阿尔茨海默病(痴呆的主要原因)的药物开发一直是一个长期的挑战。皂苷是具有各种药理活性的类固醇或三萜类糖苷,在治疗阿尔茨海默病方面显示出治疗潜力。
- Alzheimer’s disease
- saponin
- amyloid beta
- inflammation
- oxidative stress
- apoptosis
1. 简介Introduction
Alzheimer’s disease (AD), the leading cause of dementia, is a progressive neurodegenerative disease that is clinically characterized by memory loss, cognitive impairment, and behavioral disturbances [2]. Since its discovery in 1906, AD has emerged as one of the most costly, fatal, and burdensome diseases of this century [3]. The pathogenesis of AD involves various biological processes [4] involving the abnormal deposition of amyloid beta peptide (Aβ) [5], the accumulation of neurofibrillary tangles (NFTs) [6], neuroinflammation [7], neuronal apoptosis [8], neurotransmitter abnormities [9], and oxidative stress [10]. Despite considerable efforts, drug discovery for the treatment of AD has been slow, with only acetylcholinesterase (AChE)/butyrylcholinesterase (BChE) inhibitors [11] such as galantamine, donepezil, tacrine, and rivastigmine currently available as therapies [12]. However, these treatments only delay the onset of symptoms and cannot halt disease progression and are often associated with significant side effects [13]. Therefore, the development of new therapeutic drugs is urgently needed. Saponins, a type of natural compound, have been extensively studied for their various pharmacological properties [14]. Of particular interest is their potential to enhance learning and memory in individuals with AD [15].
Saponins are naturally occurring compounds that are widely distributed in various plants [16], and they can be divided into two major groups based on their chemical structure: triterpenoid saponins and steroidal saponins [17]. Triterpenoid saponins are further subdivided into tetracyclic triterpenes and pentacyclic triterpenes and are mainly found in plants such as Pentaaceae, Leguminosae, Poria, and Platycodonaceae [18]. The main saponin skeletons of the triterpenoid saponins include dammarane, oleanane, ursane, and lupane (Figure 1 A, B, C, D) [19]. Steroidal saponins, on the other hand, are mainly found in plants such as Dioscoreaceae, Liliaceae, and Scrophulariaceae [20]. The main saponin metaskeletons of steroidal saponins include spirostane, furostane, cholestane, and cardenolide (Figure 1 E, F, G, H) [21]. Saponins possess multiple bioactivities, such as reduction of amyloid beta (Aβ) deposition [22], inhibition of tau protein phosphorylation [23], antioxidation [24], antiapoptosis [25], and anti-inflammation [26]. These properties make saponins promising therapeutic candidates for AD and other neurological disorders [27]. Meanwhile, the diversity of saponins found in different plants [28] makes them a valuable source of potential drugs for the treatment of AD. However, there is a current lack of comprehensive and systematic review in this field. To address this gap, we thoroughly searched the relevant literature in the major databases, including PubMed and Web of Science, using keywords “Alzheimer’s disease” or “AD” and “saponin”. By browsing all the relevant studies from May 2007 to May 2023, excluding review articles, 63 references were selected, involving 40 saponins extracted from different herbs. The selected references are known to be effective in the treatment of AD, present clear mechanisms, authentic and reliable information, and contain the latest findings in the field. The studies highlighted that saponins have exhibited various beneficial effects such as reducing Aβ levels, reducing NETs, exerting antioxidative, antiapoptotic, anti-inflammatory, and increased neurotransmitter-enhancing effects [25]. The underlying mechanisms behind these effects include reducing amyloid precursor protein (APP) production [29], improving tau protein phosphorylation [30], and reducing reactive oxygen species (ROS) generation [31], inhibiting the apoptotic and inflammatory signaling pathways [31,32], and increasing neurotransmitter expression [33]. This comprehensive review sheds light on the potential saponins as therapeutic agents for AD and related neurological disorders.
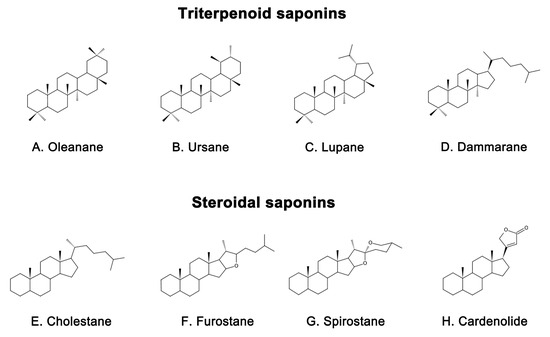
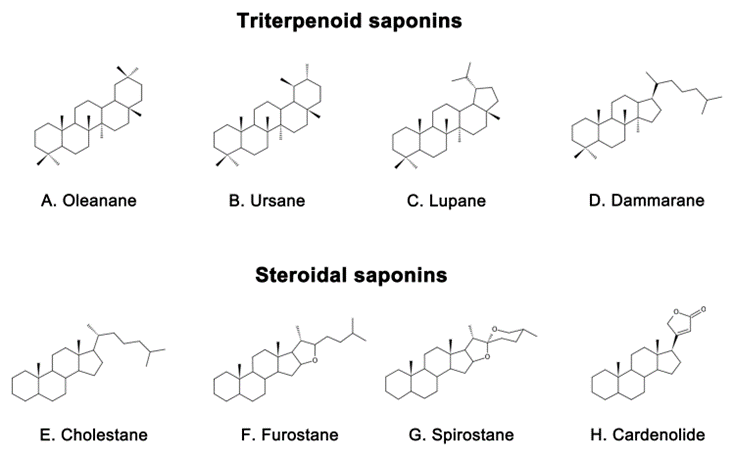
Representative triterpenoid saponins include (
) Oleanane, (
) Ursane, (
) Lupane, (
) Dammarane and steroidal saponins include (
) Cholestane, (
) Furostane, (
) Spirostane, (
) Cardenolide.
2. Mechanism of Saponins 皂苷治疗阿尔茨海默病的机制in Treating Alzheimer’s Disease
2.1. 抑制Inhibition of Aβ沉积和神经毒性 Deposition and Neurotoxicity
Aβ is a peptide that is a major component of the senile plaques found in the brains of people with AD [34]. Its accumulation is highly neurotoxic and is considered a hallmark of AD [35], which can result in impaired cognitive function, including spatial memory [36]. Aβ is generated from the APP and is typically enclosed by microglia and dystrophic synapses that aggregate around neurons [37,38]. APP is a transmembrane protein located in the synapse of neurons, which can be cleaved by both amyloid and nonamyloid pathways [39]. In the nonamyloid pathway, APP is sequentially cleaved by α-secretase (mainly ADAM10) and γ-secretase [40], resulting in P3 peptide (P3), C83 carboxy-terminal fragment (C83), APP intracellular domain (AICD), and soluble amyloid precursor protein-α (sAPPα) with beneficial neurotrophic effects [41]. Conversely, in the amyloid pathway, APP is first cleaved by β-secretase and sAPPβ is secreted [42]. Subsequently, γ-secretase cleaves the C-terminal fragment (C99) of the residual APP and eventually leads to the release of peptides of different lengths [43]. The most prevalent of them are Aβ1-40 and Aβ1-42 [44], which are neurotoxic fragments capable of oligomerization, aggregation, and subsequent plaque formation [45]. , Aβ is degraded by a variety of proteases, most notably insulin-degrading enzymes (IDE) and neprilysin (NEP) [46]. However, the production or activity of these clearance enzymes may decrease with age, leading to a failure to clear Aβ in a timely manner [47]. This phenomenon has been linked to the reduced activity of the peroxisome proliferator-activated receptor γ (PPARγ) [48], which is a transcription factor that regulates the expression of IDE and Bace1, reduces Aβ production [49], promotes Aβ clearance [50], and exerts neuroprotective effects [51]. An imbalance between Aβ production and clearance is responsible for the accumulation of Aβ in the brain [52]. In order to develop novel treatments for AD, it may be beneficial to inhibit Aβ production, enhance its clearance, or directly combat its neurotoxicity [53]
Natural saponins have shown potential in affecting Aβ metabolism through different pathways [54]. For example, some natural saponins are able to inhibit the formation of Aβ by reducing APP production. Ginsenoside Rg1, a tetracyclic triterpenoid saponin extracted from ginseng [55], has been shown to reduce Aβ deposition in APP/presenilin 1(PS1) double transgenic AD model mice by lowering APP levels [56]. Pharmacokinetic studies have also shown that ginsenoside Rg1 can cross the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB), which provides theoretical support for its ability to improve learning and memory abilities [57]. Xanthoceraside, another tetracyclic triterpenoid saponin, extracted from the bark of the Xanthoceras sorbifolia Bunge commonly used in traditional Chinese medicine (TCM) to treat rheumatism [58]
,在脑室内注射诱导的AD小鼠模型中,已被证明可降低APP蛋白水平和Aβ沉积在大脑皮层和海马体中,从而改善AD小鼠模型的认知功能障碍[59]。此外,据报道,人参皂苷Rh2是中药中使用的另一种人参衍生物,通过降低胆固醇和脂筏水平来调节APP表达。这最终导致sAPPα水平升高和Aβ浓度降低[60]。has been shown to reduce APP protein levels and Aβ deposition in the cerebral cortex and hippocampus, thereby ameliorating cognitive function dysfunction in an AD mouse model induced by Aβ intracerebroventricular (ICV) injection [59]. In addition, ginsenoside Rh2, another ginseng derivative used in TCM, has been reported to regulate APP expression by reducing cholesterol and lipid raft levels. This ultimately led to an increase in sAPPα levels and a decrease in Aβ concentrations [60].
Most saponins have been shown to inhibit Aβ deposition by modulating the activity of APP-processing enzymes, such as ginsenoside Rg1 [61], RAPO-1-3 [62], onjisaponin B [62], pseudoginsenoside-F11 (PF11) [63], theasaponin E1 [64], anginsenoside (20S)-Rg3 [65], and ginsenoside C-K (CK) [66]. Ginsenoside Rg1 reduces the γ-secretase responsible for Aβ production by attenuating the Aβ-mediated inhibition of cAMP response element-binding protein (CREB) phosphorylation and protein kinase A (PKA) activity [61]. It also upregulates ADAM10 while reducing BACE1 in Wistar rat models of AD induced by ovariectomy (OVX) and D-galactose (D-gal) [67]. In sAPPα-transfected HT22 cells and neuroblastoma (SH-SY5Y) cells, ginsenoside Rg1 has been found to increase the levels of sAPPα and estrogen receptor (ER) α, elevate α-secretase activity, and decrease extracellular release of Aβ. Further studies have shown that these effects are mediated by the upregulation of phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2) and phosphorylated protein kinase B (pAkt) [68] but can be reversed by ER antagonists and potentially attenuated by inhibitors of protein kinase C (PKC), MAPK, and phosphatidyl 3-kinase (PI3K) [68]. Yuan Zhi (RAPO) is a TCM formulation that is commonly used to treat dementia due to its neuroprotective effects [69]. Its active ingredient, RAPO-1-3, and onjisaponin B, an acyl saponin with a similar constituent to RAPO-1-3, have been found to reduce Aβ production by promoting the degradation of APP protein through interference with the PS1/BACE1 interaction [62]. Another compound, PF11, which is a pentacyclic triterpene abundant in ginseng [70], has also demonstrated efficacy in inhibiting APP amyloidogenic processing. By reducing the expression of BACE1, PF11 reduced Aβ deposition and ameliorated cognitive impairment and synaptic dysfunction in SAMP8 mice [63]. Theasaponin E1, a pentacyclic triterpene extracted from green tea seeds [71], has been shown to reduce the production of Aβ by increasing the activity of NEP and ADAM10 in APP (SweAPP N2a) cells while inhibiting the expression of BACE1, APP, and PS1 [64]. Furthermore, CK, which is produced through the degradation of protopanaxadiol saponins by the gut microbiota [72], has been studied for its neuroprotective properties. Currently, it is primarily obtained by glycosyl hydrolysis of proto-ginsenoside diol-type saponins [73]. In scopolamine-induced ICR mice, CK was found to reduce the expression of BACE1 and PS1, increase IDE activity, reduce Aβ expression, and improve memory function [66]. In addition, ginsenoside (20S)-Rg3, a component of heat-processed ginseng, has been found to reduce Aβ levels by increasing phosphatidylinositol 4-kinase IIα(PI4KIIα) activity and ultimately decrease the expression of γ-secretase by decreasing the association of PS1 fragments and lipid rafts in cultured primary neurons and in the brains of an AD mouse model [65].
Aside from inhibiting Aβ production, certain saponins have been found to promote the clearance of Aβ. For example, minor ginsenoside F1, a trace ginsenoside derived from Panax ginseng [74], has been shown to effectively reduce Aβ plaques and alleviate cognitive impairment in APP/PS1 mice by enhancing the expression of pCREB [75]. Similarly, Bacopaside I (BS-I), a tetracyclic triterpene and standardized extract of Bacopa monnieri [76], has been shown to be neuroprotective and improve cognitive function [77]. The aglycones of Bacopa monnieri and its derivatives have good intestinal absorption and BBB permeability [78]. Recent studies suggest that BS-I induces sufficient innate immune stimulation and phagocytosis to promote amyloid clearance, thereby reducing amyloid plaque burden in APP/PS1 mice [79]. In addition, Aβ deposition in the brain is linked to the dysfunction of the endosomal–lysosomal system dysfunction [80], which is regulated by the transcription factor EB (TFEB) [81]. PF11 has been observed to increase Aβ clearance by promoting the mammalian target of rapamycin (mTOR)-dependent TFEB-mediated lysosome biogenesis and alleviating endosomal–lysosomal system dysfunction through the conversion of Rab5 to Rab7 [82].
2.2. 抑制异常Inhibiting Aberrantau蛋白磷酸化 Tau Protein Phosphorylation
NFTs, a well-known pathological hallmark of AD [104], are composed of excessively phosphorylated proteins linked by helical filaments known as tubulin-associated unit (tau) protein [105]. Tau protein, mainly found in neuronal axons, is a microtubule-associated protein involved in various functions such as axonal microtubule maintenance, cytoplasmic transport, and regulation of neuronal signaling [106,107]. Its optimal function depends on the low levels of normal phosphorylation [108]. However, overphosphorylation of tau protein leads to its detachment from microtubules, resulting in microtubule depolymerization, aggregation into NFTs, and ultimately neurodegeneration and cell death [109]. The correlation between NFT density and cognitive decline suggests that tau protein plays a central role in AD [110]. Furthermore, the prevalence of tau protein pathology positively correlates with cognitive impairment in AD, where a person with AD has increased levels of tau protein, hyperphosphorylated tau protein, and aggregated tau in their brain [111]. Abnormal tau protein phosphorylation plays a greater role in AD than the total tau protein [112]. As such, it is considered a critical pathogenic step in the development of NFTs and, subsequently, AD [113].
Several saponins have been found to inhibit the hyperphosphorylation of tau proteins, protect synaptic structures, and reduce neuronal damage. An octillol-type saponin, PF11, has been found to increase the activity of phosphatase-2A (PP2A) by enhancing leucine carboxyl methyltransferase-1 (LCMT-1) and improving cognitive impairment in SAMP/8 mice by inhibiting the hyperphosphorylation of tau protein at serine 396 and tyrosine 205 in the brain [63]. Streptozotocin (STZ) is a glucosamine–nitrosourea compound commonly used in AD studies to induce tau proteins’ hyperphosphorylation and cognitive impairment [114]. Tau protein phosphorylation occurs at multiple sites, usually associated with glycogen synthase kinase (GSK) 3 and cyclin-dependent kinase 5 (CDK5). PF11 was also shown to attenuate STZ-induced tau hyperphosphorylation in the brain of male Wistar rats by correcting the dysregulation of the insulin receptor substrate 1/PI3K/Akt/GSK-3β and calpain I/CDK5 signaling pathways [70]. Additionally, PF11 ameliorated STZ-induced learning and memory deficits, synaptic damage, and neuronal death [70]. Another active compound, Ginsenoside Rb1, found in ginseng [115], exhibits neuroprotective properties against different neurotoxins [116]. Research has shown that ginsenoside Rb1 counters cognitive impairment induced by aluminum oxide in ICR mice while reducing Ser396 tau phosphorylation by increasing PP2A levels and decreasing p-GSK levels [111,117]. Ginsenoside Rd, another compound found in ginseng [118], has been shown to have potential therapeutic applications in neurodegenerative diseases [119]. It has been demonstrated to reduce tau proteins’ hyperphosphorylation and neurotoxicity induced by okadaic acid (OA) by increasing PP2A activity in both rat and cortical neuronal models of AD [120].
NFT 是 AD 的一个众所周知的病理标志 [104],由称为微管蛋白相关单元 (tau) 蛋白的螺旋丝连接的过度磷酸化蛋白质组成 [105]。Tau蛋白主要存在于神经元轴突中,是一种微管相关蛋白,参与多种功能,如轴突微管维持、细胞质转运和神经元信号传导调节[106,107]。其最佳功能取决于正常磷酸化的低水平[108]。然而,tau蛋白的过度磷酸化导致其与微管分离,导致微管解聚,聚集成NFT,最终导致神经变性和细胞死亡[109]。NFT密度与认知能力下降之间的相关性表明tau蛋白在AD中起核心作用[110]。此外,tau蛋白病理学的患病率与AD患者的认知障碍呈正相关,AD患者的大脑中tau蛋白、过度磷酸化tau蛋白和聚集tau蛋白水平升高[111]。异常tau蛋白磷酸化在AD中的作用大于总tau蛋白[112]。因此,它被认为是 NFT 以及随后 AD 发展的关键致病步骤[113]。 已经发现几种皂苷可以抑制tau蛋白的过度磷酸化,保护突触结构,并减少神经元损伤。研究发现,辛替洛尔型皂苷PF11通过增强亮氨酸羧基甲基转移酶-2(LCMT-2)来增加磷酸酶-1A(PP1A)的活性,并通过抑制大脑中丝氨酸8和酪氨酸396处tau蛋白的过度磷酸化来改善SAMP/205小鼠的认知障碍[63]。链脲佐菌素(STZ)是一种氨基葡萄糖-亚硝基脲化合物,常用于AD研究,以诱导tau蛋白的过度磷酸化和认知障碍[114]。Tau蛋白磷酸化发生在多个位点,通常与糖原合酶激酶(GSK)3和细胞周期蛋白依赖性激酶5(CDK5)相关。PF11还通过纠正胰岛素受体底物1/PI3K/Akt/GSK-3β和钙蛋白酶I/CDK5信号通路的失调,减弱STZ诱导的雄性Wistar大鼠大脑中的tau过度磷酸化[70]。此外,PF11改善了STZ诱导的学习和记忆缺陷、突触损伤和神经元死亡[70]。在人参中发现的另一种活性化合物人参皂苷Rb1[115]对不同的神经毒素具有神经保护特性[116]。研究表明,人参皂苷Rb1可对抗ICR小鼠氧化铝诱导的认知障碍,同时通过增加PP396A水平和降低p-GSK水平来减少Ser2tau磷酸化[111,117]。人参中发现的另一种化合物人参皂苷Rd[118]已被证明在神经退行性疾病中具有潜在的治疗应用[119]。在AD的大鼠和皮质神经元模型中,已经证明可以通过增加PP2A活性来减少冈田酸(OA)诱导的tau蛋白过度磷酸化和神经毒性[120]。Theasaponin E1, a vital compound found in green tea seeds, shows remarkable potential in reducing p-tau protein levels and inhibiting AD-promoting genes while activating AD-remitting genes [121]. This potential has been observed in AD models constructed from SHY5Y and glioblastoma (HTB2) cells. Notably, theasaponin E1 successfully reduced p-tau protein by inhibiting several enzymes, including GSK-3β, CDK5, c-Jun NH2-terminal kinase (JNK), MAPK, ERK1/MARK, and calmodulin-dependent protein kinase II alpha [121]. Similarly, Xanthoceraside, a triterpene saponin extracted from the shell of Xanthoceras sorbifolia Bunge, has been shown to exert neuroprotective effects by inhibiting the phosphorylation of tau protein [122]. In a study conducted on mice with AD, the administration of Xanthoceraside significantly decreased the expression of pGSK-3β and tau protein at Ser396 and Ser404, thereby effectively inhibiting the phosphorylation of tau protein [59].
茶皂苷E1是一种在绿茶种子中发现的重要化合物,在降低p-tau蛋白水平和抑制AD促进基因方面显示出显着的潜力,同时激活AD缓解基因[121]。在由SHY5Y和胶质母细胞瘤(HTB2)细胞构建的AD模型中已经观察到这种潜力。值得注意的是,茶皂苷E1通过抑制几种酶(包括GSK-3β、CDK5、c-Jun NH2末端激酶(JNK)、MAPK、ERK1/MARK和钙调蛋白依赖性蛋白激酶IIΑ)成功还原p-tau蛋白[121]。同样,黄花芍是一种从山梨黄芪壳中提取的三萜皂苷,已被证明通过抑制tau蛋白的磷酸化来发挥神经保护作用[122]。在一项针对AD小鼠的研究中,给予黄尾花苷显著降低了pGSK-3β和tau蛋白在Ser396和Ser404处的表达,从而有效抑制了tau蛋白的磷酸化[59]。2.3. 抗炎作用Anti-Inflammatory Effect
In recent years, neuroinflammation has become recognized as a third neuropathological hallmark of AD, in addition to Aβ and NFTs [123]. Neuroinflammation is an inflammatory response to pathological insults that occur within the central nervous system (CNS), such as infection, trauma, ischemia, and toxin accumulation [124]. This response is characterized by the proliferation and activation of microglia and astrocytes in the brain [125]. Such activation is usually accompanied by the secretion of inflammatory cytokines such as interleukin-1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) [126]. Microglia are innate immune cells that reside within the CNS and are thought to be important regulators in the inflammatory response within the brain [127]. In the early stages of AD, activated microglia can exert protective effects by phagocytosing and degrading Aβ [128,129]. However, when Aβ accumulates excessively, it binds various receptors on the surface of microglia such as cluster of differentiation (CD) 14, CD36 [130], Toll-like receptor (TLR) 4, and TLR2, to promote the release of inflammatory factors [131]. This response leads to neuroinflammation and further impairment of cognitive function. Similarly, astrocyte activation is also associated with Aβ [132]. Activated astrocytes produce various inflammatory mediators [133] and secrete small amounts of Aβ [134], resulting in a chronic vicious cycle [133]. These reactions can exacerbate neuroinflammation and damage neurons.
近年来,神经炎症已成为除Aβ和NFTs之外AD的第三个神经病理学标志[123]。神经炎症是对中枢神经系统内发生的病理性损伤(如感染、创伤、缺血和毒素积累)的炎症反应[124]。这种反应的特征是大脑中小胶质细胞和星形胶质细胞的增殖和活化[125]。这种激活通常伴有炎症细胞因子的分泌,例如IL-1β、白细胞介素1(IL-6)和肿瘤坏死因子α(TNF-α)[6]。小胶质细胞是存在于CNS内的先天免疫细胞,被认为是大脑内炎症反应的重要调节因子[126]。在AD的早期阶段,活化的小胶质细胞可以通过吞噬和降解Aβ发挥保护作用[127,128]。然而,当Aβ过度积累时,它会结合小胶质细胞表面的各种受体,如分化簇(CD)129、CD14[36]、Toll样受体(TLR)130和TLR4,促进炎症因子的释放[2]。这种反应导致神经炎症和认知功能的进一步损害。同样,星形胶质细胞活化也与Aβ有关[131]。活化的星形胶质细胞产生各种炎症介质[132]并分泌少量Aβ[133],导致慢性恶性循环[134]。这些反应会加剧神经炎症并损害神经元。 小胶质细胞和星形胶质细胞介导的神经炎症涉及多种途径[135],其中一条是核因子κ-B(NF-κB)途径[136]。该途径激活TNF-α、IL-1β和IL-6的转录,从而加剧炎症[137]。小胶质细胞激活还取决于其他信号通路,例如PI3K/Akt/mTOR通路[138],其可促进炎症介质NO合酶(NOS)和环氧合酶-2(Cox-2)的表达[139,140]。此外,MAPK/p38[141]、JAK激酶/信号转导器、转录激活剂(STAT)和其他通路也有助于神经炎症[142]。因此,调节炎症信号通路和抑制促炎因子的产生可能为治疗AD提供一种有希望的方法。Multiple pathways are involved in microglia- and astrocyte-mediated neuroinflammation [135], one of which is the nuclear factor kappa-B (NF-κB) pathway [136]. This pathway activates the transcription of TNF-α, IL-1β, and IL-6, which exacerbates inflammation [137]. Microglial activation also depends on other signaling pathways such as the PI3K/Akt/mTOR pathway [138]which can promote the expression of the inflammatory mediators NO synthase (NOS) and cyclooxygenase-2 (Cox-2) [139,140]. Furthermore, MAPK/p38 [141], Janus kinase (JAK)/signal transducer, and activator of transcription (STAT) and other pathways also contribute to neuroinflammation [142]. Therefore, modulating inflammatory signaling pathways and inhibiting the production of proinflammatory factors may offer a promising approach to the treatment of AD.
一般来说,天然皂苷具有抗炎生理活性,从而通过调节炎症相关的信号通路来抑制炎症因子的表达或释放来保护神经细胞。2.4. 改善线粒体功能和抗氧化应激Improvement in Mitochondrial Function and Antioxidative Stress
Oxidative stress refers to an imbalance between oxidation and antioxidation in the body [174], which ismainly characterized by the increased production of reactive oxygen species and reduced ability of antioxidants to combat them [175]. The brain is susceptible to oxidative damage due to its high lipid content and lacks effective antioxidant defense mechanisms, despite high oxygen consumption [176,177]. It is now well established that the level of oxidation in the brain also increases with age [178]. Interestingly, extensive oxidative damage can be observed in the stage of mild cognitive impairment that precedes the typical clinical manifestations of AD, suggesting that oxidative stress may be a central mechanism in driving the disease [179].
线粒体作为有氧代谢的必需细胞器,可显著促进氧化应激的产生[180]。同时,现在已经表明,线粒体氧化应激可导致轴突和神经元损伤,并最终导致神经功能障碍[181]。当线粒体内发生氧化应激时,可导致线粒体肿胀、外膜破裂和钙离子丢失,导致ROS的进一步产生和释放[182]。在生理条件下,ROS可以刺激细胞生长[183],并被抗氧化酶和其他抗氧化防御系统[184]消除,如超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)和过氧化氢酶(CAT)[185]。然而,当发生氧化还原失衡状态时,自由基大量积聚,导致脂质过氧化,导致丙二醛(MDA)的产生[186]、细胞膜损伤、线粒体膜损伤,甚至神经元死亡[187]。此外,氧化应激可能与大脑中的Aβ聚集[188]、tau蛋白磷酸化[189]和金属离子微环境损伤相互作用,放大神经元损伤,最终导致AD的发病机制[190,191]。 Several natural saponins may act as exogenous antioxidants to combat AD by managing oxidative stress [192,193]. Ginsenoside Rh2, a rare ginsenoside with few reports on its neuroprotective effects compared to other similar molecules [194], has shown significant neuroprotective effects in a scopolamine-induced model of memory impairment in ICR mice. Ginsenoside Rh2 effectively managed MDA levels while increasing glutathione (GSH) levels and SOD activity in the brain, effectively inhibiting oxidative stress [195]. These findings suggest that ginsenoside Rh2 has great potential for the treatment of AD by inhibiting oxidative stress in the brain. Crude saponins from BF showed potent antioxidant effects in addition to anti-inflammatory effects. In addition, saponins from BF alleviated oxidative stress, reduced ROS, and MDA levels by restoring GSH-Px, GSH, and CAT levels in the Aβ ICV-induced BALB/c AD mouse model [161]. Similarly, ginsenoside Rd was found to reduce protein carbonyls and 4-hydroxy-2-nonenal (4-HNE) levels by decreasing the glutathione disulfide (GSSG)/GSH ratio, thereby counteracting oxidative stress in the brain of Aβ-induced AD mouse models [170]. Ginsenoside Rg3, a tetracyclic triterpenoid saponin, is the major bioactive component of ginseng and is widely used for the treatment of tumors [196]. Results from in vivo studies have shown that ginsenoside Rg3 can improve mitochondrial dysfunction and enhance ROS scavenging ability by increasing SOD, CAT, and GSH-Px levels, thus ameliorating oxidative stress in the brain of D-gal-induced AD rats [197]. PF11 was found to inhibit the production of both APP and Aβ in the cortex and hippocampus, while restoring the activity of SOD and GSH-Px in the brains of AD mice and reducing MDA levels in the cortex [198]. Ginsenoside Rg1 has also been found to protect primary cortical neurons against Aβ-induced increases in ROS and H2O2 levels by inhibiting oxidative stress 通过抑制氧化应激来降低水平[199]. This results in a decrease in mitochondrial membrane potential and an increase in cytochrome 。这导致线粒体膜电位降低,细胞色素C (Cyt-c) oxidase activity, ultimately leading to a decrease in Cyt-c release and indicating improved mitochondrial function (Cyt-c)氧化酶活性增加,最终导致Cyt-c释放减少,表明线粒体功能得到改善[199]. Another newly discovered saponin compound, huangjingsterol 。另一种新发现的皂苷化合物,来自虎杖的黄晶甾醇B from Polygonatum cyrtonema Hua, showed significant antioxidant activity [200] by reducing oxidative stress and protecting cells from Aβ-induced damage through modulating the activity of ,通过调节GSH-Px and 和SOD 的活性来减少氧化应激并保护细胞免受Aβ诱导的损伤,显示出显着的抗氧化活性[200]. 。同样,研究人员发现,来自Similarly, researchers have found that a saponin from the fruit of Soolanum anguivi can protect the P2 region of rat brain synaptosomes from oxidative damage induced by ferrous iron and sodium nitroprusside, reduce ROS generation, restore total thiol levels, and protect mitochondrial function 果实的皂苷可以保护大鼠脑突触体的P200区域免受亚铁和硝普钠诱导的氧化损伤,减少ROS生成,恢复总硫醇水平,并保护线粒体功能[192]. This suggests that saponins from Solanum anguivi fruits may have antioxidant properties that benefit mitochondrial function 。这表明来自茄果实的皂苷可能具有抗氧化特性,有益于线粒体功能[19201]. 。PGS has a similar effect with its ability to upregulate intracellular heme oxygenase-1 (HO-1), SOD, CAT, and 具有类似的效果,能够上调细胞内血红素加氧酶-201(HO-1)、SOD、CAT和GSH-Px to inhibit Aβ-induced ROS production and reduce oxidative damage in AD brains ,以抑制Aβ诱导的ROS产生并减少AD大脑中的氧化损伤[153]。尽管人参具有神经保护作用,但其抗氧化特性的机制仍然知之甚少. Despite the demonstrated neuroprotective effects of Panax ginseng, the mechanisms underlying its antioxidant properties remain poorly understood. However, a recent study has shown that the primary bioactive compound in Panax ginseng, NTR1, is able to prevent the loss of mitochondrial membrane potential and reduce the accumulation of ROS induced by Aβ 然而,最近的一项研究表明,人参中的主要生物活性化合物NTR153能够防止线粒体膜电位的丧失,并减少Aβ诱导的ROS积累[2021]. This suggests that 。这表明NTR1 may exert neuroprotective effects by inhibiting oxidative stress. Furthermore, most previous experiments have focused on saponins extracted from Panax ginseng roots, and the pharmacological effects of other parts, such as saponins in P. notoginseng leaves (LPNS), are largely unknown 202可能通过抑制氧化应激来发挥神经保护作用。此外,以前的大多数实验都集中在从人参根中提取的皂苷上,其他部分的药理作用,如三七叶(LPNS)中的皂苷,在很大程度上是未知的[2031]. 。LPNS, which mainly include the ginsenosides Rb1, Rb2, Rb3, and Rc, have been shown to have potent neuroprotective properties 主要包括人参皂苷Rb203、Rb1、Rb2和Rc,已被证明具有有效的神经保护特性[203].。在 In H2O2- or oxygen and glucose deprivation (OGD)-induced astrocytes from the cortex of 或来自SD rats and 大鼠和SH-SY5Y cells, LPNS increased the levels of nuclear factor erythroid2-related factor 2 (Nrf2) and its downstream HO-1 and glutathione S-transferase pi 1 (GSTP1) in response to increased ROS, as well as significantly reducing lactate dehydrogenase (LDH) expression and increasing the cellular activity of astrocytes 细胞皮层的氧和葡萄糖剥夺(OGD)诱导的星形胶质细胞,LPNS增加了核因子红细胞2相关因子2(Nrf2)及其下游HO-1和谷胱甘肽S-转移酶pi 1(GSTP1)的水平,以响应ROS升高,以及显着降低乳酸脱氢酶(LDH)表达并增加星形胶质细胞的细胞活性[203].。