| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leonardo Schena | + 3162 word(s) | 3162 | 2021-03-16 05:18:07 | | | |
| 2 | Vicky Zhou | Meta information modification | 3162 | 2021-03-23 04:48:45 | | |
Video Upload Options
Pomegranate Peel Extracts as a source of bioactive components are natural alternative means to control plant diseases and prevent food spoilage.
1. Introduction
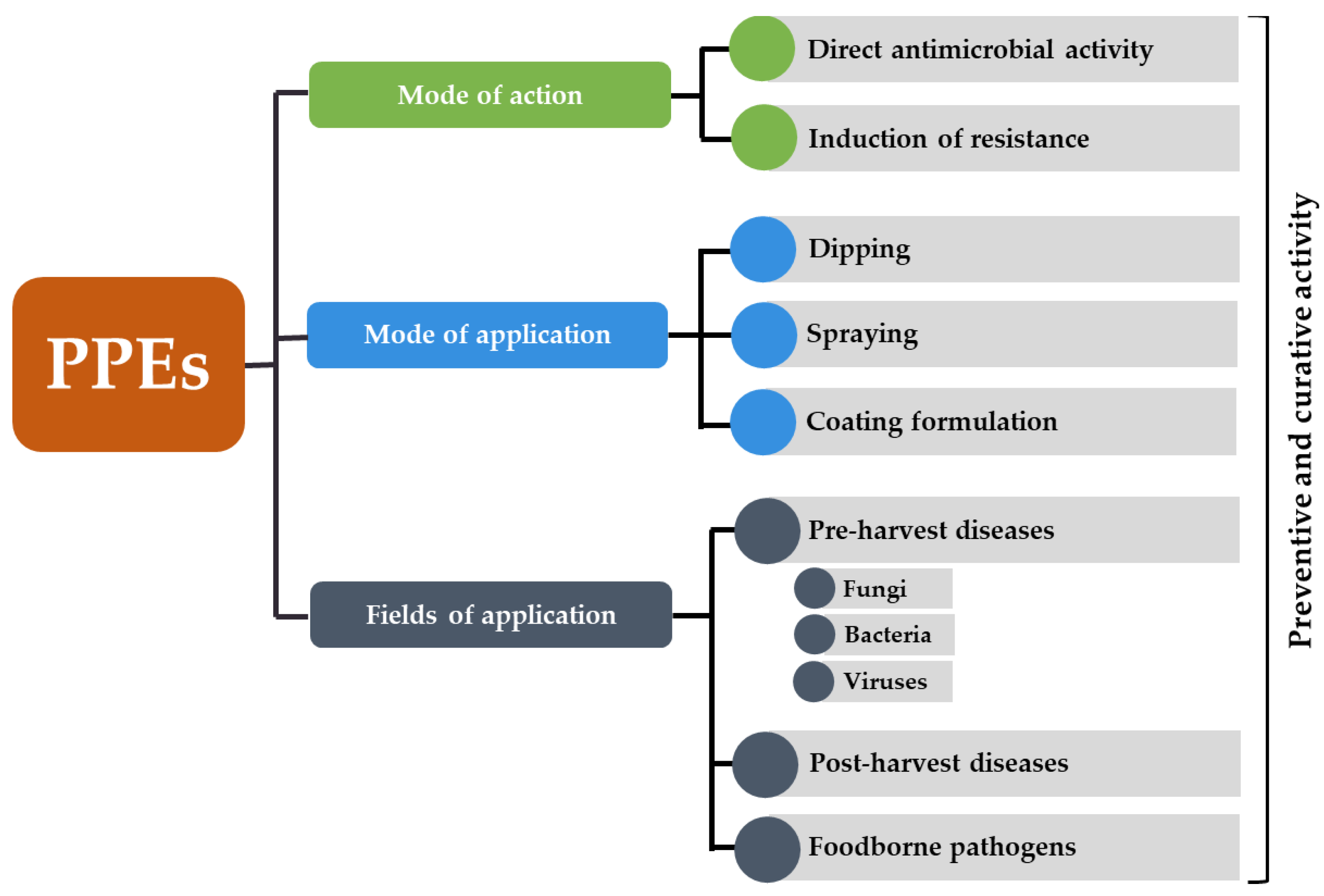
Starting from the Green Revolution, the development of modern and high yielding crop varieties has led to intensive monoculture cropping systems, loss of biodiversity and more susceptibility to diseases [1][2]. Together with climate change and market globalization, the emergence and spread of resistant pathogens has become a serious concern [2][3]. For decades, the Green Revolution has been accompanied by the intensive use of chemicals which reduces agricultural production stability and sustainability [2][4]. Chemical pesticides pose serious risks to human and environmental health, with negative impacts on non-target microorganisms and increasing selection for antimicrobial resistant strains [5][6]. With the latest European legislative restrictions and the rise of consumer awareness in food safety and healthy living, the development of safe and environmentally friendly alternative control means to control plant diseases has become an imperative need [7][8][9]. Several alternative methods have been proposed, including plant extracts, which are usually applied alone or as a part of integrated pest management programs [10][11][12][13]. In this regard, extracts from pomegranate peel have emerged as a source of very promising antimicrobial substances to control plant and foodborne pathogens. Pomegranate peels have been used in folk medicine since ancient times because of their health benefits due to the presence of various useful compounds [14]. A number of scientific evidences have proved the therapeutic and antioxidant activity of pomegranate peel extracts (PPEs) against many critical maladies including cancer, inflammation, diabetes, cardiovascular diseases, etc. [15][16][17][18] (Figure 1).
Figure 1. Schematic representation of fields of application, mode of application and mode of action of pomegranate peel extracts (PPEs) to control plant diseases and increase the shelf-life and safety of fresh fruits and vegetables.
2. Practical Applications
2.1. Post-Harvest Diseases
The control of postharvest diseases and the extension of the shelf-life of fresh fruits and vegetables are the most investigated fields of application of PPEs (Table 1). Fresh fruits and vegetables are very perishable and their quality can quickly deteriorate due to the high respiratory metabolism, biosynthesis and action of ethylene, transpiration and decay which is mainly caused by fungi [13]. Postharvest decay can be caused by latent and quiescent infections established in the field between flowering and fruit maturity and by wound infections that occur during harvesting and subsequent handling and storage [19]. Currently, synthetic fungicides are still used to control postharvest diseases; however, the growing health and environmental concerns over pesticide disposal and residue levels have led to more and more stringent regulations that withdraw most postharvest fungicides. For instance, in many European countries the postharvest use of fungicides is completely prohibited or limited to just a few chemicals registered on specific commodities. Furthermore, the few currently authorized chemicals are increasingly threatened by the development of pathogen resistant strains.
Table 1. Overview of different applications of PPEs to control plant diseases and foodborne pathogens.
| Field of Application | Pathogens | Host | References |
|---|---|---|---|
| Pre-harvest diseases |
Fusarium oxysporum | Tomato | [20] |
| Colletotrichum acutatum | Olive | [21] | |
| Uncinula necator | Grape | [22] | |
| Pseudomonas syringae pv. tomato | Tomato | [23] | |
| Xylella fastidiosa | Olive | [24] | |
| Tomato Spotted Wilt Virus | Tobacco, carnation | [22] | |
| Post-harvest diseases |
Fusarium sambucinum | Potato tubers | [25] |
| Botrytis cinerea | Lemon, strawberry, grape | [26][27][28] | |
| Monilinia laxa | Sweet cherries, apple | [27] | |
| Monilinia fructigena | Apple | [29] | |
| Penicillium digitatum | Lemon, grapefruit, orange | [30][27][31][32] | |
| Penicillium italicum | Lemon, grapefruit | [27][31] | |
| Penicillium expansum | Apple | [27] | |
| Colletotrichum gloeosporioides | Capsicum | [33] | |
| Colletotrichum acutatum | Olive | [21] | |
| Foodborne pathogens | Listeria monocytogenes | In vitro and in vivo (pear, apple, melon) | [34][35][36][37][38] |
| Salmonella spp. | In vitro | [39][34] | |
| Escherichia coli | In vitro | [39][34][36][37] | |
| Staphylococcus aureus | In vitro | [39][34][36][40] | |
| Clostridia | In vitro | [41] | |
| Yersinia enterocolitica | In vitro | [34] | |
| Bacillus subtilis | In vitro | [39][36] | |
| Bacillus cereus | In vitro | [36] | |
| Vibrio parahaemolyticus | In vitro | [38] |
2.2. Pre-Harvest Diseases
2.2.1. Control of Fungal Field Diseases
Currently, there are few reports on the use of PPEs to control field fungal diseases; however, the high efficacy and broad range of antifungal activity demonstrated by different PPEs in in vitro and laboratory experiments encouraged scientists to further investigate this field of application (Table 1). Indeed, available field data suggest the possible use of PPEs to control a broad range of diseases caused by necrotrophic, hemibiotrophic and biotrophic fungal pathogens. For instance, the incorporation of a PPE in soils artificially inoculated with F. oxysporum f. sp. lycopersici significantly reduced the population of the pathogen and increased the number of healthy tomato plants [20][28]. Results showed high efficiency of the extract, similar to the standard fungicide dicloran (Marisan 50 PB). In another study, the application of a methanolic PPE as seed or soil treatment significantly deceased pre- and post-emergence damping off of tomato caused by F. oxysporum under greenhouse conditions [42]. Soil treatment was more effective than seedling treatment. It is worth mentioning that it was reported that a high concentration of the extract may induce allelopathic activity in tomato plants [20].
Regarding hemibiotrophic fungi, field trials conducted in commercial olive orchards to control olive anthracnose demonstrated a very high efficacy of an ethanolic PPE that proved significantly more effective than copper, traditionally used to control this disease [21]. In particular, the application of the extract in the early ascending phase of the disease outbreak completely inhibited the development of natural rots. Authors speculated that since their extract is obtained using safe chemicals with no apparent phytotoxic effect on treated olive fruit, it may be regarded as a safe and effective natural antifungal preparation.
Recently, the use of an ethanolic PPE to control the grape powdery mold fungus Uncinula necator has been patented, highlighting the possible use of PPEs also against biotrophic pathogens (Figure 2) [22]. On grapevine cv. Aglianico, three treatments at intervals of 15 days, during the phenological phases of fruit set, pre-bunch closure and bunch closure, reduced the disease incidence by 71%, reaching an efficacy equal to that of the systemic fungicide Spiroxamine (Prosper®, Bayer Crop Science Italy).
Figure 2. Representative results of trials conducted on grapevine (cv. Aglianico) to control the powdery mold fungus Uncinula necator. Treatments were made on 8 July 2016 with wettable sulphur (TIOVIT®) at 2 g/l, Spiroxamine (PROSPER®) at 0.8 mL/l and PGE at 6 g/l. Untreated grapes were used as control. Photos were made at the beginning of the grape veraison, on 17 August 2016.
The use of PPEs to control fungal field diseases is very intriguing particularly because they may significantly contribute to reduce or replace the use of synthetic fungicides in agriculture. In fact, the broad spectrum of activity and the high level of efficacy suggest a potential market not restricted to organic productions since it may also include conventional and/or integrated farming systems.
2.2.2. Control of Bacterial Field Diseases
The possible use of PPEs to control plant bacterial diseases is particularly interesting since copper is the only authorized effective bactericide in most countries and frequently does not provide an accurate level of protection. Copper treatments are protectants without curative or systemic action and, therefore, must be applied prior to infection. Furthermore, copper can have serious ecological drawbacks. It accumulates in the soil and toxifies the natural microbial population and fauna. It may also have harmful effects on humans. This led to its insertion in the list of substances identified as “candidates for substitution” by the European Commission under Regulation (EC) No 1107/2009.
The use of PPEs to control plant bacteria was first proposed in 2013 [23]. Authors reported a good efficacy against the tomato bacterial speck caused by Pseudomonas syringae pv. tomato and speculated on the importance of their findings considering the lack of valid alternative compounds and the non-availability of commercial resistant cultivars of tomato. More recently, a hydroalcoholic PPE was utilized to treat olive trees affected by Xylella fastidiosa, a systemic bacterium that colonizes the xylem tissues. Experiments carried out over a four-year period (2016–2019) showed a general improvement of the plant’s health after trunk injections [24][43]. The induction of resistance in plant host tissues treated with PPEs and the broad range of antibacterial activity demonstrated in in vitro studies suggest the potential use of PPEs as safe control means in other plant bacterial pathosystems.
2.2.3. Control of Viral Field Diseases
An Italian patent has recently covered the use of an alcoholic extract of pomegranate peel to control plant viruses [22]. The artificial inoculations of the extract on tobacco and carnation plantlets prevented the infection of tomato spotted wilt virus (TSWV). According to the authors, the extract activates specific resistance responses in the plant and prevents viral infections. Although further investigations are needed to confirm the possible implementation of PPEs in plant virus control strategies, these preliminary results are highly important. In fact, plant viruses are challenging pathogens hard to control and only in rare cases can be controlled through the application of pesticides or other chemicals [44]. Therefore, rigorous research should be dedicated to investigate the application of PPEs to control plant virus diseases.
3. Mechanisms of Action
The mechanisms by which the bioactive components of PPEs exert their activity have not been completely elucidated, although a consistent quantity of information is now available suggesting both a direct antimicrobial activity and the activation of resistance responses in treated plant tissues. The direct antimicrobial activity is one of the most investigated features of PPEs, although most studies have focused on human-associated microorganisms [34][39][45]. In vitro trials showed strong inhibitory activity against the germination of conidia and the mycelium growth of major plant fungal pathogens including Botrytis cinerea, Penicillium digitatum, Penicillium expansum, Penicillium italicum, Alternaria alternata, Stemphylium botryosum, Colletotrichum acutatum sensu stricto, Fusarium oxysporum, Aspergillus parasiticus, Monilinia laxa and Monilinia fructigena [20][26][30][21][46][47][45][27]. The level of antifungal activity can greatly vary according to extract type and pathogen species. For instance, an ethanolic PPE completely inhibited the germination of conidia of B. cinerea and C. acutatum, while it was less effective against P. digitatum and P. expansum which were reduced by 91.0% and 82.7%, respectively [21][27]. An aqueous PPE inhibited the mycelial growth of A. alternata, S. botryosum and Fusarium spp. but it was ineffective against P. expansum, P. digitatum and B. cinerea [47].
Additionally, since PPEs combine a direct antifungal activity with the inhibition of the toxin biosynthesis, they can be used against mycotoxigenic fungi. In a recent study, a methanolic PPE significantly delayed conidial germination and hyphal elongation rate of Aspergillus flavus and Fusarium proliferatum. Furthermore, the production of aflatoxins was reduced by 97% using the extract alone, and it was completely inhibited combining the PPE with the azole fungicide prochloraz (PRZ) [48].
Furthermore, PPEs have also been reported to exert a high antimicrobial activity against both Gram-positive and negative bacteria [39][49][50][45][51]. They proved effective against important plant pathogens such as Clavibacter michiganensis subsp. michiganensis, Pseudomonas syringae pv. actinidiae, Pseudomonas syringae pv. syringae, Erwinia carotovora and Xanthomonas campestris [52][53]and against food-borne pathogens such as Salmonella spp. and L. monocytogenes [34][35]. Belgacem et al. [35] showed strong and quick bactericidal and bacteriostatic activity against L. monocytogenes. Strong activity against bacteria was also confirmed by analyzing the epiphytic population of olive drupes and citrus fruits after PPE treatments [21][54].
Numerous studies correlated the antifungal and antibacterial activity of PPEs to their high concentration of polyphenols, particularly punicalagins and ellagic acids. Rongai et al. [55] found that punicalagins are responsible for the inhibition of the mycelial growth of Fusarium oxysporum f. sp. lycopersici and highlighted that PPEs are among the most effective plant extracts in preventing the germination of F. oxysporum. Similar results were reported for the conidial germination and hyphal growth of the mycotoxigenic fungi A. flavus and F. proliferatum [48]. Microscopic observation of Fusarium sambucinum mycelium treated with methanol PPE revealed hyphal morphological modifications including curling, twisting, and collapsing [25]. Cell empty cavities and disintegration of cytoplasmic organelles were also observed. Similarly, an abnormal mycelia structure of M. laxa and M. fructigena was recorded following a PPE treatment [29]. Furthermore, analysis of the sterol composition of A. flavus showed potential inhibition by PPE of the ergosterol biosynthesis, a pathway responsible for fungal cell membrane fluidity and permeability and required for hyphal elongation. Akhtar et al. [56] and Foss et al. [57] reported that PPE polyphenolic compounds combine with proteins of the fungal cell membrane and cause the cell death by increasing permeability. Furthermore, PPEs can decrease the pH gradient around the cell membrane and cause the cell death by increasing permeability [26][49]. On the other hand, Wu and Kim [58] and Dey et al. [59] highlighted that the reaction of polyphenols with sulfhydryl groups may induce enzymatic inhibition and microbial starvation. In this regard, Sudharsan et al. [48] observed that PPEs can inhibit aflatoxin production in A. flavus by inhibiting specific enzymes in the pathway of aflatoxin biosynthesis. In addition, a specific study on the proteomic effects of punicalagin on S. aureus showed that it adversely alters bacterial growth by disrupting iron homeostasis and inducing SOS responses, possibly through DNA biosynthesis inhibition [60]. Although some reports suggested that the presence of the outer lipopolysaccharide membrane in Gram-negative bacteria could reduce the ability of PPEs to alter and affect cells, other investigations have shown a high efficacy against Gram-negative bacteria such as Salmonella Enteritidis [34].
Besides the direct antimicrobial activity, there is clear evidence showing that PPEs induce resistance in plant tissues. Pangallo et al. [21][31] indirectly demonstrated that PPE could activate the plant defense responses in olive drupes inoculated with C. acutatum and in citrus fruits inoculated with P. digitatum and P. italicum by observing a reduction in disease incidence without a direct contact between the pathogens and the extract. Furthermore, on grapefruit, an increase in reactive oxygen species (ROS) was detected following the PPE treatment, reaching a peak after 24 h post-treatment [31]. The same study revealed the activation of several genes involved in plant defense responses such as CHI, CHS, MAPK, MAPKK and PAL. More recently, a transcriptomic analyses of citrus fruit revealed the activation of many genes and pathways involved in plant defense responses [61]. Particularly, the study showed the induction of nine enzymes implicated in the biosynthesis of antibiotics. The authors suggested that the induction of this pathway might be one of the main mechanisms of action of PPE to counteract microbial infections and correlated the activation of these enzymes with the extract composition since polyphenols are proved to induce resistance in plant tissues [62].
The activation of resistance responses may explain the observed long persistence of efficacy after PPEs treatments [27][54]. However, specific investigations are needed to determine the persistence of host resistance responses after PPE treatment and their degradation rate within the host tissues.
3.1. Preventive and Curative Actions
An important feature of PPEs is their high efficacy in both preventive and curative treatments [27][31][25]. The control of already established infections is important since most of the alternative control means are only effective when applied before the infection takes place. On olives artificially inoculated with C. acutatum sensu stricto, PPE treatments made 6, 12 and 24 h after inoculations significantly reduced the incidence of rots suggesting the possible control of already established infections. Similar findings were obtained on apples inoculated with P. expansum and on grapefruits and lemons inoculated with P. digitatum and P. italicum [27]. Furthermore, a strong curative action was also confirmed in field trials to control anthracnose in olive orchards characterized by a high incidence of latent infections [21]. Authors speculated on the importance of this feature since latent infections play a fundamental role in the epidemiology of olive anthracnose [63][64]. These results also suggested the potential use of PPEs to increase the shelf-life of fresh fruits and vegetables since treatments applied just before or soon after harvest could be used to reduce latent infections and protect fruits during harvesting, packaging and storage [30][65].
The exact mechanisms by which PPE exerts its curative action is not completely understood. The ability of the extract to rapidly activate resistance response in plants and induce the production of antifungal compounds is likely to play a major role [61]. Additionally, a direct antifungal activity of the extract against the colonizing fungi might be possible, but currently there is no evidence regarding the penetration and diffusion of PPE active components into the host tissues.
4. Foodborne Pathogens Associated with Fruits and Vegetables
Much effort is devoted to plant associated pathogens as they affect the health and productivity of many important crops. However, some of these pathogens directly infect also human beings, resulting in cross-kingdom pathogenicity [66][67]. The risk of human borne pathogens is very high, leading to human illness and in some cases to death. Despite the wide range of preservation techniques, food borne diseases remain an important global health problem. Serious food-borne outbreaks and food recalls were recorded on fresh produce resulting in acute food poisoning and huge economic losses [68]. This is mainly attributed to many factors, including cross contamination, mechanical wounding and especially the development of antibiotic resistant foodborne pathogens which has been an emerging public-health threat [67][69]. As processed foods are very susceptible to physical and biological deterioration, the need to develop natural preservation techniques is highly required from the consumer who is moving toward a healthier diet. In this regard, PPEs were reported to exert strong bactericidal and bacteriostatic activity against several Gram-positive and negative foodborne bacteria including Salmonella spp., L. monocytogenes, E. coli, Pseudomonas aeruginosa, Clostridia, S. aureus, Y. enterocolitica, Bacillus subtilis, Bacillus cereus and Vibrio parahaemolyticus (Table 1) [56][34][36][38][70]. It has been demonstrated that PPEs can extend the shelf-life and maintain the microbiological, chemical and sensorial quality of food products when applied individually or in combination with other antimicrobial agents. For instance, a strong activity of PPE against L. monocytogenes was shown on fresh-cut pear, melon and apple fruits, suggesting its possible implementation in the industry of ready-to-eat fresh-cut products to guarantee high levels of control and safety [35]. Interestingly, PPEs showed synergetic action with several other bioactive agents including chitosan, alginate, biocontrol agents and other plant extracts [32][71][72][73][74][75][76]. Furthermore, the possible use of PPEs in edible coating formulations is particularly interesting in light of the rapidly increasing interest of food industries for new active packaging materials [77][78]. For instance, the incorporation of a PPE in gelatine film-forming solution improved the antioxidant properties and antimicrobial activity of the active packaging against S. aureus, L. monocytogenes and E. coli [40]. Similarly, the incorporating of PPE and sodium dehydroacetate in a PVA film increased the antioxidant activity of the film and the bacteriostatic effect against E. coli and S. aureus [79]. Recently, PPE immobilized electrospun active nanofibers were proposed as an excellent food wrapping material to preserve the sensory properties and extend the shelf-life of meat and other food product [80]. Currently, many industries are interested in introducing PPEs in their food products as a functional ingredient because of their excellent antioxidant, anti-inflammatory and antibacterial effects, and their wide range of application which is not restricted to fresh fruits and vegetables but also many other food products including meat, sausages, fish, bread, juice, etc.
Interestingly, research showed that the application of PPEs on food products is not only useful for their antimicrobial activity against foodborne pathogens but also for their beneficial effect in stimulating the growth of human gut microbiota. In fact, one key finding on PPEs is their potential prebiotic effect, mainly due to their richness in polyphenols, particularly ellagitannins, which are reported to selectively modulate the growth of susceptible microorganisms [41][81][82][83]. It has been reported that PPEs stimulate the growth of Bifidobacterium and Lactobacillus strains, some of the most important taxa involved in food microbiology and human nutrition [82][84]. Neyrinck et al. [83] found that PPE combined with the probiotic Lactobacillus rhamnosus significantly reduces lipid accumulation, suggesting its potential implementation in obesity prevention diets.
References
- Evenson, R.E.; Gollin, D. Assessing the impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762.
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 332–342.
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544.
- Larsen, A.E.; Gaines, S.D.; Deschênes, O. Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat. Commun. 2017, 8, 302.
- Sanzani, S.M.; Schena, L.; De Girolamo, A.; Ippolito, A.; González-Candelas, L. Characterization of genes associated with induced resistance against Penicillium expansum in apple fruit treated with quercetin. Postharvest Biol. Technol. 2010, 56, 1–11.
- Gill, H.K.; Garg, H. Pesticides: Environmental impacts and management strategies. In Pesticides-Toxic Aspects; IntechOpen: Rijeka, Croatia, 2014.
- Dilucia, F.; Lacivita, V.; Conte, A.; Nobile, M.A.D. Sustainable use of fruit and vegetable by-products to enhance food packaging performance. Foods 2020, 9, 857.
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255.
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10.
- Spadaro, D.; Gullino, M.L. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 2004, 91, 185–194.
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59.
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant-and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52.
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81.
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A review study on Punica granatum L. J. Evid. Based Complement. Altern. Med. 2016, 21, 221–227.
- Li, Y.; Ye, T.; Yang, F.; Hu, M.; Liang, L.; He, H.; Li, Z.; Zeng, A.; Li, Y.; Yao, Y. Punica granatum (pomegranate) peel extract exerts potent antitumor and anti-metastasis activity in thyroid cancer. RSC Adv. 2016, 6, 84523–84535.
- Stojanović, I.; Šavikin, K.; Đedović, N.; Živković, J.; Saksida, T.; Momčilović, M.; Koprivica, I.; Vujičić, M.; Stanisavljević, S.; Miljković, Đ. Pomegranate peel extract ameliorates autoimmunity in animal models of multiple sclerosis and type 1 diabetes. J. Funct. Foods 2017, 35, 522–530.
- Negi, P.; Jayaprakasha, G.; Jena, B. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397.
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 2019, 63.
- Eckert, J.W.; Eaks, I. Postharvest disorders and diseases of citrus fruits. Citrus Ind. 1989, 5, 179–260.
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur. J. Plant Pathol. 2017, 147, 229–238.
- Pangallo, S.; Li Destri Nicosia, M.G.; Agosteo, G.E.; Abdelfattah, A.; Romeo, F.V.; Cacciola, S.O.; Rapisarda, P.; Schena, L. Evaluation of a Pomegranate Peel Extract (PGE) as Alternative Mean to Control Olive Anthracnose. Phytopathology 2017, 107, 1462–1467.
- Agosteo, G.E.; Li Destri Nicosia, M.G.; Schena, L.; Romeo, F.V.; Rapisarda, P.; Tiberini, A.; Pangallo, S.; Timpanaro, N.; Amenta, M.; Ballistreri, G.; et al. Metodo di Difesa di Colture Agrarie nei Confronti di Patologie Causate da Microrganismi e Virus. Italian Patent N. 102018000004605, 17 April 2018.
- Quattrucci, A.; Ovidi, E.; Tiezzi, A.; Vinciguerra, V.; Balestra, G. Biological control of tomato bacterial speck using Punica granatum fruit peel extract. Crop Prot. 2013, 46, 18–22.
- Rongai, D.; Sabatini, N.; Marco, C.D. Preliminary studies to control Olive Quick Decline Syndrome (OQDS) caused by Xylella fastidiosa by endotherapy treatments with Punica granatum extract. In Proceedings of the Atti, Giornate Fitopatologiche, Chianciano Terme, Italy, 6–9 March 2018; Volume 2, pp. 303–309.
- Elsherbiny, E.A.; Amin, B.H.; Baka, Z.A. Efficiency of pomegranate (Punica granatum L.) peels extract as a high potential natural tool towards Fusarium dry rot on potato tubers. Postharvest Biol. Technol. 2016, 111, 256–263.
- Rongai, D.; Sabatini, N.; Pulcini, P.; Di Marco, C.; Storchi, L.; Marrone, A. Effect of pomegranate peel extract on shelf life of strawberries: Computational chemistry approaches to assess antifungal mechanisms involved. J. Food Sci. Technol. 2018, 55, 2702–2711.
- Li Destri Nicosia, M.G.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Droby, S.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61.
- Rongai, D.; Pulcini, P.; Pesce, B.; Marco, C.D.; Milano, F. Effects of pomegranate (Punica granatum) peel extract on fusarium wilt of tomato (Fusarium oxysporum f sp. lycopersici) and on grey mould (Botrytis cinerea). In Proceedings of the Atti, Giornate Fitopatologiche, Chianciano Terme, Italy, 8–11 March 2016; Volume 2, pp. 303–310.
- Elkhetabi, A.; Lahlali, R.; Askarne, L.; Ezrari, S.; El Ghadaroui, L.; Tahiri, A.; Hrustić, J.; Amiri, S. Efficacy assessment of pomegranate peel aqueous extract for brown rot (Monilinia spp.) disease control. Physiol. Mol. Plant Pathol. 2020, 110, 101482.
- Tayel, A.; El-Baz, A.; Salem, M.; El-Hadary, M. Potential applications of pomegranate peel extract for the control of citrus green mould. J. Plant Dis. Prot. 2009, 116, 252–256.
- Pangallo, S.; Li Destri Nicosia, M.; Raphael, G.; Levin, E.; Ballistreri, G.; Cacciola, S.; Rapisarda, P.; Droby, S.; Schena, L. Elicitation of resistance responses in grapefruit and lemon fruits treated with a pomegranate peel extract. Plant Pathol. 2017, 66, 633–640.
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsi’ pomegranate peel extracts. Ind. Crop. Prod. 2018, 111, 345–352.
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and antifungal activity of pomegranate peel extract and its use in polysaccharide-based edible coatings to extend the shelf-life of capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327.
- Al-Zoreky, N. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248.
- Belgacem, I.; Schena, L.; Teixidó, N.; Romeo, F.; Ballistreri, G.; Abadias, M. Effectiveness of a pomegranate peel extract (PGE) in reducing Listeria monocytogenes in vitro and on fresh-cut pear, apple and melon. Eur. Food Res. Technol. 2020, 246, 1765–1772.
- Hayrapetyan, H.; Hazeleger, W.C.; Beumer, R.R. Inhibition of Listeria monocytogenes by pomegranate (Punica granatum) peel extract in meat paté at different temperatures. Food Control 2012, 23, 66–72.
- Hanani, Z.N.; Husna, A.A.; Syahida, S.N.; Khaizura, M.N.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211.
- Wu, J.; Goodrich, K.M.; Eifert, J.D.; Jahncke, M.L.; O’Keefe, S.F.; Welbaum, G.E.; Neilson, A.P. Inhibiting foodborne pathogens Vibrio parahaemolyticus and Listeria monocytogenes using extracts from traditional medicine: Chinese gallnut, pomegranate peel, Baikal skullcap root and forsythia fruit. Open Agric. 2018, 3, 163–170.
- Nuamsetti, T.; Dechayuenyong, P.; Tantipaibulvut, S. Antibacterial activity of pomegranate fruit peels and arils. Sci. Asia 2012, 38, 319–322.
- Hanani, Z.N.; Yee, F.C.; Nor-Khaizura, M. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259.
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349.
- Mohamad, T.G.; Khalil, A.A. Effect of agriculture waste: Pomegranate (Punica granatum L.) fruits peel on some important phytopathogenic fungi and control of tomato damping-off. J. Appl. Life Sci. Int. 2015, 3, 103–113.
- Rongai, D.; Pulcini, P.; Di Lernia, D.; Nota, P.; Milano, F.; Di Marco, C. Ulteriori studi sul contenimento dell’olive quick decline syndrome syndrome (OQDS) tramite trattamenti endoterapici con estratto di melograno. AttiGiornate Fitopatol. 2020, 2, 109–114.
- Valkonen, J. The challenge of controlling plant viruses. Ann. Appl. Biol. 2014, 164, 313–317.
- Oraki, H.H.; Demirci, A.Ş.; Gümüş, T. Antibacterial and antifungal activity of pomegranate (Punica granatum L. cv.) peel. Electron. J. Environ. Agric. Food Chem. 2011, 10, 1958–1969.
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sci. 2015, 10, 409–416.
- Glazer, I.; Masaphy, S.; Marciano, P.; Bar-Ilan, I.; Holland, D.; Kerem, Z.; Amir, R. Partial identification of antifungal compounds from Punica granatum peel extracts. J. Agric. Food Chem. 2012, 60, 4841–4848.
- Sudharsan, S.; Shapiro, O.; Ziv, C.; Barda, O.; Zakin, V.; Sionov, E. Synergistic Inhibition of Mycotoxigenic Fungi and Mycotoxin Production by Combination of Pomegranate Peel Extract and Azole Fungicide. Front. Microbiol. 2019, 10, 1919.
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 2019,54, 959–965.
- Manapure, N.; Naik, R.; Satbhai, R.; Mohite, S. Evaluation of Antioxidant Activity of Solvent Extracted Pomegranate Peel and its Effect against Plant Pathogenic Bacteria and Fungi. J. Pure Appl. Microbiol. 2015, 9, 1081–1089.
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutr. 2020, 1-18.
- Hassan, N.A.; El-Feky, G.S.; Elegami, H.M. Antibacterial Activity of Thirty Two Pomegranate (Punica granatum L.) Accessions Growing in Egypt Fruit Peels. World Appl. Sci. J. 2013, 21, 960–967.
- Rongai, D.; Pucci, N. Esperienze preliminari sul contenimento di alcuni batteri fitopatogeni attraverso l’utilizzo dell’estratto di Punica granatum. AttiGiornate Fitopatol. 2016, 2, 311–314.
- Pangallo, S.; Li Destri Nicosia, M.; Scibettaa, S.; Strano, M.; Cacciola, S.; Belgacem, I.; Agosteo, G.; Schena, L. Pre- and postharvest applications of a pomegranate peel extract to control citrus fruit decay during storage and shelf life. Plant Dis. 2020, doi:10.1094/pdis-01-20-0178-re.
- Rongai, D.; Milano, F.; Sciò, E. Inhibitory effect of plant extracts on conidial germination of the phytopathogenic fungus Fusarium oxysporum. Am. J. Plant Sci. 2012, 3, 1693–1698.
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425.
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.; Endo, E.H.; Dias Filho, B.P. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32.
- Wu, V.C.; Kim, B. Effect of a simple chlorine dioxide method for controlling five foodborne pathogens, yeasts and molds on blueberries. Food Microbiol. 2007, 24, 794–800.
- Dey, D.; Debnath, S.; Hazra, S.; Ghosh, S.; Ray, R.; Hazra, B. Pomegranate pericarp extract enhances the antibacterial activity of ciprofloxacin against extended-spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) producing Gram-negative bacilli. Food Chem. Toxicol. 2012, 50, 4302–4309.
- Cooper, B.; Islam, N.; Xu, Y.; Beard, H.S.; Garrett, W.M.; Gu, G.; Nou, X. Quantitative proteomic analysis of staphylococcus aureus treated with punicalagin, a natural antibiotic from pomegranate that disrupts iron homeostasis and induces SOS. Proteomics 2018, 18, 1700461.
- Belgacem, I.; Pangallo, S.; Abdelfattah, A.; Romeo, F.V.; Cacciola, S.O.; Li Destri Nicosia, M.G.; Ballistreri, G.; Schena, L. Transcriptomic Analysis of Orange Fruit Treated with Pomegranate Peel Extract (PGE). Plants 2019, 8, 101.
- Sanzani, S.M.; Schena, L.; Ippolito, A. Effectiveness of phenolic compounds against citrus green mould. Molecules 2014, 19, 12500–12508.
- Cacciola, S.; Faedda, R.; Sinatra, F.; Agosteo, G.; Schena, L.; Frisullo, S.; di San Lio, G.M. Olive anthracnose. J. Plant Pathol. 2012, 94, 29–44.
- Moral, J.M.; Castillo, L.F.R.; Romero, J.; Rodríguez, M.P.; Bello, J.J.; Xaviér, C.; Cabello, D.; Casas, A.T. Gestión integrada de la antracnosis del olivo. Vida Rural 2014, 379, 56–60.
- Ippolito, A.; Nigro, F. Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot. 2000, 19, 715–723.
- Hasnain, S.; Rehman, Y.; Mahmood, S. Cross-Kingdom Pathogenicity across Plants and Human Beings. J. Bacteriol. Parasitol. 2015, 6, 124.
- Barak, J.D.; Schroeder, B.K. Interrelationships of food safety and plant pathology: The life cycle of human pathogens on plants. Annu. Rev. Phytopathol. 2012, 50, 241–266.
- CDC. List of Selected Multistate Foodborne Outbreak Investigations. Availabe online: https://www.cdc.gov/foodsafety/outbreaks/multistate-outbreaks/outbreaks-list.html (accessed on 26 February 2021).
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935.
- Sorrenti, V.; Randazzo, C.L.; Caggia, C.; Ballistreri, G.; Romeo, F.V.; Fabroni, S.; Timpanaro, N.; Raffaele, M.; Vanella, L. Beneficial Effects of Pomegranate Peel Extract and Probiotics on Pre-adipocyte Differentiation. Front. Microbiol. 2019, 10, 660.
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and antifungal activity of pomegranate peel extract and its use in
- polysaccharide-based edible coatings to extend the shelf-life of capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327.
- Taberner, V.; Sanchís, E.; Mateos, M.; Palou, L.; Pérez-Gago, M. Pectin-based edible coatings formulated with pomegranate peel
- extracts and other antibrowning agents to extend shelf life of fresh-cut'Rojo Brillante'persimmon. In Proceedings of the ISHS Acta Horticulturae 1194—VIII International Postharvest Symposium: Enhancing Supply Chain and Consumer Benefits-Ethical and Technological Issues, Cartagena, Murcia, Spain, 21–24 June 2016; pp. 887–894.
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252.
- Cruz, V.; Rojas, R.; Saucedo-Pompa, S.; Martínez, D.G.; Aguilera-Carbó, A.F.; Alvarez, O.B.; Rodríguez, R.; Ruiz, J.; Aguilar, C.N. Improvement of shelf life and sensory quality of pears using a specialized edible coating. J. Chem. 2015, 2015.
- Farris, S.; Vartiainen, J. Functional Coatings for Food Packaging Applications; MDPI AG: Basel, Switzerland, 2021.
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56.
- He, L.; Lan, W.; Ahmed, S.; Qin, W.; Liu, Y. Electrospun polyvinyl alcohol film containing pomegranate peel extract and sodium dehydroacetate for use as food packaging. Food Packag. Shelf Life 2019, 22, 100390.
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439.
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352.
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.-P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168.
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809.
- Felis, G.E.; Dellaglio, F. Taxonomy of lactobacilli and bifidobacteria. Curr. Issues Intest. Microbiol. 2007, 8, 44.