Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Geriatrics & Gerontology
阿尔茨海默病(痴呆的主要原因)的药物开发一直是一个长期的挑战。皂苷是具有各种药理活性的类固醇或三萜类糖苷,在治疗阿尔茨海默病方面显示出治疗潜力。
- Alzheimer’s disease
- saponin
- amyloid beta
- inflammation
- oxidative stress
- apoptosis
1. 简介
阿尔茨海默病(AD)是痴呆的主要原因,是一种进行性神经退行性疾病[1],临床特征为记忆丧失、认知障碍和行为障碍[2]。自1906年发现以来,AD已成为本世纪最昂贵,最致命和最繁重的疾病之一[3]。AD的发病机制涉及各种生物学过程[4],涉及淀粉样蛋白β肽(Aβ)的异常沉积[5],神经原纤维缠结(NFT)的积累[6],神经炎症[7],神经元凋亡[8],神经递质异常[9]和氧化应激[10].尽管付出了相当大的努力,但治疗AD的药物发现进展缓慢,目前只有AChE/丁酰胆碱酯酶(BChE)抑制剂[11],如加兰他敏、多奈哌齐、他克林和卡巴拉汀[12]。然而,这些治疗只能延缓症状的发作,不能阻止疾病进展,并且通常伴有明显的副作用[13]。因此,迫切需要开发新的治疗药物。皂苷是一种天然化合物,因其各种药理学特性而被广泛研究[14]。特别令人感兴趣的是它们增强AD患者学习和记忆的潜力[15]。
皂苷是天然存在的化合物,广泛分布于各种植物中[16],根据其化学结构可分为两大类:三萜皂苷和甾体皂苷[17]。三萜皂苷进一步细分为四环三萜和五环三萜,主要存在于五萜科、豆科、茯苓科和鸭嘴科等植物中[18]。三萜皂苷的主要皂苷骨架包括达玛烷、苹果酸、熊烷和鲁帕烷(图 1A-D)[19]。另一方面,甾体皂苷主要存在于薯蓣科、百合科和蝙蝠科等植物中[20]。甾体皂苷的主要皂苷代谢物包括螺糠烷、呋喃烷、胆甾烷和卡氏内酯(图 1E-H)[21]。皂苷具有多种生物活性,如减少β淀粉样蛋白沉积[22]、抑制tau蛋白磷酸化[23]、抗氧化[24]、抗细胞凋亡[25]和抗炎[26]。这些特性使皂苷成为AD和其他神经系统疾病的候选药物[27]。同时,在不同植物中发现的皂苷的多样性[28]使它们成为治疗AD的潜在药物的宝贵来源。
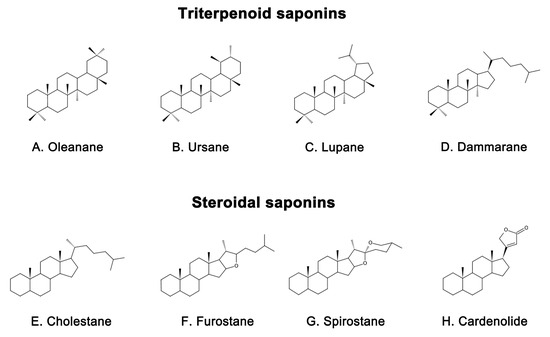
图1.具有代表性的皂苷骨架结构包括(A)Oleanane、(B)Ursane、(C)Lupane、(D)达玛烷和甾体皂苷包括(E)胆甾烷、(F)呋喃烷、(G)螺烷、(H)羧内酯。
2. 皂苷治疗阿尔茨海默病的机制
2.1. 抑制Aβ沉积和神经毒性
Aβ是一种肽,是AD患者大脑中老年斑块的主要成分[34]。其积累具有高度神经毒性,被认为是AD的标志[35],可导致认知功能受损,包括空间记忆[36]。Aβ由APP产生,通常被聚集在神经元周围的小胶质细胞和营养不良突触包围[37,38]。APP是一种位于神经元突触中的跨膜蛋白,可被淀粉样蛋白和非淀粉样蛋白途径切割[39]。在九淀粉样蛋白通路中,APP被α分泌酶(主要是ADAM10)和γ分泌酶[40]顺序裂解,产生P3肽(P3)、C83羧基末端片段(C83)、APP细胞内结构域(AICD)和可溶性淀粉样蛋白前体蛋白-α(sAPPα),具有有益的神经营养作用[41]。相反,在淀粉样蛋白通路中,APP首先被β分泌酶切割,然后分泌sAPPβ[42]。随后,γ分泌酶切割残留APP的C末端片段(C99),最终导致不同长度的肽的释放[43]。其中最普遍的是Aβ1-40和Aβ1-42[44],它们是能够寡聚化、聚集和随后形成斑块的神经毒性片段[45]。在体内,Aβ被多种蛋白酶降解,最明显的是胰岛素降解酶(IDE)和脑啡肽酶(NEP)[46]。然而,这些清除酶的产生或活性可能随着年龄的增长而下降,导致无法及时清除Aβ[47]。这种现象与过氧化物酶体增殖物激活受体γ(PPARγ)活性降低有关[48],PPARγ是一种转录因子,可调节IDE和Bace1的表达,减少Aβ的产生[49],促进Aβ清除[50],并发挥神经保护作用[51]。Aβ产生和清除之间的不平衡是Aβ在大脑中积累的原因[52]。为了开发AD的新疗法,抑制Aβ生成、增强其清除率或直接对抗其神经毒性可能有益[53]。
天然皂苷已显示出通过不同途径影响Aβ代谢的潜力[54]。例如,一些天然皂苷能够通过减少APP的产生来抑制Aβ的形成。人参皂苷Rg1是一种从人参中提取的四环三萜皂苷[55],已被证明可以通过降低APP水平来减少APP/早老素1(PS1)双转基因AD模型小鼠的Aβ沉积[56]。药代动力学研究还表明,人参皂苷Rg1可以穿过血脑屏障(BBB)和血脑脊液屏障(BCSFB),这为其提高学习和记忆能力提供了理论支持[57]。黄芜苷,另一种四环三萜皂苷,提取自山梨黄芩的树皮,常用于治疗风湿病[58],在脑室内注射诱导的AD小鼠模型中,已被证明可降低APP蛋白水平和Aβ沉积在大脑皮层和海马体中,从而改善AD小鼠模型的认知功能障碍[59]。此外,据报道,人参皂苷Rh2是中药中使用的另一种人参衍生物,通过降低胆固醇和脂筏水平来调节APP表达。这最终导致sAPPα水平升高和Aβ浓度降低[60]。
大多数皂苷已被证明通过调节APP加工酶的活性来抑制Aβ沉积,例如人参皂苷Rg1 [61],RAPO-1-3 [62],皂苷B [62],假人参皂苷-F11(PF11)[63],茶皂苷E1[64],心绞痛皂苷(20S)-Rg3 [65]和人参皂苷C-K(CK)[66].人参皂苷Rg1通过减弱Aβ介导的cAMP反应元件结合蛋白(CREB)磷酸化和蛋白激酶A(PKA)活性的抑制,降低负责Aβ产生的γ分泌酶[61]。在卵巢切除术(OVX)和D-半乳糖(D-gal)诱导的AD的Wistar大鼠模型中,它还可以上调ADAM10,同时降低BACE1[67]。在sAPPα转染的HT22细胞和神经母细胞瘤(SH-SY5Y)细胞中,人参皂苷Rg1被发现可以增加sAPPα和雌激素受体(ER)α的水平,提高α分泌酶活性,并减少Aβ的细胞外释放。进一步的研究表明,这些效应是由磷酸化细胞外信号调节激酶1/2(pERK1/2)和磷酸化蛋白激酶B(pAkt)的上调介导的[68],但可以被ER拮抗剂逆转,并可能被蛋白激酶C(PKC)、MAPK、和磷脂酰3-激酶(PI3K)抑制剂减弱[68]。元智(RAPO)是一种中药制剂,由于其神经保护作用,常用于治疗痴呆[69]。已发现其活性成分RAPO-1-3和onjisoponin B(一种与RAPO-1-3成分相似的酰基皂苷)通过干扰PS1/BACE1相互作用促进APP蛋白的降解来减少Aβ的产生[62]。另一种化合物PF11是一种富含人参的五环三萜[70],也显示出抑制APP淀粉样蛋白生成加工的功效。通过降低BACE1的表达,PF11减少了SAMP8小鼠的Aβ沉积,改善了认知障碍和突触功能障碍[63]。茶皂苷E1是一种从绿茶种子中提取的五环三萜[71],已被证明通过增加APP(SweAPP N10a)细胞中NEP和ADAM2的活性来减少Aβ的产生,同时抑制BACE1、APP和PS1的表达[64]。此外,CK是通过肠道微生物群降解原托他二醇皂苷产生的[72],其神经保护特性已被研究。目前,它主要通过原人参皂苷二醇型皂苷的糖基水解获得[73]。在东莨菪碱诱导的ICR小鼠中,发现CK可降低BACE1和PS1的表达,增加IDE活性,降低Aβ表达并改善记忆功能[66]。此外,人参皂苷(20S)-Rg3是热加工人参的一种成分,已发现通过增加磷脂酰肌醇4激酶IIα(PI4KIIα)活性来降低Aβ水平,并通过减少培养的原代神经元和AD小鼠模型大脑中PS1片段和脂筏的关联来最终降低γ分泌酶的表达[65]。
除了抑制Aβ的产生外,还发现某些皂苷可以促进Aβ的清除。例如,次要人参皂苷F1(一种来源于人参的微量人参皂苷[74])已被证明可以通过增强pCREB的表达来有效减少APP/PS1小鼠的Aβ斑块并缓解认知障碍[75]。同样,假马齿苋苷I(BS-I)是一种四环三萜和假马齿苋的标准化提取物[76],已被证明具有神经保护作用并改善认知功能[77]。假马齿苋及其衍生物的糖苷元具有良好的肠道吸收和BBB通透性[78]。最近的研究表明,BS-I诱导足够的先天免疫刺激和吞噬作用以促进淀粉样蛋白清除,从而减少APP/PS1小鼠的淀粉样斑块负荷[79]。此外,Aβ在大脑中的沉积与内体-溶酶体系统功能障碍有关[80],后者受转录因子EB(TFEB)的调节[81]。据观察,PF11通过促进哺乳动物雷帕霉素(mTOR)依赖性TFEB介导的溶酶体生物发生靶标,并通过将Rab5转化为Rab7来缓解内体-溶酶体系统功能障碍,从而增加Aβ清除率[82]。
2.2. 抑制异常tau蛋白磷酸化
NFT 是 AD 的一个众所周知的病理标志 [104],由称为微管蛋白相关单元 (tau) 蛋白的螺旋丝连接的过度磷酸化蛋白质组成 [105]。Tau蛋白主要存在于神经元轴突中,是一种微管相关蛋白,参与多种功能,如轴突微管维持、细胞质转运和神经元信号传导调节[106,107]。其最佳功能取决于正常磷酸化的低水平[108]。然而,tau蛋白的过度磷酸化导致其与微管分离,导致微管解聚,聚集成NFT,最终导致神经变性和细胞死亡[109]。NFT密度与认知能力下降之间的相关性表明tau蛋白在AD中起核心作用[110]。此外,tau蛋白病理学的患病率与AD患者的认知障碍呈正相关,AD患者的大脑中tau蛋白、过度磷酸化tau蛋白和聚集tau蛋白水平升高[111]。异常tau蛋白磷酸化在AD中的作用大于总tau蛋白[112]。因此,它被认为是 NFT 以及随后 AD 发展的关键致病步骤[113]。
已经发现几种皂苷可以抑制tau蛋白的过度磷酸化,保护突触结构,并减少神经元损伤。研究发现,辛替洛尔型皂苷PF11通过增强亮氨酸羧基甲基转移酶-2(LCMT-2)来增加磷酸酶-1A(PP1A)的活性,并通过抑制大脑中丝氨酸8和酪氨酸396处tau蛋白的过度磷酸化来改善SAMP/205小鼠的认知障碍[63]。链脲佐菌素(STZ)是一种氨基葡萄糖-亚硝基脲化合物,常用于AD研究,以诱导tau蛋白的过度磷酸化和认知障碍[114]。Tau蛋白磷酸化发生在多个位点,通常与糖原合酶激酶(GSK)3和细胞周期蛋白依赖性激酶5(CDK5)相关。PF11还通过纠正胰岛素受体底物1/PI3K/Akt/GSK-3β和钙蛋白酶I/CDK5信号通路的失调,减弱STZ诱导的雄性Wistar大鼠大脑中的tau过度磷酸化[70]。此外,PF11改善了STZ诱导的学习和记忆缺陷、突触损伤和神经元死亡[70]。在人参中发现的另一种活性化合物人参皂苷Rb1[115]对不同的神经毒素具有神经保护特性[116]。研究表明,人参皂苷Rb1可对抗ICR小鼠氧化铝诱导的认知障碍,同时通过增加PP396A水平和降低p-GSK水平来减少Ser2tau磷酸化[111,117]。人参中发现的另一种化合物人参皂苷Rd[118]已被证明在神经退行性疾病中具有潜在的治疗应用[119]。在AD的大鼠和皮质神经元模型中,已经证明可以通过增加PP2A活性来减少冈田酸(OA)诱导的tau蛋白过度磷酸化和神经毒性[120]。
茶皂苷E1是一种在绿茶种子中发现的重要化合物,在降低p-tau蛋白水平和抑制AD促进基因方面显示出显着的潜力,同时激活AD缓解基因[121]。在由SHY5Y和胶质母细胞瘤(HTB2)细胞构建的AD模型中已经观察到这种潜力。值得注意的是,茶皂苷E1通过抑制几种酶(包括GSK-3β、CDK5、c-Jun NH2末端激酶(JNK)、MAPK、ERK1/MARK和钙调蛋白依赖性蛋白激酶IIΑ)成功还原p-tau蛋白[121]。同样,黄花芍是一种从山梨黄芪壳中提取的三萜皂苷,已被证明通过抑制tau蛋白的磷酸化来发挥神经保护作用[122]。在一项针对AD小鼠的研究中,给予黄尾花苷显著降低了pGSK-3β和tau蛋白在Ser396和Ser404处的表达,从而有效抑制了tau蛋白的磷酸化[59]。
2.3. 抗炎作用
近年来,神经炎症已成为除Aβ和NFTs之外AD的第三个神经病理学标志[123]。神经炎症是对中枢神经系统内发生的病理性损伤(如感染、创伤、缺血和毒素积累)的炎症反应[124]。这种反应的特征是大脑中小胶质细胞和星形胶质细胞的增殖和活化[125]。这种激活通常伴有炎症细胞因子的分泌,例如IL-1β、白细胞介素1(IL-6)和肿瘤坏死因子α(TNF-α)[6]。小胶质细胞是存在于CNS内的先天免疫细胞,被认为是大脑内炎症反应的重要调节因子[126]。在AD的早期阶段,活化的小胶质细胞可以通过吞噬和降解Aβ发挥保护作用[127,128]。然而,当Aβ过度积累时,它会结合小胶质细胞表面的各种受体,如分化簇(CD)129、CD14[36]、Toll样受体(TLR)130和TLR4,促进炎症因子的释放[2]。这种反应导致神经炎症和认知功能的进一步损害。同样,星形胶质细胞活化也与Aβ有关[131]。活化的星形胶质细胞产生各种炎症介质[132]并分泌少量Aβ[133],导致慢性恶性循环[134]。这些反应会加剧神经炎症并损害神经元。
小胶质细胞和星形胶质细胞介导的神经炎症涉及多种途径[135],其中一条是核因子κ-B(NF-κB)途径[136]。该途径激活TNF-α、IL-1β和IL-6的转录,从而加剧炎症[137]。小胶质细胞激活还取决于其他信号通路,例如PI3K/Akt/mTOR通路[138],其可促进炎症介质NO合酶(NOS)和环氧合酶-2(Cox-2)的表达[139,140]。此外,MAPK/p38[141]、JAK激酶/信号转导器、转录激活剂(STAT)和其他通路也有助于神经炎症[142]。因此,调节炎症信号通路和抑制促炎因子的产生可能为治疗AD提供一种有希望的方法。
一般来说,天然皂苷具有抗炎生理活性,从而通过调节炎症相关的信号通路来抑制炎症因子的表达或释放来保护神经细胞。
2.4. 改善线粒体功能和抗氧化应激
线粒体作为有氧代谢的必需细胞器,可显著促进氧化应激的产生[180]。同时,现在已经表明,线粒体氧化应激可导致轴突和神经元损伤,并最终导致神经功能障碍[181]。当线粒体内发生氧化应激时,可导致线粒体肿胀、外膜破裂和钙离子丢失,导致ROS的进一步产生和释放[182]。在生理条件下,ROS可以刺激细胞生长[183],并被抗氧化酶和其他抗氧化防御系统[184]消除,如超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)和过氧化氢酶(CAT)[185]。然而,当发生氧化还原失衡状态时,自由基大量积聚,导致脂质过氧化,导致丙二醛(MDA)的产生[186]、细胞膜损伤、线粒体膜损伤,甚至神经元死亡[187]。此外,氧化应激可能与大脑中的Aβ聚集[188]、tau蛋白磷酸化[189]和金属离子微环境损伤相互作用,放大神经元损伤,最终导致AD的发病机制[190,191]。
Several natural saponins may act as exogenous antioxidants to combat AD by managing oxidative stress [192,193]. Ginsenoside Rh2, a rare ginsenoside with few reports on its neuroprotective effects compared to other similar molecules [194], has shown significant neuroprotective effects in a scopolamine-induced model of memory impairment in ICR mice. Ginsenoside Rh2 effectively managed MDA levels while increasing glutathione (GSH) levels and SOD activity in the brain, effectively inhibiting oxidative stress [195]. These findings suggest that ginsenoside Rh2 has great potential for the treatment of AD by inhibiting oxidative stress in the brain. Crude saponins from BF showed potent antioxidant effects in addition to anti-inflammatory effects. In addition, saponins from BF alleviated oxidative stress, reduced ROS, and MDA levels by restoring GSH-Px, GSH, and CAT levels in the Aβ ICV-induced BALB/c AD mouse model [161]. Similarly, ginsenoside Rd was found to reduce protein carbonyls and 4-hydroxy-2-nonenal (4-HNE) levels by decreasing the glutathione disulfide (GSSG)/GSH ratio, thereby counteracting oxidative stress in the brain of Aβ-induced AD mouse models [170]. Ginsenoside Rg3, a tetracyclic triterpenoid saponin, is the major bioactive component of ginseng and is widely used for the treatment of tumors [196]. Results from in vivo studies have shown that ginsenoside Rg3 can improve mitochondrial dysfunction and enhance ROS scavenging ability by increasing SOD, CAT, and GSH-Px levels, thus ameliorating oxidative stress in the brain of D-gal-induced AD rats [197]. PF11 was found to inhibit the production of both APP and Aβ in the cortex and hippocampus, while restoring the activity of SOD and GSH-Px in the brains of AD mice and reducing MDA levels in the cortex [198].
Ginsenoside Rg1 has also been found to protect primary cortical neurons against Aβ-induced increases in ROS and H2O2 levels by inhibiting oxidative stress [199]. This results in a decrease in mitochondrial membrane potential and an increase in cytochrome C (Cyt-c) oxidase activity, ultimately leading to a decrease in Cyt-c release and indicating improved mitochondrial function [199]. Another newly discovered saponin compound, huangjingsterol B from Polygonatum cyrtonema Hua, showed significant antioxidant activity [200] by reducing oxidative stress and protecting cells from Aβ-induced damage through modulating the activity of GSH-Px and SOD [200]. Similarly, researchers have found that a saponin from the fruit of Solanum anguivi can protect the P2 region of rat brain synaptosomes from oxidative damage induced by ferrous iron and sodium nitroprusside, reduce ROS generation, restore total thiol levels, and protect mitochondrial function [192]. This suggests that saponins from Solanum anguivi fruits may have antioxidant properties that benefit mitochondrial function [201]. PGS has a similar effect with its ability to upregulate intracellular heme oxygenase-1 (HO-1), SOD, CAT, and GSH-Px to inhibit Aβ-induced ROS production and reduce oxidative damage in AD brains [153]. Despite the demonstrated neuroprotective effects of Panax ginseng, the mechanisms underlying its antioxidant properties remain poorly understood. However, a recent study has shown that the primary bioactive compound in Panax ginseng, NTR1, is able to prevent the loss of mitochondrial membrane potential and reduce the accumulation of ROS induced by Aβ [202]. This suggests that NTR1 may exert neuroprotective effects by inhibiting oxidative stress. Furthermore, most previous experiments have focused on saponins extracted from Panax ginseng roots, and the pharmacological effects of other parts, such as saponins in P. notoginseng leaves (LPNS), are largely unknown [203]. LPNS, which mainly include the ginsenosides Rb1, Rb2, Rb3, and Rc, have been shown to have potent neuroprotective properties [203]. In H2O2- or oxygen and glucose deprivation (OGD)-induced astrocytes from the cortex of SD rats and SH-SY5Y cells, LPNS increased the levels of nuclear factor erythroid2-related factor 2 (Nrf2) and its downstream HO-1 and glutathione S-transferase pi 1 (GSTP1) in response to increased ROS, as well as significantly reducing lactate dehydrogenase (LDH) expression and increasing the cellular activity of astrocytes [203].
2.5. Antiapoptotic Effect
It is widely accepted that neuronal apoptosis contributes significantly to the pathogenesis of AD [213] and is even a pathological hallmark of the disease [214]. Neuronal apoptosis has been found to be abundant in AD autopsy samples [215] and several AD models have successfully reproduced this apoptosis [216]. The biochemical signature of apoptosis is mainly the activation of cysteinase triggered by intrinsic or extrinsic apoptotic pathways [217]. Apoptosis triggered by external cellular signals is called the “death receptor“ pathway, which involves the activation of caspase-8 followed by cleavage of caspase-3, leading to apoptosis [218]. In contrast, the intrinsic apoptotic pathway is driven by the mitochondria [219]. Mitochondrial damage induces the release of Cyt-c and other apoptotic factors into the cytoplasm, which then activate caspase-9 and caspase-3, resulting in apoptosis [220]. The B-cell lymphoma-2 (Bcl-2) protein family includes proapoptotic proteins, BCL2-associated X (BAX), Bcl-2 antagonist killer 1 (BAK), Bcl-2-like protein 11 (BIM), p53 upregulated modulator of apoptosis (PUMA), and Bcl2 modifying factor (BMF), and antiapoptotic proteins, such as Bcl-2, lymphoma-extra-large (Bcl-xL), Bcl-2 like 2 (Bcl-w), and myeloid cell leukemia-1 (Mcl-1), which tightly regulates this apoptotic process by controlling the ratio of proapoptotic and antiapoptotic proteins [221,222], particularly the Bax/Bcl-2 ratio [223]. In AD patients, Bcl-2 expression is reduced in the hippocampus [224], while Bax accumulates near senile plaques and tau protein fiber tangles in an AD animal model, suggesting that mitochondria-mediated apoptosis is closely linked to the pathology of AD [225].
Numerous saponins, including astragaloside (AST), NTR2, PGS, AS-IV, NTR1, Ginsenoside Rg2, and Ginsenoside Rg1, have shown antiapoptotic effects in AD-associated models. AST, a small molecule saponin derived from Astragalus membranaceus, has been widely used in China for the treatment of chronic diseases [226]. In in vivo and in vitro experiments, AST can prevent Aβ-induced apoptosis in cortical neurons by modulating the PI3K/Akt and MAPK (or ERK) pathway [227]. This effect is reversed by the PI3K inhibitor LY294002 and mimicked by the inhibition of ERK and enhanced by its inhibitor U0126, suggesting that the neuroprotective effect of AST may also be related to the inhibition of the ERK pathways [227]. Similarly, NTR2 can reverse the Aβ-induced apoptosis of primary cortical neurons via miR-27a/SOX8/axis and ameliorate cognitive dysfunction in AD mice [159]. PGS may provide neuroprotection not only by reducing inflammation but also by inhibiting apoptosis. PGS increased the expression of Bcl-2 family proteins, resulting in a reduction in Cyt-c release and caspase-9 and -3 expression, thereby inhibiting Aβ-induced apoptosis in HT22 cells. PGS achieved this by inhibiting p38, ERK, and JNK activation, as evidenced by decreased levels of p-p38/p38, p-ERK/ERK, and p-JNK/JNK, and by downregulating MAPK signaling [153]. In vitro and in vivo experiments constructed in HT-22 cells and C57BL/6 induced by amyloid beta protein fragment 1-42 oligomers (AβO) have shown that AS-IV reduced the Aβ-induced inhibition of the brain-derived neurotrophic factor (BDNF)–tyrosine kinase receptor B (TrkB) signaling pathway by regulating PPARγ [228]. This mechanism leads to the reduction in the number and fluorescence density of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells and caspase-3 levels, ultimately alleviating hippocampal neuronal apoptosis and memory impairment [228]. In Aβ-induced PC12 cells, NTR1 has also been shown to exert an antiapoptotic effect, reducing the percentage of TUNEL-positive cells and the levels of caspase-3, Bax, and the Bax/Bcl-2 ratio, and exerted an antiapoptotic effect by suppressing the MAPK pathway [202].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241310505
This entry is offline, you can click here to edit this entry!
