice. ASN Neuro. 2015, 7, 1759091415618916.
- Hongo, N.; Takamura, Y.; Nishimaru, H.; Matsumoto, J.; Tobe, K.; Saito, T.; Saido, T.C.; Nishijo, H. Astaxanthin Ameliorated Parvalbumin-Positive Neuron Deficits and Alzheimer’s Disease-Related Pathological Progression in the Hippocampus of App (NL-G-F/NL-G-F) Mice. Front. Pharmacol. 2020, 11, 307.
- Sasaguri, H.; Hashimoto, S.; Watamura, N.; Sato, K.; Takamura, R.; Nagata, K.; Tsubuki, S.; Ohshima, T.; Yoshiki, A.; Sato, K.; et al. Recent Advances in the Modeling of Alzheimer’s Disease. Front. Neurosci. 2022, 16, 807473.
- Lu, X.Y.; Huang, S.; Chen, Q.B.; Zhang, D.; Li, W.; Ao, R.; Leung, F.C.; Zhang, Z.; Huang, J.; Tang, Y.; et al. Metformin Ameliorates Aβ Pathology by Insulin-Degrading Enzyme in a Transgenic Mouse Model of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2020, 2020, 2315106.
- Moosecker, S.; Gomes, P.; Dioli, C.; Yu, S.; Sotiropoulos, I.; Almeida, O. Activated PPARγ Abrogates Misprocessing of Amyloid Precursor Protein, Tau Missorting and Synaptotoxicity. Front. Cell. Neurosci. 2019, 13, 239.
- Medrano-Jiménez, E.; Jiménez-Ferrer Carrillo, I.; Pedraza-Escalona, M.; Ramírez-Serrano, C.E.; Álvarez-Arellano, L.; Cortés-Mendoza, J.; Herrera-Ruiz, M.; Jiménez-Ferrer, E.; Zamilpa, A.; Tortoriello, J.; et al. Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-γ-dependent mechanism. J. Neuroinflammation 2019, 16, 143.
- Dinda, B.; Dinda, M.; Kulsi, G.; Chakraborty, A.; Dinda, S. Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. Eur. J. Med. Chem. 2019, 169, 185–199.
- Kumar, A.P.; Prabhakaran, P.; Kumar, B.R.P.; Jeyarani, V.; Dhanabal, S.P.; Justin, A. Glitazones, PPAR-γ and Neuroprotection. Mini Rev. Med. Chem. 2021, 21, 1457–1464.
- Mohamed, L.A.; Qosa, H.; Kaddoumi, A. Age-Related Decline in Brain and Hepatic Clearance of Amyloid-β is Rectified by the Cholinesterase Inhibitors Donepezil and Rivastigmine in Rats. ACS Chem. Neurosci. 2015, 6, 725–736.
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608.
- Pagano, K.; Tomaselli, S.; Molinari, H.; Ragona, L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020, 14, 619667.
- Alolga, R.N.; Nuer-Allornuvor, G.F.; Kuugbee, E.D.; Yin, X.; Ma, G. Ginsenoside Rg1 and the control of inflammation implications for the therapy of type 2 diabetes: A review of scientific findings and call for further research. Pharmacol. Res. 2020, 152, 104630.
- Li, F.; Wu, X.; Li, J.; Niu, Q. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer’s disease model. Mol. Med. Rep. 2016, 13, 4904–4910.
- Xue, W.; Liu, Y.; Qi, W.Y.; Gao, Y.; Li, M.; Shi, A.X.; Li, K.X. Pharmacokinetics of ginsenoside Rg1 in rat medial prefrontal cortex, hippocampus, and lateral ventricle after subcutaneous administration. J. Asian Nat. Prod. Res. 2016, 18, 587–595.
- Kunisawa, K.; Shan, J.; Lu, Q.; Yang, Y.; Kosuge, A.; Kurahashi, H.; Saito, K.; Zou, L.; Nabeshima, T.; Mouri, A. Loureirin C and Xanthoceraside Attenuate Depression-Like Behaviors and Expression of Interleukin-17 in the Prefrontal Cortex Induced by Chronic Unpredictable Mild Stress in Mice. Neurochem. Res. 2022, 47, 2880–2889.
- Jin, G.; Wang, L.H.; Ji, X.F.; Chi, T.Y.; Qi, Y.; Jiao, Q.; Xu, Q.; Zhou, X.Y.; Zhang, R.; Zou, L.B. Xanthoceraside rescues learning and memory deficits through attenuating β-amyloid deposition and tau hyperphosphorylation in APP mice. Neurosci. Lett. 2014, 573, 58–63.
- Qiu, J.; Li, W.; Feng, S.H.; Wang, M.; He, Z.Y. Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid precursor protein via a cholesterol-dependent pathway. Genet. Mol. Res. 2014, 13, 3586–3598.
- Fang, F.; Chen, X.; Huang, T.; Lue, L.F.; Luddy, J.S.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta 2012, 1822, 286–292.
- Li, X.; Cui, J.; Yu, Y.; Li, W.; Hou, Y.; Wang, X.; Qin, D.; Zhao, C.; Yao, X.; Zhao, J.; et al. Traditional Chinese Nootropic Medicine Radix Polygalae and Its Active Constituent Onjisaponin B Reduce β-Amyloid Production and Improve Cognitive Impairments. PLoS ONE 2016, 11, e151147.
- Zhang, Z.; Yang, J.; Liu, C.; Xie, J.; Qiu, S.; Yang, X.; Wu, C. Pseudoginsenoside-F11 alleviates cognitive deficits and Alzheimer’s disease-type pathologies in SAMP8 mice. Pharmacol. Res. 2019, 139, 512–523.
- Khan, M.I.; Shin, J.H.; Kim, M.Y.; Shin, T.S.; Kim, J.D. Green Tea Seed Isolated Theasaponin E1 Ameliorates AD Promoting Neurotoxic Pathogenesis by Attenuating Aβ Peptide Levels in SweAPP N2a Cells. Molecules 2020, 25, 2334.
- Kang, M.S.; Baek, S.H.; Chun, Y.S.; Moore, A.Z.; Landman, N.; Berman, D.; Yang, H.O.; Morishima-Kawashima, M.; Osawa, S.; Funamoto, S.; et al. Modulation of lipid kinase PI4KIIα activity and lipid raft association of presenilin 1 underlies γ-secretase inhibition by ginsenoside (20S)-Rg3. J. Biol. Chem. 2013, 288, 20868–20882.
- Yang, Q.; Lin, J.; Zhang, H.; Liu, Y.; Kan, M.; Xiu, Z.; Chen, X.; Lan, X.; Li, X.; Shi, X.; et al. Ginsenoside Compound K Regulates Amyloid β via the Nrf2/Keap1 Signaling Pathway in Mice with Scopolamine Hydrobromide-Induced Memory Impairments. J. Mol. Neurosci. 2019, 67, 62–71.
- Zhang, X.; Wang, J.; Xing, Y.; Gong, L.; Li, H.; Wu, Z.; Li, Y.; Wang, J.; Wang, Y.; Dong, L.; et al. Effects of ginsenoside Rg1 or 17β-estradiol on a cognitively impaired, ovariectomized rat model of Alzheimer’s disease. Neuroscience 2012, 220, 191–200.
- Shi, C.; Zheng, D.D.; Fang, L.; Wu, F.; Kwong, W.H.; Xu, J. Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim. Biophys. Acta 2012, 1820, 453–460.
- Lin, Z.; Gu, J.; Xiu, J.; Mi, T.; Dong, J.; Tiwari, J.K. Traditional Chinese medicine for senile dementia. Evid. Based Complement. Altern. Med. 2012, 2012, 692621.
- Zhu, L.; Hou, X.J.; Che, X.H.; Zhou, T.S.; Liu, X.Q.; Wu, C.F.; Yang, J.Y. Pseudoginsenoside-F11 attenuates cognitive dysfunction and tau phosphorylation in sporadic Alzheimer’s disease rat model. Acta Pharmacol. Sin. 2021, 42, 1401–1408.
- Kim, J.D.; Chaudhary, N.; Seo, H.J.; Kim, M.Y.; Shin, T.S. Theasaponin E1 as an effective ingredient for anti-angiogenesis and anti-obesity effects. Biosci. Biotechnol. Biochem. 2014, 78, 279–287.
- Oh, J.; Kim, J.S. Compound K derived from ginseng: Neuroprotection and cognitive improvement. Food Funct. 2016, 7, 4506–4515.
- Lee, S.; Kwon, M.C.; Jang, J.P.; Sohng, J.K.; Jung, H.J. The ginsenoside metabolite compound K inhibits growth, migration and stemness of glioblastoma cells. Int. J. Oncol. 2017, 51, 414–424.
- Kim, J.K.; Cui, C.H.; Yoon, M.H.; Kim, S.C.; Im, W.T. Bioconversion of major ginsenosides Rg1 to minor ginsenoside F1 using novel recombinant ginsenoside hydrolyzing glycosidase cloned from Sanguibacter keddieii and enzyme characterization. J. Biotechnol. 2012, 161, 294–301.
- Han, J.; Oh, J.P.; Yoo, M.; Cui, C.H.; Jeon, B.M.; Kim, S.C.; Han, J.H. Minor ginsenoside F1 improves memory in APP/PS1 mice. Mol. Brain 2019, 12, 77.
- Chakravarty, A.K.; Sarkar, T.; Masuda, K.; Shiojima, K.; Nakane, T.; Kawahara, N. Bacopaside I and II: Two pseudojujubogenin glycosides from Bacopa monniera. Phytochemistry 2001, 58, 553–556.
- Pham, H.T.N.; Tran, H.N.; Nguyen, P.T.; Le, X.T.; Nguyen, K.M.; Phan, S.V.; Yoneyama, M.; Ogita, K.; Yamaguchi, T.; Folk, W.R.; et al. Bacopa monnieri (L.) Wettst. Extract Improves Memory Performance via Promotion of Neurogenesis in the Hippocampal Dentate Gyrus of Adolescent Mice. Int. J. Mol. Sci. 2020, 21, 3365.
- Ramasamy, S.; Chin, S.P.; Sukumaran, S.D.; Buckle, M.J.; Kiew, L.V.; Chung, L.Y. In Silico and In Vitro Analysis of Bacoside A Aglycones and Its Derivatives as the Constituents Responsible for the Cognitive Effects of Bacopa monnieri. PLoS ONE 2015, 10, e0126565.
- Li, Y.; Yuan, X.; Shen, Y.; Zhao, J.; Yue, R.; Liu, F.; He, W.; Wang, R.; Shan, L.; Zhang, W. Bacopaside I ameliorates cognitive impairment in APP/PS1 mice via immune-mediated clearance of β-amyloid. Aging 2016, 8, 521–533.
- Seyfried, N.T.; Dammer, E.B.; Swarup, V.; Nandakumar, D.; Duong, D.M.; Yin, L.; Deng, Q.; Nguyen, T.; Hales, C.M.; Wingo, T.; et al. A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer’s Disease. Cell Syst. 2017, 4, 60–72.
- Brown, R.A.; Voit, A.; Srikanth, M.P.; Thayer, J.A.; Kingsbury, T.J.; Jacobson, M.A.; Lipinski, M.M.; Feldman, R.A.; Awad, O. mTOR hyperactivity mediates lysosomal dysfunction in Gaucher’s disease iPSC-neuronal cells. Dis. Model. Mech. 2019, 12, dmm038596.
- Yao, X.C.; Xue, X.; Zhang, H.T.; Zhu, M.M.; Yang, X.W.; Wu, C.F.; Yang, J.Y. Pseudoginsenoside-F11 alleviates oligomeric β-amyloid-induced endosome-lysosome defects in microglia. Traffic 2019, 20, 61–70.
- Usenovic, M.; Niroomand, S.; Drolet, R.E.; Yao, L.; Gaspar, R.C.; Hatcher, N.G.; Schachter, J.; Renger, J.J.; Parmentier-Batteur, S. Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells. J. Neurosci. 2015, 35, 14234–14250.
- Rubenstein, R.; Chang, B.; Grinkina, N.; Drummond, E.; Davies, P.; Ruditzky, M.; Sharma, D.; Wang, K.; Wisniewski, T. Tau phosphorylation induced by severe closed head traumatic brain injury is linked to the cellular prion protein. Acta Neuropathol. Commun. 2017, 5, 30.
- Papanikolopoulou, K.; Roussou, I.G.; Gouzi, J.Y.; Samiotaki, M.; Panayotou, G.; Turin, L.; Skoulakis, E.M.C. Drosophila Tau Negatively Regulates Translation and Olfactory Long-Term Memory, but Facilitates Footshock Habituation and Cytoskeletal Homeostasis. J. Neurosci. 2019, 39, 8315–8329.
- Lacovich, V.; Espindola, S.L.; Alloatti, M.; Pozo, D.V.; Cromberg, L.E.; Carna, M.E.; Forte, G.; Gallo, J.M.; Bruno, L.; Stokin, G.B.; et al. Tau Isoforms Imbalance Impairs the Axonal Transport of the Amyloid Precursor Protein in Human Neurons. J. Neurosci. 2017, 37, 58–69.
- Barini, E.; Antico, O.; Zhao, Y.; Asta, F.; Tucci, V.; Catelani, T.; Marotta, R.; Xu, H.; Gasparini, L. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol. Neurodegener. 2016, 11, 16.
- Candia, R.F.; Cohen, L.S.; Morozova, V.; Corbo, C.; Alonso, A.D. Importin-Mediated Pathological Tau Nuclear Translocation Causes Disruption of the Nuclear Lamina, TDP-43 Mislocalization and Cell Death. Front. Molec. Neurosci. 2022, 15, 888420.
- Noel, A.; Foveau, B.; LeBlanc, A.C. Caspase-6-cleaved Tau fails to induce Tau hyperphosphorylation and aggregation, neurodegeneration, glial inflammation, and cognitive deficits. Cell Death Dis. 2021, 12, 227.
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35.
- Pîrşcoveanu, D.F.V.; Pirici, I.; Tudorică, V.; Bălşeanu, T.A.; Albu, V.C.; Bondari, S.; Bumbea, A.M.; Pîrşcoveanu, M. Tau protein in neurodegenerative diseases—A review. Rom. J. Morphol. Embryol. 2017, 58, 1141–1150.
- Voss, K.; Gamblin, T.C. GSK-3β phosphorylation of functionally distinct tau isoforms has differential, but mild effects. Mol. Neurodegener 2009, 4, 18.
- Mahmoudi, N.; Kiasalari, Z.; Rahmani, T.; Sanaierad, A.; Afshin-Majd, S.; Naderi, G.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin Attenuates Cognitive Impairment in Streptozotocin-Induced Diabetic Rats: Underlying Mechanisms. Neuropsychobiology 2021, 80, 25–35.
- Ahmed, T.; Raza, S.H.; Maryam, A.; Setzer, W.N.; Braidy, N.; Nabavi, S.F.; de Oliveira, M.R.; Nabavi, S.M. Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Res. Bull. 2016, 125, 30–43.
- Shalaby, A.M.; Alnasser, S.M.; Ahmed Khairy, D.; Alabiad, M.A.; Alorini, M.; Jaber, F.A.; Tawfeek, S.E. The neuroprotective effect of ginsenoside Rb1 on the cerebral cortex changes induced by aluminium chloride in a mouse model of Alzheimer’s disease: A histological, immunohistochemical, and biochemical study. J. Chem. Neuroanat. 2023, 129, 102248.
- Zhao, H.H.; Di, J.; Liu, W.S.; Liu, H.L.; Lai, H.; Lu, Y.L. Involvement of GSK3 and PP2A in ginsenoside Rb1’s attenuation of aluminum-induced tau hyperphosphorylation. Behav. Brain Res. 2013, 241, 228–234.
- Nabavi, S.F.; Sureda, A.; Habtemariam, S.; Nabavi, S.M. Ginsenoside Rd and ischemic stroke; a short review of literatures. J. Ginseng Res. 2015, 39, 299–303.
- Adams, M.E.; Li, D.K.; McConkey, J.P.; Davidson, R.G.; Day, B.; Duncan, C.P.; Tron, V. Evaluation of cartilage lesions by magnetic resonance imaging at 0.15 T: Comparison with anatomy and concordance with arthroscopy. J. Rheumatol. 1991, 18, 1573–1580.
- Li, L.; Liu, J.; Yan, X.; Qin, K.; Shi, M.; Lin, T.; Zhu, Y.; Kang, T.; Zhao, G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011, 138, 135–141.
- Khan, M.I.; Khan, M.Z.; Shin, J.H.; Shin, T.S.; Lee, Y.B.; Kim, M.Y.; Kim, J.D. Neuroprotective Effects of Green Tea Seed Isolated Saponin Due to the Amelioration of Tauopathy and Alleviation of Neuroinflammation: A Therapeutic Approach to Alzheimer’s Disease. Molecules 2022, 27, 2079.
- Zhou, H.; Tai, J.; Xu, H.; Lu, X.; Meng, D. Xanthoceraside Could Ameliorate Alzheimer’s Disease Symptoms of Rats by Affecting the Gut Microbiota Composition and Modulating the Endogenous Metabolite Levels. Front. Pharmacol. 2019, 10, 1035.
- Li, F.; Eteleeb, A.M.; Buchser, W.; Sohn, C.; Wang, G.; Xiong, C.; Payne, P.R.; McDade, E.; Karch, C.M.; Harari, O.; et al. Weakly activated core neuroinflammation pathways were identified as a central signaling mechanism contributing to the chronic neurodegeneration in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 935279.
- Howe, A.M.; Burke, S.; O’Reilly, M.E.; McGillicuddy, F.C.; Costello, D.A. Palmitic Acid and Oleic Acid Differently Modulate TLR2-Mediated Inflammatory Responses in Microglia and Macrophages. Mol. Neurobiol. 2022, 59, 2348–2362.
- Kurtys, E.; Casteels, C.; Real, C.C.; Eisel, U.; Verkuyl, J.M.; Broersen, L.M.; Klein, H.C.; Dierckx, R.; Doorduin, J.; de Vries, E. Therapeutic effects of dietary intervention on neuroinflammation and brain metabolism in a rat model of photothrombotic stroke. CNS Neurosci. Ther. 2019, 25, 36–46.
- Alkhatip, A.A.A.M.M.; Kamel, M.G.; Hamza, M.K.; Farag, E.M.; Yassin, H.M.; Elayashy, M.; Naguib, A.A.; Wagih, M.; Abd-Elhay, F.A.; Algameel, H.Z.; et al. The diagnostic and prognostic role of neutrophil-to-lymphocyte ratio in COVID-19: A systematic review and meta-analysis. Expert Rev. Mol. Diagn. 2021, 21, 505–514.
- Kumar, P.; Mishra, J.; Kumar, N. Mechanistic Role of Jak3 in Obesity-Associated Cognitive Impairments. Nutrients. 2022, 14, 3715.
- Merighi, S.; Nigro, M.; Travagli, A.; Gessi, S. Microglia and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12990.
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554.
- Yu, Y.; Ye, R.D. Microglial Aβ receptors in Alzheimer’s disease. Cell. Mol. Neurobiol. 2015, 35, 71–83.
- Gambuzza, M.E.; Sofo, V.; Salmeri, F.M.; Soraci, L.; Marino, S.; Bramanti, P. Toll-like receptors in Alzheimer’s disease: A therapeutic perspective. CNS Neurol. Disord. Drug Targets 2014, 13, 1542–1558.
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights Into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408.
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and anti-inflammatory activities of allyl isothiocyanate through attenuation of JNK/NF-κB/TNF-α signaling. Int. J. Mol. Sci. 2017, 18, 1423.
- Radenovic, L.; Nenadic, M.; Ulamek-Koziol, M.; Januszewski, S.; Czuczwar, S.J.; Andjus, P.R.; Pluta, R. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging 2020, 12, 12251–12267.
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677.
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651.
- Kim, Y.; Lee, H.; Kim, S.Y.; Lim, Y. Effects of Lespedeza Bicolor Extract on Regulation of AMPK Associated Hepatic Lipid Metabolism in Type 2 Diabetic Mice. Antioxidants 2019, 8, 599.
- Urasaki, Y.; Beaumont, C.; Talbot, J.N.; Hill, D.K.; Le, T.T. Akt3 Regulates the Tissue-Specific Response to Copaiba Essential Oil. Int. J. Mol. Sci. 2020, 21, 2851.
- Tyagi, A.; Kamal, M.A.; Poddar, N.K. Integrated Pathways of COX-2 and mTOR: Roles in Cell Sensing and Alzheimer’s Disease. Front. Neurosci. 2020, 14, 693.
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681.
- Falcicchia, C.; Tozzi, F.; Arancio, O.; Watterson, D.M.; Origlia, N. Involvement of p38 MAPK in Synaptic Function and Dysfunction. Int. J. Mol. Sci. 2020, 21, 5624.
- Jain, M.; Singh, M.K.; Shyam, H.; Mishra, A.; Kumar, S.; Kumar, A.; Kushwaha, J. Role of JAK/STAT in the Neuroinflammation and its Association with Neurological Disorders. Ann. Neurosci. 2021, 28, 191–200.
- Ayalon, I.; de Barros Marangoni, L.F.; Benichou, J.I.C.; Avisar, D.; Levy, O. Red Sea corals under Artificial Light Pollution at Night (ALAN) undergo oxidative stress and photosynthetic impairment. Glob. Chang. Biol. 2019, 25, 4194–4207.
- Gargouri, B.; Yousif, N.M.; Bouchard, M.; Fetoui, H.; Fiebich, B.L. Inflammatory and cytotoxic effects of bifenthrin in primary microglia and organotypic hippocampal slice cultures. J. Neuroinflamm. 2018, 15, 159.
- Pousa, P.A.; Souza, R.M.; Melo, P.; Correa, B.; Mendonca, T.; Simoes-E-Silva, A.C.; Miranda, D.M. Telomere Shortening and Psychiatric Disorders: A Systematic Review. Cells 2021, 10, 1423.
- Muke, S.; Kaikini, A.; Peshattiwar, V.; Bagle, S.; Dighe, V.; Sathaye, S. Neuroprotective Effect of Coumarin Nasal Formulation: Kindling Model Assessment of Epilepsy. Front. Pharmacol. 2018, 9, 992.
- Ward, S.M.; Himmelstein, D.S.; Ren, Y.; Fu, Y.; Yu, X.W.; Roberts, K.; Binder, L.I.; Sahara, N. TOC1: A valuable tool in assessing disease progression in the rTg4510 mouse model of tauopathy. Neurobiol. Dis. 2014, 67, 37–48.
- Ohashi, H.; Tsuji, M.; Oguchi, T.; Momma, Y.; Nohara, T.; Ito, N.; Yamamoto, K.; Nagata, M.; Kimura, A.M.; Kiuchi, Y.; et al. Combined Treatment with Curcumin and Ferulic Acid Suppressed the Aβ-Induced Neurotoxicity More than Curcumin and Ferulic Acid Alone. Int. J. Mol. Sci. 2022, 23, 9685.
- Cui, Y.; Li, F.; Zhu, X.; Xu, J.; Muhammad, A.; Chen, Y.; Li, D.; Liu, B.; Wang, C.; Wang, Z.; et al. Alfalfa saponins inhibit oxidative stress-induced cell apoptosis through the MAPK signaling pathway. Redox Rep. 2022, 27, 1–8.
- Yoo, K.Y.; Park, S.Y. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules 2012, 17, 3524–3538.
- Hsieh, Y.H.; Deng, J.S.; Chang, Y.S.; Huang, G.J. Ginsenoside Rh2 Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 Signaling Pathways in Mice. Nutrients 2018, 10, 1208.
- Lv, J.; Lu, C.; Jiang, N.; Wang, H.; Huang, H.; Chen, Y.; Li, Y.; Liu, X. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother. Res. 2021, 35, 337–345.
- Oyeleke, M.B.; Owoyele, B.V. Saponins and flavonoids from Bacopa floribunda plant extract exhibit antioxidant and anti-inflammatory effects on amyloid β 1-42-induced Alzheimer’s disease in BALB/c mice. J. Ethnopharmacol. 2022, 288, 114997.
- Liu, J.; Yan, X.; Li, L.; Zhu, Y.; Qin, K.; Zhou, L.; Sun, D.; Zhang, X.; Ye, R.; Zhao, G. Ginsennoside rd attenuates cognitive dysfunction in a rat model of Alzheimer’s disease. Neurochem. Res. 2012, 37, 2738–2747.
- Lee, S.J.; Lee, W.J.; Chang, S.E.; Lee, G.Y. Antimelanogenic effect of ginsenoside Rg3 through extracellular signal-regulated kinase-mediated inhibition of microphthalmia-associated transcription factor. J. Ginseng Res. 2015, 39, 238–242.
- Zhang, Y.; Yang, X.; Wang, S.; Song, S. Ginsenoside Rg3 Prevents Cognitive Impairment by Improving Mitochondrial Dysfunction in the Rat Model of Alzheimer’s Disease. J. Agric. Food Chem. 2019, 67, 10048–10058.
- Wang, C.M.; Liu, M.Y.; Wang, F.; Wei, M.J.; Wang, S.; Wu, C.F.; Yang, J.Y. Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2013, 106, 57–67.
- Huang, T.; Fang, F.; Chen, L.; Zhu, Y.; Zhang, J.; Chen, X.; Yan, S.S. Ginsenoside Rg1 attenuates oligomeric Aβ (1-42)-induced mitochondrial dysfunction. Curr. Alzheimer Res. 2012, 9, 388–395.
- Huang, L.; Wu, Y.; Yin, H.; Yang, X.; Yuan, M.; Ning, H. Two new compounds from the stewed Polygonatum cyrtonema Hua and their protective effect against Aβ (25–35) induced cytotoxicity and oxidative stress damage. Nat. Prod. Res. 2021, 35, 4945–4952.
- Elekofehinti, O.O.; Kamdem, J.P.; Meinerz, D.F.; Kade, I.J.; Adanlawo, I.G.; Rocha, J.B. Saponin from the fruit of Solanum anguivi protects against oxidative damage mediated by Fe (2+) and sodium nitroprusside in rat brain synaptosome P2 fraction. Arch. Pharm. Res. 2015, 10.
- Ji, Y.J.; Kim, S.; Kim, J.J.; Jang, G.Y.; Moon, M.; Kim, H.D. Crude Saponin from Platycodon grandiflorum Attenuates Aβ-Induced Neurotoxicity via Antioxidant, Anti-Inflammatory and Anti-Apoptotic Signaling Pathways. Antioxidants 2021, 10, 1968.
- Ma, B.; Meng, X.; Wang, J.; Sun, J.; Ren, X.; Qin, M.; Sun, J.; Sun, G.; Sun, X. Notoginsenoside R1 attenuates amyloid-beta-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 2014, 22, 151–159.
- Zhou, N.; Tang, Y.; Keep, R.F.; Ma, X.; Xiang, J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine 2014, 21, 1189–1195.
- Okouchi, M.; Ekshyyan, O.; Maracine, M.; Aw, T.Y. Neuronal apoptosis in neurodegeneration. Antioxid. Redox Signal. 2007, 9, 1059–1096.
- Sharma, V.K.; Singh, T.G.; Singh, S.; Garg, N.; Dhiman, S. Apoptotic Pathways and Alzheimer’s Disease: Probing Therapeutic Potential. Neurochem. Res. 2021, 46, 3103–3122.
- Mufson, E.J.; He, B.; Nadeem, M.; Perez, S.E.; Counts, S.E.; Leurgans, S.; Fritz, J.; Lah, J.; Ginsberg, S.D.; Wuu, J.; et al. Hippocampal proNGF signaling pathways and β-amyloid levels in mild cognitive impairment and Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012, 71, 1018–1029.
- Roth, K.A. Caspases, apoptosis, and Alzheimer disease: Causation, correlation, and confusion. J. Neuropathol. Exp. Neurol. 2001, 60, 829–838.
- Castro, M.A.; Dalmolin, R.J.; Moreira, J.C.; Mombach, J.C.; de Almeida, R.M. Evolutionary origins of human apoptosis and genome-stability gene networks. Nucleic Acids Res. 2008, 36, 6269–6283.
- Park, K.R.; Lee, H.; Cho, M.; Yun, H.M. A Phytochemical Constituent, (E)-Methyl-Cinnamate Isolated from Alpinia katsumadai Hayata Suppresses Cell Survival, Migration, and Differentiation in Pre-Osteoblasts. Int. J. Mol. Sci. 2020, 21, 3700.
- Karousi, P.; Artemaki, P.I.; Sotiropoulou, C.D.; Christodoulou, S.; Scorilas, A.; Kontos, C.K. Identification of Two Novel Circular RNAs Deriving from BCL2L12 and Investigation of Their Potential Value as a Molecular Signature in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 8867.
- Asara, Y.; Marchal, J.A.; Carrasco, E.; Boulaiz, H.; Solinas, G.; Bandiera, P.; Garcia, M.A.; Farace, C.; Montella, A.; Madeddu, R. Cadmium modifies the cell cycle and apoptotic profiles of human breast cancer cells treated with 5-fluorouracil. Int. J. Mol. Sci. 2013, 14, 16600–16616.
- Lanning, M.E.; Yu, W.; Yap, J.L.; Chauhan, J.; Chen, L.; Whiting, E.; Pidugu, L.S.; Atkinson, T.; Bailey, H.; Li, W.; et al. Structure-based design of N-substituted 1-hydroxy-4-sulfamoyl-2-naphthoates as selective inhibitors of the Mcl-1 oncoprotein. Eur. J. Med. Chem. 2016, 113, 273–292.
- Valentin, R.; Grabow, S.; Davids, M.S. The rise of apoptosis: Targeting apoptosis in hematologic malignancies. Blood 2018, 132, 1248–1264.
- Öztaş, E.; Kara, M.; Boran, T.; Bişirir, E.; Karaman, E.F.; Kaptan, E.; Özhan, G. Cellular Stress Pathways Are Linked to Acetamiprid-Induced Apoptosis in SH-SY5Y Neural Cells. Biology 2021, 10, 820.
- Nagy, Z.S.; Esiri, M.M. Apoptosis-related protein expression in the hippocampus in Alzheimer’s disease. Neurobiol. Aging 1997, 18, 565–571.
- MacGibbon, G.A.; Lawlor, P.A.; Sirimanne, E.S.; Walton, M.R.; Connor, B.; Young, D.; Williams, C.; Gluckman, P.; Faull, R.L.; Hughes, P.; et al. Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer’s disease hippocampus. Brain Res. 1997, 750, 223–234.
- Costa, I.M.; Lima, F.O.V.; Fernandes, L.C.B.; Norrara, B.; Neta, F.I.; Alves, R.D.; Cavalcanti, J.R.L.P.; Lucena, E.E.S.; Cavalcante, J.S.; Rego, A.C.M.; et al. Astragaloside IV Supplementation Promotes A Neuroprotective Effect in Experimental Models of Neurological Disorders: A Systematic Review. Curr. Neuropharmacol. 2019, 17, 648–665.
- Chang, C.P.; Liu, Y.F.; Lin, H.J.; Hsu, C.C.; Cheng, B.C.; Liu, W.P.; Lin, M.T.; Hsu, S.F.; Chang, L.S.; Lin, K.C. Beneficial Effect of Astragaloside on Alzheimer’s Disease Condition Using Cultured Primary Cortical Cells Under β-amyloid Exposure. Mol. Neurobiol. 2016, 53, 7329–7340.
- Hu, Y.; Wu, L.; Jiang, L.; Liang, N.; Zhu, X.; He, Q.; Qin, H.; Chen, W. Notoginsenoside R2 reduces Aβ25-35-induced neuronal apoptosis and inflammation via miR-27a/SOX8/β-catenin axis. Hum. Exp. Toxicol. 2021, 40, S347–S358.
- Wang, X.; Xu, W.; Chen, H.; Li, W.; Li, W.; Zhu, G. Astragaloside IV prevents Aβ (1-42) oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 2020, 1747, 147041.




