Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Annachiara Pirozzi | -- | 2230 | 2023-03-23 11:33:22 | | | |
| 2 | Camila Xu | Meta information modification | 2230 | 2023-03-24 03:39:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gali, L.; Pirozzi, A.; Donsì, F. Lipid-Based Nano-Systems. Encyclopedia. Available online: https://encyclopedia.pub/entry/42469 (accessed on 03 March 2026).
Gali L, Pirozzi A, Donsì F. Lipid-Based Nano-Systems. Encyclopedia. Available at: https://encyclopedia.pub/entry/42469. Accessed March 03, 2026.
Gali, Lynda, Annachiara Pirozzi, Francesco Donsì. "Lipid-Based Nano-Systems" Encyclopedia, https://encyclopedia.pub/entry/42469 (accessed March 03, 2026).
Gali, L., Pirozzi, A., & Donsì, F. (2023, March 23). Lipid-Based Nano-Systems. In Encyclopedia. https://encyclopedia.pub/entry/42469
Gali, Lynda, et al. "Lipid-Based Nano-Systems." Encyclopedia. Web. 23 March, 2023.
Copy Citation
Lipid-based delivery systems can be similarly designed to encapsulate and protect bioactive ingredients and enhance their functionality, stability, and bioavailability. Lipid-based delivery systems are composed of lipids such as triglycerides, phospholipids, and waxes, which form a stable matrix that can encapsulate various types of bioactive ingredients such as vitamins, minerals, and flavonoids.
plant bioactive compounds
encapsulation
biopolymers
1. Introduction
Lipid-based delivery systems can be similarly designed to encapsulate and protect bioactive ingredients and enhance their functionality, stability, and bioavailability. Lipid-based delivery systems are composed of lipids such as triglycerides, phospholipids, and waxes, which form a stable matrix that can encapsulate various types of bioactive ingredients such as vitamins, minerals, and flavonoids.Lipid-based delivery systems offer several advantages in food applications. For example, lipids are natural components of the diet and are well tolerated by the body. Lipid-based delivery systems are also especially efficient in terms of protecting bioactive ingredients from environmental stresses such as light, oxygen, and temperature, which can help maintain their stability and activity. Additionally, lipids can enhance the bioavailability of bioactive ingredients by increasing their solubility and facilitating their absorption by the body [1][2].
The structural and physicochemical diversity of lipids and their biocompatibility make them an excellent choice for the fabrication and design of delivery systems, which can be tailored for different bioactive substances. Oils, glyceric lipids at different esterification degrees (mono-, di-, and triglycerides), waxes, and phospholipids are usually used as the constituent materials for lipid-based nanoparticles [2]. The use of lipids is particularly appealing for the delivery of both hydrophilic and hydrophobic compounds destined to be administered through various routes (oral, nasal, transdermal, and parenteral). More specifically, lipid-based formulations were shown to be very well adapted for local administration, either for therapeutic or cosmetics purposes, due to their high loading capacity, stability, and permeation through the skin [3]. Furthermore, lipid nanoparticles also offer many advantages in terms of the encapsulation of pharmaceutical and nutraceutical compounds for oral administration because (i) the incorporation of poorly water-soluble compounds in a lipidic core increases lipids’ solubility directly and indirectly by triggering bile secretion; additionally, (ii) the lipidic matrix structure promotes lymphatic transportation while limiting the extent of metabolization and degradation in the liver. The main types of lipid-based nanocarriers of interest for natural antioxidants include colloidal emulsions, solid lipid nanoparticles and nanostructured lipid carriers, liposomes, phytosomes, and niosomes [4] (Figure 1).
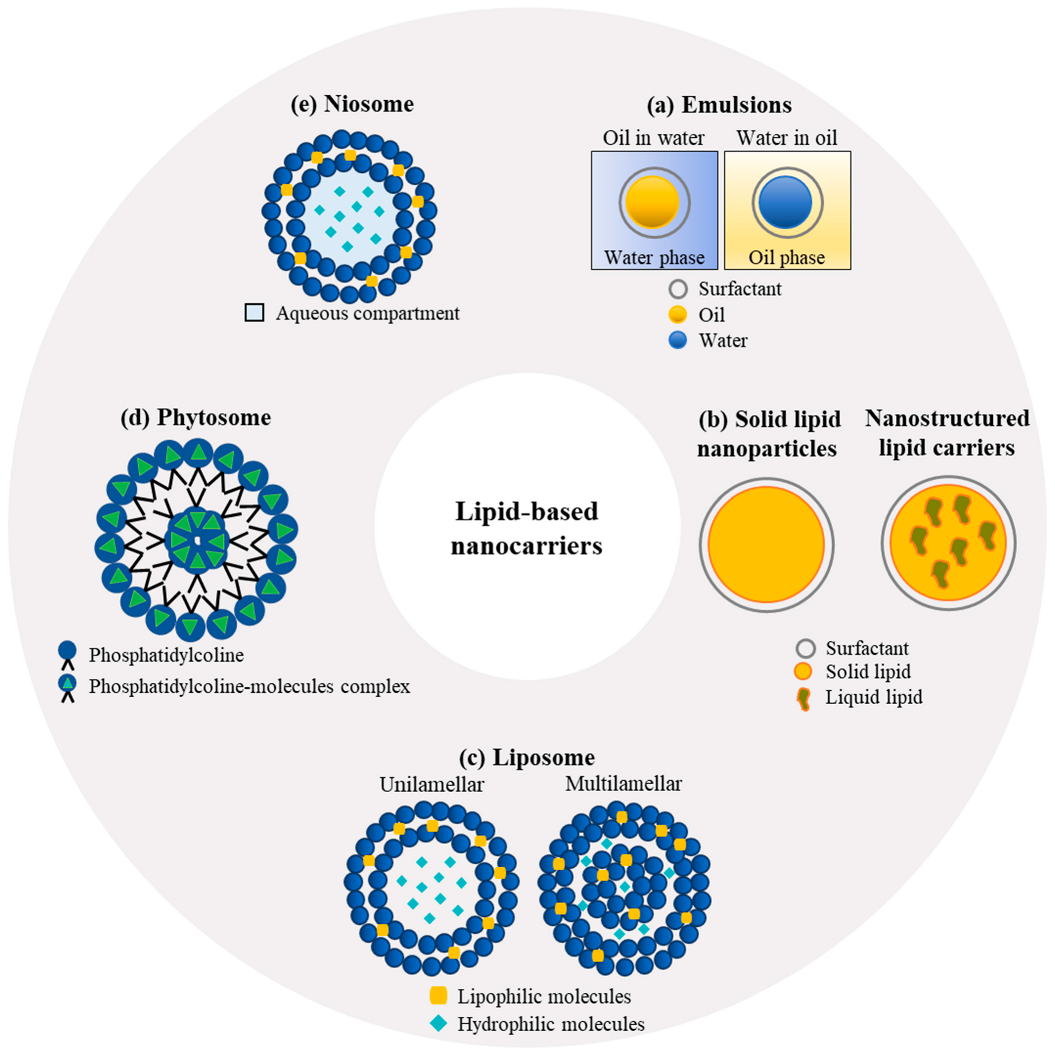
Figure 1. Main lipid-based systems for the delivery of lipophilic and hydrophilic compounds.
2. Colloidal Emulsions
Emulsions (Figure 1a) are non-homogeneous dispersions composed of two immiscible liquids, for example, an oily phase finely dispersed in a continuous aqueous phase through stabilization with a surfactant. Different categories of emulsions can be distinguished according to their mean droplet size, which is usually expressed as a mean radius, including conventional emulsions or macroemulsions (0.1–100 μm), sub-microemulsions (100–600 nm), and nano-emulsions (10–100 nm) [5][6]. Depending on the location of one phase in relation to the other, single dispersions (oil in water (O/W) or water in oil (W/O)) and multiple emulsions (O/W/O or W/O/W) can be formed [6]. O/W emulsions are more suitable for encapsulating hydrophobic compounds, whereas W/O emulsions are designed to contain hydrophilic molecules [7]. The use of biopolymers instead of surfactants as interfacial stabilizing agents provides a thicker layer on the surface of the emulsion, thereby allowing for high stability against coalescence, especially under extreme environmental conditions [8]. Additionally, biopolymeric nanoparticles (obtained from proteins, polysaccharides, and gums) can be used as stabilizing agents to produce so-called Pickering emulsions, which are characterized by excellent physical stability due to the formation of a compact and robust interfacial layer, which is rather insensitive to pH or ionic strength changes [6].
Many examples of emulsions encapsulating natural extracts or individual natural antioxidant compounds have been reported in the literature. Pomegranate peel extracts, which are rich in punicalagin, were encapsulated in double water-in-oil-in-water (W1/O/W2) emulsions, which were optimized by varying the type of oil used (castor, soybean, sunflower, Miglyol, and/or orange oil) and the fabrication method (direct membrane emulsification or mechanical agitation), using polyglycerol polyricinoleate (PGPR) and Tween 80 as lipophilic and hydrophilic emulsifiers, respectively. The double emulsion’s distinguishing features, such as its mean diameter, encapsulation efficiency, and release properties, depended largely on the type of oil and the fabrication method used [9]. In another example, different emulsions were prepared by high-pressure homogenization using different emulsifiers/stabilizers, such as Tween 80, lecithin, whey proteins, pectin, and Quillaja saponin, to encapsulate different thyme essential oils in O/W nano-emulsions in order to protect them and use them as food preservatives [10]. Nano-emulsions can be formed either using low-energy or high-energy methods. For the better stability of the nano-emulsions, a fine particle size is necessary. To this end, high-energy production methods are used, including high-pressure homogenization, microfluidizers, and sonicators. These techniques involve the disruption of the sample during emulsification, overcoming the forces of coalescence, and, hence, leading to the formation of smaller droplets. In high-pressure homogenization, coarse emulsions are passed through a narrow valve under high pressure. This passage causes the droplets to separate and reduce in size due to the action of different forces, such as shear stress, cavitation, and turbulent flow conditions. Microfluidization also relies on applying high pressure to the coarse emulsion to reduce droplet size. A microfluidizer differs from a high-pressure homogenizer in the design of the channel for emulsion flow [11]. Sonication is widely used to produce nano-emulsions through the application of ultrasonic waves. These waves lead to the generation of rapidly oscillating microbubbles that break down to produce intense cavitation forces in their vicinity. The application of this method leads to the production of small particles (<100 nm). In this method, batch processes (bath or probes) are widely used, but a continuous flow ultrasonic system has been developed that consists of a disturbance zone generated by an ultrasonic probe through which a coarse emulsion flows. This method reduces processing time [12].
3. Solid-Lipid Nanoparticles and Nanostructured Lipid Carriers
Solid-lipid nanoparticles (SLNs) (Figure 1b) are emulsions formed using lipids that are solid at ambient temperature instead of liquid oils. Hot and cold homogenization procedures are commonly used for the large-scale preparation of SLNs in the food industry, but other methods are also available, such as microemulsion templating, membrane emulsification, coacervation, and double-emulsion techniques [13]. SLNs are used to encapsulate lipophilic compounds with higher efficiency than can be achieved with ordinary emulsions and liposomes; moreover, SLNs typically ensure a slower release of the entrapped molecules, provide more extensive protection against chemical degradation, and, most importantly for their therapeutic efficiency, remain solid at body temperature [14]. However, because of the crystalline structure formed when the lipids solidify, SLNs are characterized by a lower loading capacity than emulsions, which represents an important factor limiting their application. New and improved structures, formed through the incorporation of a liquid lipid into the core of the solid matrix have been developed to overcome the loading limitations exhibited by SLNs. These carriers are referred to as nanostructured lipid carriers (NLCs) and are characterized by a lipid core made of a mixture of solid and liquid lipids, which is stabilized in an aqueous phase by the presence of a surfactant or a mixture of surfactants at their interface [15].
For example, SLNs have been developed to encapsulate Neem oil from Azadirachta indica using cholesterol as the main lipid constituent and lecithin and Tween 80 as surfactants. The particles exhibited a spherical shape, according to TEM analysis, and a mean diameter of 338 nm. The oil was successfully encapsulated with an encapsulation efficiency of 71.6% and exhibited sustained release over time. Moreover, the Neem-oil-loaded SLNs exhibited a highly toxic effect on Toxoplasma gondii tachyzoites [16]. In another study, Annona muricata fruit extract was encapsulated in SNLs prepared by high-pressure homogenization followed by ultrasonication. The loaded SNLs presented high encapsulation efficiency (83.3%) and a fine mean diameter (~135 nm) and exhibited higher cytotoxicity against MCF7 cancer cells than the free extract [17].
The preparation of NLCs, loaded with Mentha pulegium essential oil, was reported as an antibacterial and wound-healing system [18]. These NLCs were characterized by an encapsulation efficiency of 94.2% and exhibited a significantly higher antibacterial effect than the pure extract, promoting wound healing by shortening the inflammatory phase and accelerating the proliferation phase. In another study, NLCs were developed for the encapsulation of turmeric extract with enhanced antioxidative and antimicrobial effects and improved sustained release in comparison with the free extract, suggesting its efficacy as a food delivery system [19].
4. Liposomes
Liposomes (Figure 1c) are vesicles formed by one or more phospholipid bilayers surrounding an aqueous-phase core and dispersed in an aqueous phase. The size and number of layers of a liposome depend on the methods of fabrication, which usually include solvent evaporation/rehydration, surfactant displacement, solvent displacement, and homogenization. Phospholipids, mainly from eggs, sunflowers, soy, and milk, are generally used to form the lipidic shell of liposomes for food, pharmaceutical, and cosmetic applications because of their remarkable capacity to cross the biological barriers for the efficient delivery of the payload [20][21]. Moreover, the liposome structure allows for the simultaneous encapsulation of both hydrophilic (in the aqueous core) and lipophilic (within the phospholipid layer) bioactive compounds [21]. However, the application of liposomes is limited by their short half-life due to their clearance by phagocytosis (uptake by the reticuloendothelial system), oxidation or hydrolysis of phospholipids, high production costs, high tendency to release the encapsulated constituents, and low loading capacity [22]. Surface modification through the addition of polymers such as PEG has been proposed to overcome these drawbacks and enhance the properties of these systems, especially for targeted delivery [23].
A nanoliposome formulation was developed for the encapsulation of Laurus nobilis leaf extract for the preservation of minced beef. The encapsulation of the extract in nanoliposomes, characterized by a mean diameter of 99.1 nm and an encapsulation efficiency of 73.8%, enhanced the antioxidant and antimicrobial activity and the sensory properties of the extract. In another study, liposomes were developed to encapsulate myrtle extract using sunflower oil and glycerol instead of cholesterol and avoiding the use of organic solvents. The obtained liposomes, with a mean diameter in the range of 260–293 nm and an encapsulation efficiency of 68–73%, exhibited a sustained release of the extracts, which was found to be pH-sensitive [24].
Curcumin was encapsulated in a liposomal formulation presenting a mean diameter of 271.3 nm and an encapsulation efficiency of 81.1%. The curcumin-loaded liposomes showed an interesting capacity to reduce inflammatory markers such as IL-6, IL-8, IL-1β, and TNF-α in LPS-induced BCi-NS1.1 cells [25].
5. Phytosomes
Phytosomes (Figure 1d) are systems produced to incorporate plant extracts or polar phytoconstituents into a phospholipid envelope to overcome the limitations associated with their large size and hydrophilicity, which limit their passage through hydrophobic membranes and, consequently, lead to their poor absorption (in the small intestine) when consumed or upon topical application [26]. In the case of phytosomes, the interactions observed between the encapsulated constituents and the phospholipid bilayers represent the main difference with respect to liposomes, in which the phospholipids simply surround the water-soluble bioactive substance, whereas in phytosomes, the encapsulated constituents become part of the bilayer, affecting its properties [26]. The formation of vesicles of phytosomes is the result of H-bond interaction between the polyphenolic moiety of the plant extract constituents and the phosphate group of phospholipids, such as phosphatidylcholine, phosphatidylserine, and phosphatidylethanolamine, in specific stoichiometric ratios and under certain conditions [27].
Phytosomes are widely used to encapsulate plant extracts or individual compounds due to their biocompatibility, controlled release, and enhanced bioavailability owing to their diffusion properties across biological membranes. Many phytosome-based formulations, such as SiliphosR, consisting of silybin-loaded phytosomes, and MerivaR, consisting of curcumin-loaded phytosomes, are already on the market. Previous studies demonstrated the encapsulation of Moringa oleifera polyphenolic extract in phytosomes based on soy phosphatidylcholine [28]. These phytosomes, with a mean diameter of 325.7 nm, significantly increased the bioaccessibility of polyphenols and their antiproliferative effect against 4T1 cancer cells in comparison with the free extract. In another example, phospholipids from cow milk were used to prepare Aloe-vera-loaded phytosomes for cancer therapy. The obtained phytosomes, with a mean diameter of 2492 nm, exerted potent antiproliferative effects against MCF-7 cells [29]. A phytosomal preparation, designed to be incorporated in mayonnaise, was prepared to encapsulate resveratrol; with a mean diameter of 78.7 nm, it exhibited an enhanced antioxidant effect in comparison with free resveratrol [30].
6. Niosomes
Niosomes (Figure 1e) are non-ionic, surfactant-based vesicles that have been widely explored for use as a delivery system for natural extracts. They are composed of non-ionic surfactants, cholesterol, and various types of lipids, such as phospholipids, glycolipids, or sphingolipids. The unique properties of niosomes, such as their biocompatibility, high stability, and ability to encapsulate hydrophilic and hydrophobic compounds, render them an attractive delivery system for natural extracts [31].
Niosomes can be prepared by various methods, including the thin-film hydration method, the reverse phase evaporation method, and the dialysis method. The thin-film hydration method is the most common method used for the preparation of niosomes. In this method, a thin film composed of the non-ionic surfactant, cholesterol, and lipids is hydrated with an aqueous solution containing a natural extract [32].
The particle size of niosomes can be controlled by varying the type and concentration of the surfactant, the method of preparation, and the use of sonication or high-pressure homogenization [32]. Niosomes with small particle sizes (<200 nm) have been found to have improved stability and higher encapsulation efficiencies [33].
Niosomes have been used as delivery systems for a wide range of natural extracts, including flavonoids [34], terpenoids [35], alkaloids [36], and polyphenols [37], demonstrating that niosomes can improve the solubility, stability, and bioavailability of these natural extracts, thereby enhancing their therapeutic efficacy.
References
- Fernandez-Avila, C.; Hebishy, E.; Donsì, F.; Arranz, E.; Trujillo, A.J.. Nanoencapsulation in the Food Industry; Jafari, S.M., Eds.; Academic Press: Cambridge, 2019; pp. 251–340.
- Fathi, M.; Mozafari, M.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends in Food Science & Technology 2012, 23, 13–27, 10.1016/j.tifs.2011.08.003.
- Abdel-Mottaleb, M.M.; Neumann, D.; Lamprecht, A. Lipid nanocapsules for dermal application: A comparative study of lipid-based versus polymer-based nanocarriers. European Journal of Pharmaceutics and Biopharmaceutics 2011, 79, 36-42, 10.1016/j.ejpb.2011.04.009.
- Rao, S.; Prestidge, C.A. Polymer-lipid hybrid systems: Merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opinion on Drug Delivery 2016, 13, 691-707 , 10.1517/17425247.2016.1151872.
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Critical Reviews in Food Science and Nutrition 2011, 51, 285–330, 10.1080/10408398.2011.559558.
- Donsì, F.; Velikov, K.P.. Lipid-Based Nanostructures for Food Encapsulation Purposes; Jafari, S.M., Eds.; Elsevier: Amsterdam, 2019; pp. 37–87.
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Scientia Pharmaceutica 2019, 87, 17, 10.3390/scipharm87030017.
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. Journal of Biotechnology 2016, 133, 106–120, 10.1016/j.jbiotec.2016.07.005.
- Sanhueza, L.; García, P.; Giménez, B.; Benito, J.M.; Matos, M.; Gutiérrez, G. Encapsulation of Pomegranate Peel Extract (Punica granatum L.) by Double Emulsions: Effect of the Encapsulation Method and Oil Phase. Foods 2022, 11, 310, 10.3390/foods11030310.
- Jayari, A.; Donsì, F.; Ferrari, G.; Maaroufi, A. Nanoencapsulation of Thyme Essential Oils: Formulation, Characterization, Storage Stability, and Biological Activity. Foods 2022, 11, 1858, 10.3390/foods11131858.
- Hamman, J.H. Chitosan Based Polyelectrolyte Complexes as Potential Carrier Materials in Drug Delivery Systems. Marine drugs 2010, 8, 1305–1322, 10.3390/md8041305.
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Current Opinion in Colloid & Interface Science 2014, 19, 417–427, 10.1016/j.cocis.2014.07.002.
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustainable Chemistry and Pharmacy 2017, 6, 37–56, 10.1016/j.scp.2017.07.002.
- Panigrahi, S.S.; Syed, I.; Sivabalan, S.; Sarkar, P. Nanoencapsulation strategies for lipid-soluble vitamins. Chemical Papers 2018, 73, 1–16, 10.1007/s11696-018-0559-7.
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine: Nanotechnology, Biology and Medicine 2016, 12, 143–161, 10.1016/j.nano.2015.09.004.
- Nemati, S.; Rahimi, H.M.; Hesari, Z.; Sharifdini, M.; Aghdam, N.J.; Mirjalali, H.; Zali, M.R. Formulation of Neem oil-loaded solid lipid nanoparticles and evaluation of its anti-Toxoplasma activity. BMC Complementary Medicine and Therapies 2022, 22, 122, 10.1186/s12906-022-03607-z.
- Sabapati, M.; Palei, N.N.; Ashok Kumar, C.K.; Molakpogu, R.B. Solid lipid nanoparticles of Annona muricata fruit extract: Formulation, optimization and in vitro cytotoxicity studies. Drug Development and Industrial Pharmacy 2019, 45, 577–586, 10.1080/03639045.2019.1569027.
- Khezri, K.; Farahpour, M.R.; Rad, S.M. Efficacy of Mentha pulegium essential oil encapsulated into nanostructured lipid carriers as an in vitro antibacterial and infected wound healing agent. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2020, 589, 124414, 10.1016/j.colsurfa.2020.124414.
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H.S. Antioxidant, Antimicrobial and Physicochemical Properties of Turmeric Extract-Loaded Nanostructured Lipid Carrier (NLC). Colloid and Interface Science Communications 2018, 22, 18–24, 10.1016/j.colcom.2017.11.006.
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols - A review. Trends in Food Science & Technology 2010, 21, 510–523, 10.1016/j.tifs.2010.08.003.
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Advances in Colloid and Interface Science 2015, 219, 27–53, 10.1016/j.cis.2015.02.002.
- Garg, T.; Goyal, A.K. Liposomes: Targeted and Controlled Delivery System. Drug Delivery Letters 2014, 4, 62–71, 10.2174/22103031113036660015.
- Milani, D.; Athiyah, U.; Hariyadi, D.M.; Pathak, Y.V. Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Eds.; Springer: Switzerland, 2019; pp. 207–220.
- Gorjian, H.; Amiri, Z.R.; Milani, J.M.; Khaligh, N.G. Preparation and characterization of the encapsulated myrtle extract nanoliposome and nanoniosome without using cholesterol and toxic organic solvents: A comparative study. Food Chemistry 2020, 342, 128342, 10.1016/j.foodchem.2020.128342.
- Ng, Z.Y.; Wong, J.-Y.; Panneerselvam, J.; Madheswaran, T.; Kumar, P.; Pillay, V.; Hsu, A.; Hansbro, N.; Bebawy, M.; Wark, P.; et al.et al. Assessing the potential of liposomes loaded with curcumin as a therapeutic intervention in asthma. Colloids and Surfaces B: Biointerfaces 2018, 172, 51–59, 10.1016/j.colsurfb.2018.08.027.
- Ajazuddin; Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689, 10.1016/j.fitote.2010.05.001.
- Alharbi,W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif,W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475, 10.3390/pharmaceutics13091475.
- Wanjiru, J.; Gathirwa, J.; Sauli, E.; Swai, H.S. Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol- Loaded Phytosome Delivery System against Breast Cancer Cell Lines. Molecules 2022, 27, 4430, 10.3390/molecules27144430.
- Murugesan, M.P.; Ratnam, M.V.; Mengitsu, Y.; Kandasamy, K. Evaluation of anti-cancer activity of phytosomes formulated from aloe vera extract. Materials today: proceedings 2020, 42, 631–636, 10.1016/j.matpr.2020.11.047.
- Rabbani, M.; Pezeshki, A.; Ahmadi, R.; Mohammadi, M.; Tabibiazar, M.; Azar, F.A.N.; Ghorbani, M. Phytosomal nanocarriers for encapsulation and delivery of resveratrol- Preparation, characterization, and application in mayonnaise. LWT 2021, 151, 112093, 10.1016/j.lwt.2021.112093.
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. Journal of Drug Delivery Science and Technology 2020, 56, 101581, 10.1016/j.jddst.2020.101581.
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods 2021, 199, 9–15, 10.1016/j.ymeth.2021.05.004.
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Advances in Pharmacological and Pharmaceutical Sciences 2018, 2018, 6847971, 10.1155/2018/6847971.
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant—Hepatoprotective Interplay. Pharmaceutics 2020, 12, 143, 10.3390/pharmaceutics12020143.
- Vilela, J.D.M.V.; Moghassemi, S.; Dadashzadeh, A.; Dolmans, M.-M.; Azevedo, R.B.; Amorim, C.A. Safety of Lavender Oil-Loaded Niosomes for In Vitro Culture and Biomedical Applications. Nanomaterials 2022, 12, 1999, 10.3390/nano12121999.
- Poorani, V.; Vigneswaran; Kumar, G.V. Nano-Niosomal Formulation of Alkaloids from Vinca rosea for Improved Oral Delivery. Journal of Pharmaceutical and Medicinal Research 2020, 5, 102–105, 10.30799/jpmr.052.20050105.
- Al Saqr, A.; Annaji, M.; Poudel, I.; Rangari, S.; Boddu, S.H.S.; Tiwari, A.K.; Babu, R.J. Niosomal formulation of hydroxytyrosol, a polyphenolic antioxidant, for enhancing transdermal delivery across human cadaver skin. Pharmaceutical Development and Technology 2022, 27, 155–163, 10.1080/10837450.2022.2025540.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
24 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





