阿尔茨海默病(痴呆的主要原因)的药物开发一直是一个长期的挑战。皂苷是具有各种药理活性的类固醇或三萜类糖苷,在治疗阿尔茨海默病方面显示出治疗潜力。
- Alzheimer’s disease
- saponin
- amyloid beta
- inflammation
- oxidative stress
- apoptosis
1. Introduction
Alzheimer’s disease (AD), the leading cause of dementia, is a progressive neurodegenerative disease that is clinically characterized by memory loss, cognitive impairment, and behavioral disturbances [2]. Since its discovery in 1906, AD has emerged as one of the most costly, fatal, and burdensome diseases of this century [3]. The pathogenesis of AD involves various biological processes [4] involving the abnormal deposition of amyloid beta peptide (Aβ) [5], the accumulation of neurofibrillary tangles (NFTs) [6], neuroinflammation [7], neuronal apoptosis [8], neurotransmitter abnormities [9], and oxidative stress [10]. Despite considerable efforts, drug discovery for the treatment of AD has been slow, with only acetylcholinesterase (AChE)/butyrylcholinesterase (BChE) inhibitors [11] such as galantamine, donepezil, tacrine, and rivastigmine currently available as therapies [12]. However, these treatments only delay the onset of symptoms and cannot halt disease progression and are often associated with significant side effects [13]. Therefore, the development of new therapeutic drugs is urgently needed. Saponins, a type of natural compound, have been extensively studied for their various pharmacological properties [14]. Of particular interest is their potential to enhance learning and memory in individuals with AD [15].
Saponins are naturally occurring compounds that are widely distributed in various plants [16], and they can be divided into two major groups based on their chemical structure: triterpenoid saponins and steroidal saponins [17]. Triterpenoid saponins are further subdivided into tetracyclic triterpenes and pentacyclic triterpenes and are mainly found in plants such as Pentaaceae, Leguminosae, Poria, and Platycodonaceae [18]. The main saponin skeletons of the triterpenoid saponins include dammarane, oleanane, ursane, and lupane (Figure 1 A, B, C, D) [19]. Steroidal saponins, on the other hand, are mainly found in plants such as Dioscoreaceae, Liliaceae, and Scrophulariaceae [20]. The main saponin metaskeletons of steroidal saponins include spirostane, furostane, cholestane, and cardenolide (Figure 1 E, F, G, H) [21]. Saponins possess multiple bioactivities, such as reduction of amyloid beta (Aβ) deposition [22], inhibition of tau protein phosphorylation [23], antioxidation [24], antiapoptosis [25], and anti-inflammation [26]. These properties make saponins promising therapeutic candidates for AD and other neurological disorders [27]. Meanwhile, the diversity of saponins found in different plants [28] makes them a valuable source of potential drugs for the treatment of AD. However, there is a current lack of comprehensive and systematic review in this field. To address this gap, we thoroughly searched the relevant literature in the major databases, including PubMed and Web of Science, using keywords “Alzheimer’s disease” or “AD” and “saponin”. By browsing all the relevant studies from May 2007 to May 2023, excluding review articles, 63 references were selected, involving 40 saponins extracted from different herbs. The selected references are known to be effective in the treatment of AD, present clear mechanisms, authentic and reliable information, and contain the latest findings in the field. The studies highlighted that saponins have exhibited various beneficial effects such as reducing Aβ levels, reducing NETs, exerting antioxidative, antiapoptotic, anti-inflammatory, and increased neurotransmitter-enhancing effects [25]. The underlying mechanisms behind these effects include reducing amyloid precursor protein (APP) production [29], improving tau protein phosphorylation [30], and reducing reactive oxygen species (ROS) generation [31], inhibiting the apoptotic and inflammatory signaling pathways [31,32], and increasing neurotransmitter expression [33]. This comprehensive review sheds light on the potential saponins as therapeutic agents for AD and related neurological disorders.
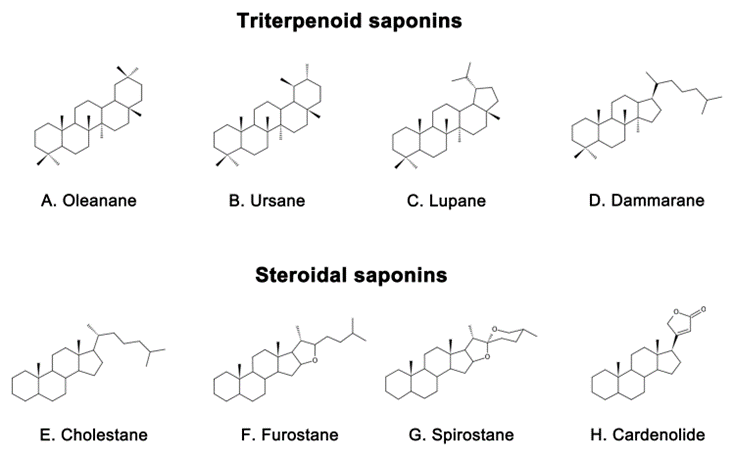
Figure 1. Representative triterpenoid saponins include (A) Oleanane, (B) Ursane, (C) Lupane, (D) Dammarane and steroidal saponins include (E) Cholestane, (F) Furostane, (G) Spirostane, (H) Cardenolide.
2. Mechanism of Saponins in Treating Alzheimer’s Disease
2.1. Inhibition of Aβ Deposition and Neurotoxicity
Aβ is a peptide that is a major component of the senile plaques found in the brains of people with AD [34]. Its accumulation is highly neurotoxic and is considered a hallmark of AD [35], which can result in impaired cognitive function, including spatial memory [36]. Aβ is generated from the APP and is typically enclosed by microglia and dystrophic synapses that aggregate around neurons [37,38]. APP is a transmembrane protein located in the synapse of neurons, which can be cleaved by both amyloid and nonamyloid pathways [39]. In the nonamyloid pathway, APP is sequentially cleaved by α-secretase (mainly ADAM10) and γ-secretase [40], resulting in P3 peptide (P3), C83 carboxy-terminal fragment (C83), APP intracellular domain (AICD), and soluble amyloid precursor protein-α (sAPPα) with beneficial neurotrophic effects [41]. Conversely, in the amyloid pathway, APP is first cleaved by β-secretase and sAPPβ is secreted [42]. Subsequently, γ-secretase cleaves the C-terminal fragment (C99) of the residual APP and eventually leads to the release of peptides of different lengths [43]. The most prevalent of them are Aβ1-40 and Aβ1-42 [44], which are neurotoxic fragments capable of oligomerization, aggregation, and subsequent plaque formation [45]. , Aβ is degraded by a variety of proteases, most notably insulin-degrading enzymes (IDE) and neprilysin (NEP) [46]. However, the production or activity of these clearance enzymes may decrease with age, leading to a failure to clear Aβ in a timely manner [47]. This phenomenon has been linked to the reduced activity of the peroxisome proliferator-activated receptor γ (PPARγ) [48], which is a transcription factor that regulates the expression of IDE and Bace1, reduces Aβ production [49], promotes Aβ clearance [50], and exerts neuroprotective effects [51]. An imbalance between Aβ production and clearance is responsible for the accumulation of Aβ in the brain [52]. In order to develop novel treatments for AD, it may be beneficial to inhibit Aβ production, enhance its clearance, or directly combat its neurotoxicity [53]
Natural saponins have shown potential in affecting Aβ metabolism through different pathways [54]. For example, some natural saponins are able to inhibit the formation of Aβ by reducing APP production. Ginsenoside Rg1, a tetracyclic triterpenoid saponin extracted from ginseng [55], has been shown to reduce Aβ deposition in APP/presenilin 1(PS1) double transgenic AD model mice by lowering APP levels [56]. Pharmacokinetic studies have also shown that ginsenoside Rg1 can cross the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB), which provides theoretical support for its ability to improve learning and memory abilities [57]. Xanthoceraside, another tetracyclic triterpenoid saponin, extracted from the bark of the Xanthoceras sorbifolia Bunge commonly used in traditional Chinese medicine (TCM) to treat rheumatism [58], has been shown to reduce APP protein levels and Aβ deposition in the cerebral cortex and hippocampus, thereby ameliorating cognitive function dysfunction in an AD mouse model induced by Aβ intracerebroventricular (ICV) injection [59]. In addition, ginsenoside Rh2, another ginseng derivative used in TCM, has been reported to regulate APP expression by reducing cholesterol and lipid raft levels. This ultimately led to an increase in sAPPα levels and a decrease in Aβ concentrations [60].
Most saponins have been shown to inhibit Aβ deposition by modulating the activity of APP-processing enzymes, such as ginsenoside Rg1 [61], RAPO-1-3 [62], onjisaponin B [62], pseudoginsenoside-F11 (PF11) [63], theasaponin E1 [64], anginsenoside (20S)-Rg3 [65], and ginsenoside C-K (CK) [66]. Ginsenoside Rg1 reduces the γ-secretase responsible for Aβ production by attenuating the Aβ-mediated inhibition of cAMP response element-binding protein (CREB) phosphorylation and protein kinase A (PKA) activity [61]. It also upregulates ADAM10 while reducing BACE1 in Wistar rat models of AD induced by ovariectomy (OVX) and D-galactose (D-gal) [67]. In sAPPα-transfected HT22 cells and neuroblastoma (SH-SY5Y) cells, ginsenoside Rg1 has been found to increase the levels of sAPPα and estrogen receptor (ER) α, elevate α-secretase activity, and decrease extracellular release of Aβ. Further studies have shown that these effects are mediated by the upregulation of phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2) and phosphorylated protein kinase B (pAkt) [68] but can be reversed by ER antagonists and potentially attenuated by inhibitors of protein kinase C (PKC), MAPK, and phosphatidyl 3-kinase (PI3K) [68]. Yuan Zhi (RAPO) is a TCM formulation that is commonly used to treat dementia due to its neuroprotective effects [69]. Its active ingredient, RAPO-1-3, and onjisaponin B, an acyl saponin with a similar constituent to RAPO-1-3, have been found to reduce Aβ production by promoting the degradation of APP protein through interference with the PS1/BACE1 interaction [62]. Another compound, PF11, which is a pentacyclic triterpene abundant in ginseng [70], has also demonstrated efficacy in inhibiting APP amyloidogenic processing. By reducing the expression of BACE1, PF11 reduced Aβ deposition and ameliorated cognitive impairment and synaptic dysfunction in SAMP8 mice [63]. Theasaponin E1, a pentacyclic triterpene extracted from green tea seeds [71], has been shown to reduce the production of Aβ by increasing the activity of NEP and ADAM10 in APP (SweAPP N2a) cells while inhibiting the expression of BACE1, APP, and PS1 [64]. Furthermore, CK, which is produced through the degradation of protopanaxadiol saponins by the gut microbiota [72], has been studied for its neuroprotective properties. Currently, it is primarily obtained by glycosyl hydrolysis of proto-ginsenoside diol-type saponins [73]. In scopolamine-induced ICR mice, CK was found to reduce the expression of BACE1 and PS1, increase IDE activity, reduce Aβ expression, and improve memory function [66]. In addition, ginsenoside (20S)-Rg3, a component of heat-processed ginseng, has been found to reduce Aβ levels by increasing phosphatidylinositol 4-kinase IIα(PI4KIIα) activity and ultimately decrease the expression of γ-secretase by decreasing the association of PS1 fragments and lipid rafts in cultured primary neurons and in the brains of an AD mouse model [65].
Aside from inhibiting Aβ production, certain saponins have been found to promote the clearance of Aβ. For example, minor ginsenoside F1, a trace ginsenoside derived from Panax ginseng [74], has been shown to effectively reduce Aβ plaques and alleviate cognitive impairment in APP/PS1 mice by enhancing the expression of pCREB [75]. Similarly, Bacopaside I (BS-I), a tetracyclic triterpene and standardized extract of Bacopa monnieri [76], has been shown to be neuroprotective and improve cognitive function [77]. The aglycones of Bacopa monnieri and its derivatives have good intestinal absorption and BBB permeability [78]. Recent studies suggest that BS-I induces sufficient innate immune stimulation and phagocytosis to promote amyloid clearance, thereby reducing amyloid plaque burden in APP/PS1 mice [79]. In addition, Aβ deposition in the brain is linked to the dysfunction of the endosomal–lysosomal system dysfunction [80], which is regulated by the transcription factor EB (TFEB) [81]. PF11 has been observed to increase Aβ clearance by promoting the mammalian target of rapamycin (mTOR)-dependent TFEB-mediated lysosome biogenesis and alleviating endosomal–lysosomal system dysfunction through the conversion of Rab5 to Rab7 [82].
2.2. Inhibiting Aberrant Tau Protein Phosphorylation
NFTs, a well-known pathological hallmark of AD [104], are composed of excessively phosphorylated proteins linked by helical filaments known as tubulin-associated unit (tau) protein [105]. Tau protein, mainly found in neuronal axons, is a microtubule-associated protein involved in various functions such as axonal microtubule maintenance, cytoplasmic transport, and regulation of neuronal signaling [106,107]. Its optimal function depends on the low levels of normal phosphorylation [108]. However, overphosphorylation of tau protein leads to its detachment from microtubules, resulting in microtubule depolymerization, aggregation into NFTs, and ultimately neurodegeneration and cell death [109]. The correlation between NFT density and cognitive decline suggests that tau protein plays a central role in AD [110]. Furthermore, the prevalence of tau protein pathology positively correlates with cognitive impairment in AD, where a person with AD has increased levels of tau protein, hyperphosphorylated tau protein, and aggregated tau in their brain [111]. Abnormal tau protein phosphorylation plays a greater role in AD than the total tau protein [112]. As such, it is considered a critical pathogenic step in the development of NFTs and, subsequently, AD [113].
Several saponins have been found to inhibit the hyperphosphorylation of tau proteins, protect synaptic structures, and reduce neuronal damage. An octillol-type saponin, PF11, has been found to increase the activity of phosphatase-2A (PP2A) by enhancing leucine carboxyl methyltransferase-1 (LCMT-1) and improving cognitive impairment in SAMP/8 mice by inhibiting the hyperphosphorylation of tau protein at serine 396 and tyrosine 205 in the brain [63]. Streptozotocin (STZ) is a glucosamine–nitrosourea compound commonly used in AD studies to induce tau proteins’ hyperphosphorylation and cognitive impairment [114]. Tau protein phosphorylation occurs at multiple sites, usually associated with glycogen synthase kinase (GSK) 3 and cyclin-dependent kinase 5 (CDK5). PF11 was also shown to attenuate STZ-induced tau hyperphosphorylation in the brain of male Wistar rats by correcting the dysregulation of the insulin receptor substrate 1/PI3K/Akt/GSK-3β and calpain I/CDK5 signaling pathways [70]. Additionally, PF11 ameliorated STZ-induced learning and memory deficits, synaptic damage, and neuronal death [70]. Another active compound, Ginsenoside Rb1, found in ginseng [115], exhibits neuroprotective properties against different neurotoxins [116]. Research has shown that ginsenoside Rb1 counters cognitive impairment induced by aluminum oxide in ICR mice while reducing Ser396 tau phosphorylation by increasing PP2A levels and decreasing p-GSK levels [111,117]. Ginsenoside Rd, another compound found in ginseng [118], has been shown to have potential therapeutic applications in neurodegenerative diseases [119]. It has been demonstrated to reduce tau proteins’ hyperphosphorylation and neurotoxicity induced by okadaic acid (OA) by increasing PP2A activity in both rat and cortical neuronal models of AD [120].
Theasaponin E1, a vital compound found in green tea seeds, shows remarkable potential in reducing p-tau protein levels and inhibiting AD-promoting genes while activating AD-remitting genes [121]. This potential has been observed in AD models constructed from SHY5Y and glioblastoma (HTB2) cells. Notably, theasaponin E1 successfully reduced p-tau protein by inhibiting several enzymes, including GSK-3β, CDK5, c-Jun NH2-terminal kinase (JNK), MAPK, ERK1/MARK, and calmodulin-dependent protein kinase II alpha [121]. Similarly, Xanthoceraside, a triterpene saponin extracted from the shell of Xanthoceras sorbifolia Bunge, has been shown to exert neuroprotective effects by inhibiting the phosphorylation of tau protein [122]. In a study conducted on mice with AD, the administration of Xanthoceraside significantly decreased the expression of pGSK-3β and tau protein at Ser396 and Ser404, thereby effectively inhibiting the phosphorylation of tau protein [59].
2.3. Anti-Inflammatory Effect
In recent years, neuroinflammation has become recognized as a third neuropathological hallmark of AD, in addition to Aβ and NFTs [123]. Neuroinflammation is an inflammatory response to pathological insults that occur within the central nervous system (CNS), such as infection, trauma, ischemia, and toxin accumulation [124]. This response is characterized by the proliferation and activation of microglia and astrocytes in the brain [125]. Such activation is usually accompanied by the secretion of inflammatory cytokines such as interleukin-1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) [126]. Microglia are innate immune cells that reside within the CNS and are thought to be important regulators in the inflammatory response within the brain [127]. In the early stages of AD, activated microglia can exert protective effects by phagocytosing and degrading Aβ [128,129]. However, when Aβ accumulates excessively, it binds various receptors on the surface of microglia such as cluster of differentiation (CD) 14, CD36 [130], Toll-like receptor (TLR) 4, and TLR2, to promote the release of inflammatory factors [131]. This response leads to neuroinflammation and further impairment of cognitive function. Similarly, astrocyte activation is also associated with Aβ [132]. Activated astrocytes produce various inflammatory mediators [133] and secrete small amounts of Aβ [134], resulting in a chronic vicious cycle [133]. These reactions can exacerbate neuroinflammation and damage neurons.
Multiple pathways are involved in microglia- and astrocyte-mediated neuroinflammation [135], one of which is the nuclear factor kappa-B (NF-κB) pathway [136]. This pathway activates the transcription of TNF-α, IL-1β, and IL-6, which exacerbates inflammation [137]. Microglial activation also depends on other signaling pathways such as the PI3K/Akt/mTOR pathway [138]which can promote the expression of the inflammatory mediators NO synthase (NOS) and cyclooxygenase-2 (Cox-2) [139,140]. Furthermore, MAPK/p38 [141], Janus kinase (JAK)/signal transducer, and activator of transcription (STAT) and other pathways also contribute to neuroinflammation [142]. Therefore, modulating inflammatory signaling pathways and inhibiting the production of proinflammatory factors may offer a promising approach to the treatment of AD.
2.4. Improvement in Mitochondrial Function and Antioxidative Stress
Oxidative stress refers to an imbalance between oxidation and antioxidation in the body [174], which ismainly characterized by the increased production of reactive oxygen species and reduced ability of antioxidants to combat them [175]. The brain is susceptible to oxidative damage due to its high lipid content and lacks effective antioxidant defense mechanisms, despite high oxygen consumption [176,177]. It is now well established that the level of oxidation in the brain also increases with age [178]. Interestingly, extensive oxidative damage can be observed in the stage of mild cognitive impairment that precedes the typical clinical manifestations of AD, suggesting that oxidative stress may be a central mechanism in driving the disease [179].
2.5. 抗凋亡作用
This entry is adapted from the peer-reviewed paper 10.3390/ijms241310505
