
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pavel A. Solopov | -- | 3866 | 2023-02-01 21:42:27 | | | |
| 2 | Lindsay Dong | -34 word(s) | 3832 | 2023-02-02 02:02:55 | | |
Video Upload Options
Since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, pharmaceutical companies and research institutions have been actively working to develop vaccines, and the mass roll-out of vaccinations against COVID-19 began in January 2021. At the same time, during lockdowns, the consumption of alcoholic beverages increased. During the peak of vaccination, consumption remained at high levels around the world, despite the gradual relaxation of quarantine restrictions. Two of the popular queries on search engines were whether it is safe to drink alcohol after vaccination and whether this will affect the effectiveness of vaccines. Many studies have been published suggesting that excessive drinking not only worsens the course of an acute respiratory distress syndrome caused by the SARS-CoV-2 virus but can also exacerbate post-COVID-19 syndrome. Despite all sorts of online speculation, there is no specific scientific data on alcohol-induced complications after vaccination in the literature. Most of the published vaccine clinical trials do not include groups of patients with a history of alcohol-use disorders.
1. Introduction
2. The Impacts of Alcohol Consumption on the Immune System
3. “Spike Effect” of COVID-19 Vaccines and Alcohol
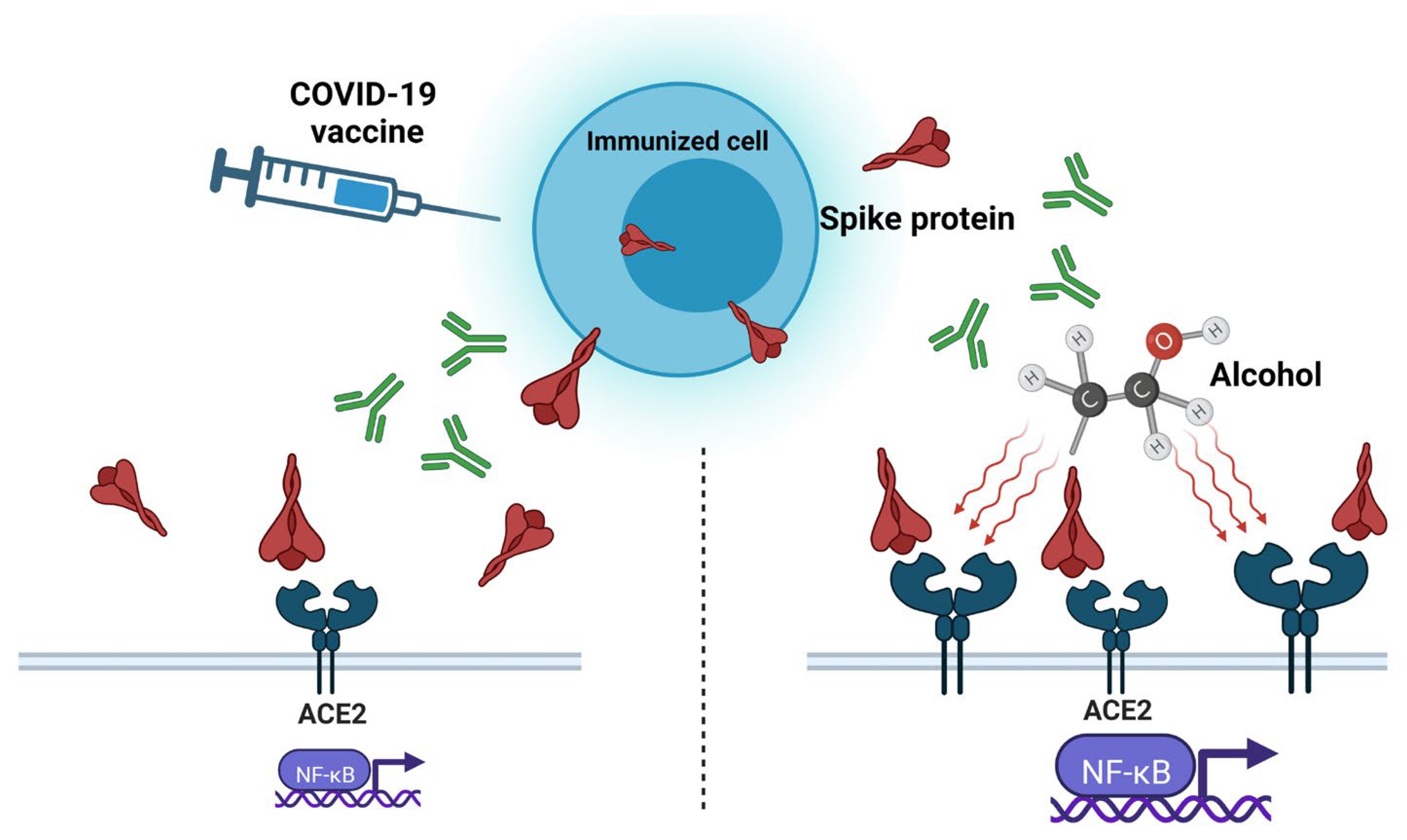
4. Protein Subunit Vaccines and Alcohol
5. Inactivated Whole-Virus Vaccines and Alcohol
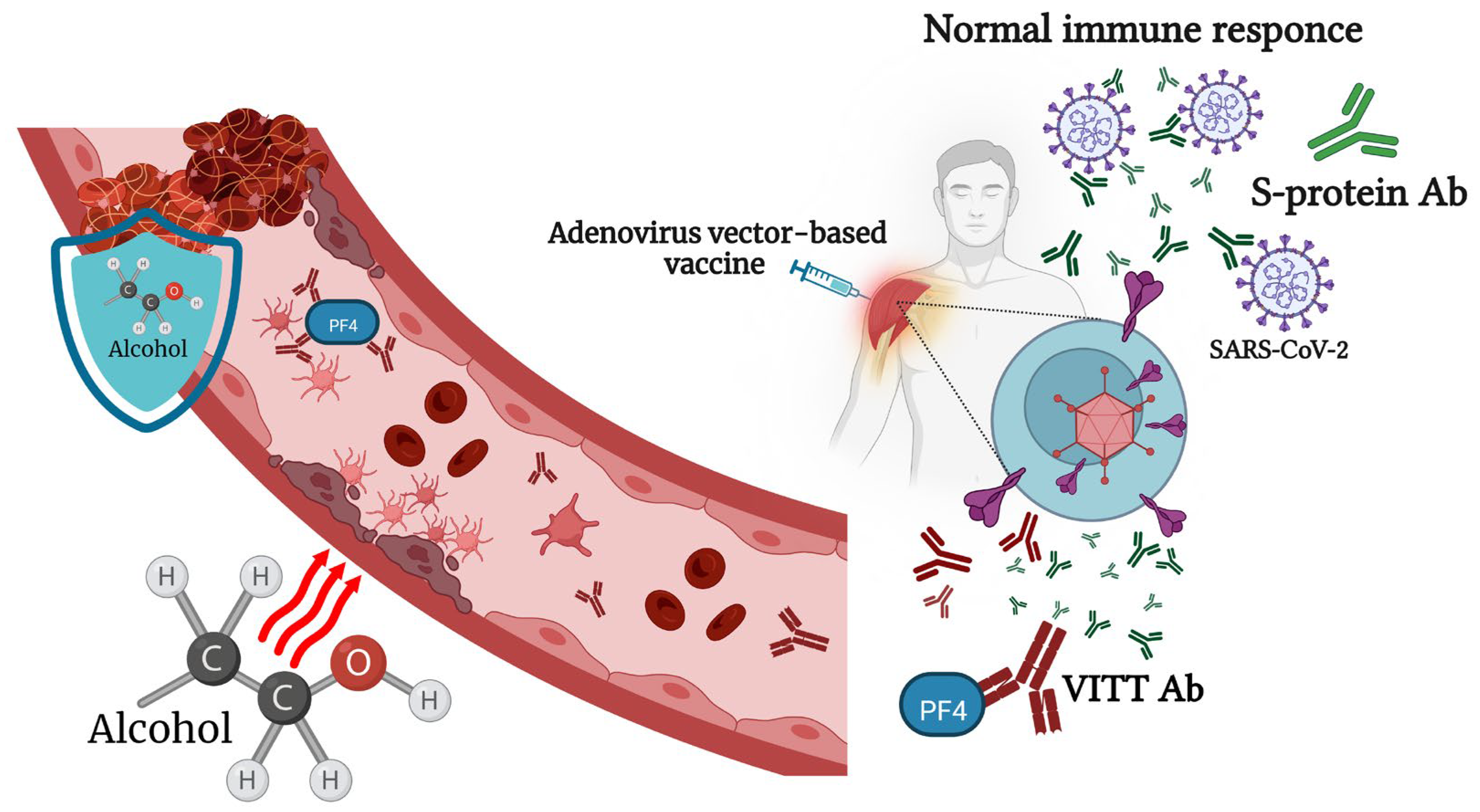
6. Viral-Vector-Based COVID-19 Vaccines and Alcohol
7. mRNA-Based Vaccines and Alcohol Consumption
The first report of protein production following reporter gene mRNA in mice was published by Wolff at al. in 1990 [94]. During that period, pharmaceutical companies did not consider mRNA a prospective technology because of doubts about its stability and its low efficacy [95]. Despite mRNA vaccines representing only 11% of all the developed COVID-19 vaccines, two mRNA vaccines, mRNA-1273 and BNT162b, were the first vaccines approved by the FDA and EUA for COVID-19 [96]. Both new mRNA vaccines, BNT162b2, manufactured by Pfizer/BioNTech, and mRNA-1273, produced by Moderna, contain molecules of RNA, modified with pseudo-uridine and encapsulated in a lipid nanoparticle vehicle. The Pfizer–BioNTech and Moderna vaccine constructs do not contain an S-protein S1/S2 furin cleavage site. Ribonucleic acid is endowed to be rapidly translated into nonactive SARS-CoV-2 S proteins in a stable closed structure in order to induce the immune response without causing cell damage due to its interaction with the ACE2 receptor [97]. However, these two vaccines were the most feared among people at the initial stage of vaccination due to the lack of data on their long-term side effects.
8. Conclusions
References
- Messaoudi, I.; Pasala, S.; Grant, K. Could moderate alcohol intake be recommended to improve vaccine responses? Expert. Rev. Vaccines 2014, 13, 817–819.
- Sarkar, D.; Jung, M.K.; Wang, H.J. Alcohol and the Immune System. Alcohol Res. Curr. Rev. 2015, 37, 153–155.
- Alraiyes, A.H.; Shaheen, K.; Alraies, M.C. Alcoholic leukopenic pneumococcal sepsis. Avicenna J. Med. 2013, 3, 53–55.
- Trannesen, H.; Andersen, J.R.; Pedersen, A.E.; Kaiser, A.H. Lymphopenia in heavy drinkers--reversibility and relation to the duration of drinking episodes. Ann. Med. 1990, 22, 229–231.
- Gonzalez-Quintela, A.; Alende, R.; Gude, F.; Campos-Franco, J.; Rey, J.; Meijide, L.M.; Fernandez-Merino, C.; Vidal, C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008, 151, 42–50.
- Mili, F.; Flanders, W.D.; Boring, J.R.; Annest, J.L.; DeStefano, F. The associations of alcohol drinking and drinking cessation to measures of the immune system in middle-aged men. Alcohol. Clin. Exp. Res. 1992, 16, 688–694.
- Barr, T.; Helms, C.; Grant, K.; Messaoudi, I. Opposing effects of alcohol on the immune system. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 65, 242–251.
- Meadows, G.G.; Zhang, H. Effects of Alcohol on Tumor Growth, Metastasis, Immune Response, and Host Survival. Alcohol Res. Curr. Rev. 2015, 37, 311–322.
- Castaldelli-Maia, J.M.; Segura, L.E.; Martins, S.S. The concerning increasing trend of alcohol beverage sales in the U.S. during the COVID-19 pandemic. Alcohol 2021, 96, 37–42.
- Nadkarni, A.; Kapoor, A.; Pathare, S. COVID-19 and forced alcohol abstinence in India: The dilemmas around ethics and rights. Int. J. Law Psychiatry 2020, 71, 101579.
- Pedrosa, A.L.; Bitencourt, L.; Fróes, A.C.F.; Cazumbá, M.L.B.; Campos, R.G.B.; de Brito, S.B.C.S.; Simões e Silva, A.C. Emotional, Behavioral, and Psychological Impact of the COVID-19 Pandemic. Front. Psychol. 2020, 11, 566212.
- Anker, J.J.; Kushner, M.G. Co-Occurring Alcohol Use Disorder and Anxiety: Bridging Psychiatric, Psychological, and Neurobiological Perspectives. Alcohol Res. 2019, 40.
- Aghababaeian, H.; Hamdanieh, L.; Ostadtaghizadeh, A. Alcohol intake in an attempt to fight COVID-19: A medical myth in Iran. Alcohol 2020, 88, 29–32.
- Awijen, H.; Ben Zaied, Y.; Nguyen, D.K. Covid-19 vaccination, fear and anxiety: Evidence from Google search trends. Soc. Sci. Med. 2022, 297, 114820.
- Bendau, A.; Plag, J.; Petzold, M.B.; Ströhle, A. COVID-19 vaccine hesitancy and related fears and anxiety. Int. Immunopharmacol. 2021, 97, 107724.
- Chen, S.; Aruldass, A.R.; Cardinal, R.N. Mental health outcomes after SARS-CoV-2 vaccination in the United States: A national cross-sectional study. J. Affect. Disord. 2022, 298, 396–399.
- McNeil, A.; Purdon, C. Anxiety disorders, COVID-19 fear, and vaccine hesitancy. J. Anxiety Disord. 2022, 90, 102598.
- Wang, L.; Wang, Q.; Davis, P.B.; Volkow, N.D.; Xu, R. Increased risk for COVID-19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry 2022, 21, 124–132.
- Solopov, P.; Colunga Biancatelli, R.; Sharlow, E.; Lazo, J.; Catravas, J. Single intratracheal exposure to SARS-CoV-2 S1 spike protein induces acute lung injury in K18-hACE2 transgenic mice. FASEB J. 2021, 35.
- Solopov, P.A.; Biancatelli, R.M.L.C.; Catravas, J.D. Alcohol Increases Lung Angiotensin-Converting Enzyme 2 Expression and Exacerbates Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein Subunit 1-Induced Acute Lung Injury in K18-hACE2 Transgenic Mice. Am. J. Pathol. 2022, 192, 990–1000.
- Bautista, A.P. Free radicals, chemokines, and cell injury in HIV-1 and SIV infections and alcoholic hepatitis. Free Radic. Biol. Med. 2001, 31, 1527–1532.
- Daniluk, J.; Szuster-Ciesielska, A.; Drabko, J.; Kandefer-Szerszeń, M. Serum cytokine levels in alcohol-related liver cirrhosis. Alcohol 2001, 23, 29–34.
- González-Quintela, A.; Dominguez-Santalla, M.; Pérez, L.; Vidal, C.; Lojo, S.; Barrio, E. Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine 2000, 12, 1437–1440.
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254.
- Yeligar, S.M.; Chen, M.M.; Kovacs, E.J.; Sisson, J.H.; Burnham, E.L.; Brown, L.A.S. Alcohol and lung injury and immunity. Alcohol 2016, 55, 51–59.
- Gentilello, L.M.; Cobean, R.A.; Walker, A.P.; Moore, E.E.; Wertz, M.J.; Dellinger, E.P. Acute ethanol intoxication increases the risk of infection following penetrating abdominal trauma. J. Trauma Inj. Infect. Crit. Care 1993, 34, 669–675.
- Wyatt, T.A.; Gentry-Nielsen, M.J.; Pavlik, J.A.; Sisson, J.H. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol. Clin. Exp. Res. 2004, 28, 998–1004.
- Cohen, S.; Tyrrell, D.A.; Russell, M.A.; Jarvis, M.J.; Smith, A. Smoking, alcohol consumption, and susceptibility to the common cold. Am. J. Public Health 1993, 83, 1277–1283.
- Quesada-Molina, M.; Muñoz-Garach, A.; Tinahones, F.J.; Moreno-Indias, I. A New Perspective on the Health Benefits of Moderate Beer Consumption: Involvement of the Gut Microbiota. Metabolites 2019, 9, 272.
- Diaz, L.E.; Montero, A.; Gonzalez-Gross, M.; Vallejo, A.I.; Romeo, J.; Marcos, A. Influence of alcohol consumption on immunological status: A review. Eur. J. Clin. Nutr. 2002, 56, S50–S53.
- Magrone, T.; Candore, G.; Caruso, C.; Jirillo, E.; Covelli, V. Polyphenols from Red Wine Modulate Immune Responsiveness: Biological and Clinical Significance. Curr. Pharm. Des. 2008, 14, 2733–2748.
- Romeo, J.; Wärnberg, J.; Nova, E.; Díaz, L.E.; González-Gross, M.; Marcos, A. Changes in the immune system after moderate beer consumption. Ann. Nutr. Metab. 2007, 51, 359–366.
- Solopov, P.; Biancatelli, R.M.L.C.; Dimitropoulou, C.; Catravas, J.D. Dietary Phytoestrogens Ameliorate Hydrochloric Acid-Induced Chronic Lung Injury and Pulmonary Fibrosis in Mice. Nutrients 2021, 13, 3599.
- Romeo, J.; Wärnberg, J.; Nova, E.; Díaz, L.E.; Gómez-Martinez, S.; Marcos, A. Moderate alcohol consumption and the immune system: A review. Br. J. Nutr. 2007, 98, S111–S115.
- Van de Loo, A.; Raasveld, S.; Hogewoning, A.; Zeeuw, R.; Bosma, E.; Bouwmeester, N.; Lukkes, M.; Knipping, K.; Mackus, M.; Kraneveld, A.; et al. Immune Responses after Heavy Alcohol Consumption: Cytokine Concentrations in Hangover-Sensitive and Hangover-Resistant Drinkers. Healthcare 2021, 9, 395.
- Mittal, A.; Manjunath, K.; Ranjan, R.K.; Kaushik, S.; Kumar, S.; Verma, V. COVID-19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020, 16, e1008762.
- Almehdi, A.M.; Khoder, G.; Alchakee, A.S.; Alsayyid, A.T.; Sarg, N.H.; Soliman, S.S. SARS-CoV-2 spike protein: Pathogenesis, vaccines, and potential therapies. Infection 2021, 49, 855–876.
- Martínez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Reséndiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 701501.
- Angeli, F.; Reboldi, G.; Trapasso, M.; Zappa, M.; Spanevello, A.; Verdecchia, P. COVID-19, vaccines and deficiency of ACE(2) and other angiotensinases. Closing the loop on the “Spike effect”. Eur. J. Intern. Med. 2022, 103, 23–28.
- Cognetti, J.S.; Miller, B.L. Monitoring Serum Spike Protein with Disposable Photonic Biosensors Following SARS-CoV-2 Vaccination. Sensors 2021, 21, 5857.
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861.
- Kim, E.S.; Jeon, M.T.; Kim, K.S.; Lee, S.; Kim, S.; Kim, D.G. Spike Proteins of SARS-CoV-2 Induce Pathological Changes in Molecular Delivery and Metabolic Function in the Brain Endothelial Cells. Viruses 2021, 13, 2021.
- Li, J.; Wang, P.; Yang, N.; Huang, J.; Ou, J.; Xu, T.; Zhao, X.; Liu, T.; Huang, X.; Wang, Q.; et al. SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166260.
- Khan, S.; Shafiei, M.; Longoria, C.; Schoggins, J.; Savani, R.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife 2021, 10, e68563.
- Biancatelli, R.M.L.C.; Solopov, P.A.; Gregory, B.; Khodour, Y.; Catravas, J.D. HSP90 Inhibitors Modulate SARS-CoV-2 Spike Protein Subunit 1-Induced Human Pulmonary Microvascular Endothelial Activation and Barrier Dysfunction. Front. Physiol. 2022, 13, 812199.
- Biancatelli, R.M.C.; Solopov, P.A.; Sharlow, E.R.; Lazo, J.S.; Marik, P.E.; Catravas, J.D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L477–L484.
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326.
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Strömdahl, A.; Cerps, S.; Uller, L.; Kjellström, S.; Bond, P.; Schmidtchen, A. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020, 12, 916–932.
- Zhu, G.; Lyu, L.; Yang, H.; Liu, G.; Yang, S.; Gu, C.; Wang, L.; Yan, H.; Hu, M.; Che, C. SARS-CoV-2 spike protein-induced host inflammatory response signature in human corneal epithelial cells. Mol. Med. Rep. 2021, 24, 584.
- Ryu, J.K.; Sozmen, E.G.; Dixit, K.; Montano, M.; Matsui, Y.; Liu, Y.E.; Helmy, T.J.; Deerinck, Z.; Yan, R.; Schuck, R.M.; et al. SARS-CoV-2 spike protein induces abnormal inflammatory blood clots neutralized by fibrin immunotherapy. bioRxiv 2021.
- Zheng, Y.; Zhao, J.; Li, J.; Guo, Z.; Sheng, J.; Ye, X.; Jin, G.; Wang, C.; Chai, W.; Yan, J.; et al. SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate. Int. J. Biol. Macromol. 2021, 193, 1124–1129.
- Boschi, C.; Scheim, D.E.; Bancod, A.; Militello, M.; Le Bideau, M.; Colson, P.; Fantini, J.; La Scola, B. SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects. Int. J. Mol. Sci. 2022, 23, 15480.
- Balasubramanian, N.; James, T.D.; Pushpavathi, S.G.; Marcinkiewcz, C.A. Repeated ethanol exposure and withdrawal alters ACE2 expression in discrete brain regions: Implications for SARS-CoV-2 infection. bioRxiv 2022.
- Wang, F.; Yang, J.L.; Yu, K.K.; Xu, M.; Xu, Y.Z.; Chen, L.; Lu, Y.-m.; Fang, H.-s.; Wang, X.-y.; Hu, Z.-q.; et al. Wang, Activation of the NF-κB pathway as a mechanism of alcohol enhanced progression and metastasis of human hepatocellular carcinoma. Mol. Cancer 2015, 14, 10.
- Vandenbulcke, M.; Janssens, J. Acute axonal polyneuropathy in chronic alcoholism and malnutrition. Acta Neurol. Belg. 1999, 99, 198–201.
- Worner, T.M. Guillain-Barré’s syndrome in alcoholics. Drug Alcohol Depend. 1989, 23, 93.
- Kim, S.Y.; Oh, D.J.; Park, B.; Choi, H.G. Bell’s palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci. Rep. 2020, 10, 4248.
- Hansson, M.; Nygren, P.A.K.; Ståhl, S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000, 32, 95–107.
- Heidary, M.; Kaviar, V.H.; Shirani, M.; Ghanavati, R.; Motahar, M.; Sholeh, M.; Ghahramanpour, H.; Khoshnood, S. A Comprehensive Review of the Protein Subunit Vaccines Against COVID-19. Front. Microbiol. 2022, 13, 927306.
- Heath, P.T.; Galiza, E.P.; Baxter, D.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of the NVX-CoV2373 COVID-19 Vaccine at Completion of the Placebo-Controlled Phase of a Randomized Controlled Trial. Clin. Infect Dis. 2022, ciac803.
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372.
- Rydyznski Moderbacher, C.; Kim, C.; Mateus, J.; Plested, J.; Zhu, M.; Cloney-Clark, S.; Weiskopf, D.; Sette, A.; Fries, L.; Glenn, G.; et al. NVX-CoV2373 vaccination induces functional SARS-CoV-2-specific CD4+ and CD8+ T cell responses. J. Clin. Investig. 2022, 132, e160898.
- Oh, J.; Cho, W.H.; Barcelon, E.; Kim, K.H.; Hong, J.; Lee, S.J. SARS-CoV-2 spike protein induces cognitive deficit and anx-iety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci. Rep. 2022, 12, 5496.
- Barrett, P.N.; Mundt, W.; Kistner, O.; Howard, M.K. Vero cell platform in vaccine production: Moving towards cell cul-ture-based viral vaccines. Expert Rev. Vaccines 2009, 8, 607–618.
- Sanders, B.P.; Koldijk, M.; Schuitemaker, H. Inactivated viral vaccines. In Vaccine Analysis: Strategies, Principles, and Control; Springer: Berlin/Heidelberg, Germany, 2015; pp. 45–80.
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833.
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Per-spectives. Vaccines 2022, 10, 819.
- Wang, C.; Chen, L.Y.; Lu, Q.B.; Cui, F. Vaccination with the Inactivated Vaccine (Sinopharm BBIBP-CorV) Ensures Pro-tection against SARS-CoV-2 Related Disease. Vaccines 2022, 10, 920.
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81.
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192.
- Ahi, M.; Farahani, R.H.; Basiri, P.; Rahjerdi, A.K.; Sheidaei, A.; Gohari, K.; Rahimi, Z.; Gholami, F.; Moradi, M.; Naeeni, F.G.; et al. Com-parison of the Safety and Immunogenicity of FAKHRAVAC and BBIBP-CorV Vaccines when Administrated as Booster Dose: A Parallel Two Arms, Randomized, Double Blind Clinical Trial. Vaccines 2022, 10, 1800.
- Ai, J.; Wang, J.; Liu, D.; Xiang, H.; Guo, Y.; Lv, J.; Zhang, Q.; Li, J.; Zhang, X.; Li, Q.; et al. Safety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin. Gastroenterol. Hepatol. 2022, 20, 1516–1524.e2.
- Dal-Ré, R.; Launay, O. Public trust on regulatory decisions: The European Medicines Agency and the AstraZeneca COVID-19 vaccine label. Vaccine 2021, 39, 4029–4031.
- Franchini, M.; Liumbruno, G.M.; Pezzo, M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur. J. Haematol. 2021, 107, 173–180.
- Kelton, J.G.; Arnold, D.M.; Nazy, I. Lessons from vaccine-induced immune thrombotic thrombocytopenia. Nat. Rev. Immunol. 2021, 21, 753–755.
- Gordon, S.F.; Clothier, H.J.; Morgan, H.; Buttery, J.P.; Phuong, L.K.; Monagle, P.; Chunilal, S.; Wood, E.M.; Tran, H.; Szer, J.; et al. Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia. Vaccine 2021, 39, 7052–7057.
- Shen, C.J.; Kao, C.H.; Hsu, T.Y.; Chen, C.Y.; Lin, C.L.; Shih, H.M. Effect of alcohol intoxication on the risk of venous thromboembolism: A nationwide retrospective cohort study. Medicine 2017, 96, e8041.
- Aggarwal, A.; Puri, K.; Liangpunsakul, S. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: Systematic review. World J. Gastroenterol. 2014, 20, 5737–5745.
- Pahor, M.; Guralnik, J.M.; Havlik, R.J.; Carbonin, P.; Salive, M.E.; Ferrucci, L.; Corti, M.-C.; Hennekens, C.H. Alcohol Con-sumption and Risk of Deep Venous Thrombosis and Pulmonary Embolism in Older Persons. J. Am. Geriatr. Soc. 1996, 44, 1030–1037.
- Kasuda, S.; Sakurai, Y.; Shima, M.; Morimura, Y.; Kudo, R.; Hatake, K.; Yoshioka, A. Ethanol inhibits microaggregate for-mation of platelets in human whole blood. HAEMA 2004, 7, 200–204.
- Abolmaali, M.; Rezania, F.; Behnagh, A.; Hamidabad, N.; Gorji, A.; Mirzaasgari, Z. Guillain-Barré syndrome in asso-ciation with COVID-19 vaccination: A systematic review. Immunol. Res. 2022, 70, 752–764.
- Shay, D.K.; Gee, J.; Su, J.; Myers, T.; Marquez, P.; Liu, R.; Zhang, B.; Licata, C.; Clark, T.; Shimabukuro, T. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March-April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 680–684.
- Idiculla, P.S.; Gurala, D.; Palanisamy, M.; Vijayakumar, R.; Dhandapani, S.; Nagarajan, E. Cerebral Venous Thrombosis: A Comprehensive Review. Eur. Neurol. 2020, 83, 369–379.
- MacNeil, J.R.; Su, J.R.; Broder, K.R.; Guh, A.Y.; Gargano, J.W.; Wallace, M.; Hadler, S.C.; Scobie, H.M.; Blain, A.E.; Moulia, D.; et al. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients—United States, April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 651–656.
- Oliver, S.E.; Wallace, M.; See, I.; Mbaeyi, S.; Godfrey, M.; Hadler, S.; Jatlaoui, T.; Twentyman, E.; Hughes, M.; Rao, A.; et al. Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Rec-ommendations from the Advisory Committee on Immunization Practices—United States, December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 90–95.
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897.
- Cazzola, M.; Rogliani, P.; Mazzeo, F.; Matera, M.G. Controversy surrounding the Sputnik V vaccine. Respir. Med. 2021, 187, 106569.
- Moore, J.P. Approaches for Optimal Use of Different COVID-19 Vaccines: Issues of Viral Variants and Vaccine Efficacy. JAMA 2021, 325, 1251–1252.
- Perreau, M.; Pantaleo, G.; Kremer, E.J. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an im-proved environment for replication of HIV in T cells. J. Exp. Med. 2008, 205, 2717–2725.
- Tumban, E. Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval. Viruses 2020, 13, 54.
- Heinz, R.; Waltenbaugh, C. Ethanol Consumption Modifies Dendritic Cell Antigen Presentation in Mice. Alcohol Clin. Exp. Res. 2007, 31, 1759–1771.
- Thompson, M.G.; Navarro, F.; Chitsike, L.; Ramirez, L.; Kovacs, E.J.; Watkins, S.K. Alcohol exposure differentially effects anti-tumor immunity in females by altering dendritic cell function. Alcohol 2016, 57, 1–8.
- Polina Nikolskaya, A.O.; Reuters. Don’t Mix Sputnik Vaccine with Alcohol, Says Russian Official. Some Recoil. Available online: https://www.reuters.com/article/health-coronavirus-russia-vaccine-alcoho-idUSKBN28J239 (accessed on 19 December 2022).
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468.
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838.
- Park, J.W.; Lagniton, P.N.; Liu, Y.; Xu, R.-H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460.
- Chirumbolo, S. Vaccination hesitancy and the “myth” on mRNA-based vaccines in Italy in the COVID-19 era: Does urgency meet major safety criteria? J. Med. Virol. 2021, 93, 4049–4053.
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554.
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479.
- Yamamoto, S.; Tanaka, A.; Ohmagari, N.; Yamaguchi, K.; Ishitsuka, K.; Morisaki, N.; Kojima, M.; Nishikimi, A.; Tokuda, H.; Inoue, M.; et al. Use of heated tobacco products, moderate alcohol drinking, and anti-SARS-CoV-2 IgG antibody titers after BNT162b2 vaccination among Japanese healthcare workers. Prev. Med. 2022, 161, 107123.
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484.
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.; et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation 2022, 146, 743–754.
- Mirijello, A.; Tarli, C.; Vassallo, G.A.; Sestito, L.; Antonelli, M.; D’Angelo, C.; Ferrulli, A.; De Cosmo, S.; Gasbarrini, A.; Addolorato, G. Alcoholic cardiomyopathy: What is known and what is not known. Eur. J. Intern. Med. 2017, 43, 1–5.
- Wilke, A.; Kaiser, A.; Ferency, I.; Maisch, B. Alcohol and myocarditis. Herz 1996, 21, 248–257.
- George, A.; Figueredo, V.M. Alcoholic Cardiomyopathy: A Review. J. Card. Fail. 2011, 17, 844–849.
- Awaya, T.; Moroi, M.; Nakamura, F.; Toi, S.; Wakiya, M.; Enomoto, Y.; Kunimasa, T.; Nakamura, M. A Possibility of Vaso-spastic Angina after mRNA COVID-19 Vaccination. Vaccines 2022, 10, 1998.
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593.
- John, B.V.; Deng, Y.; Scheinberg, A.; Mahmud, N.; Taddei, T.H.; Kaplan, D.; Labrada, M.; Baracco, G.; Dahman, B. Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis. JAMA Intern. Med. 2021, 181, 1306–1314.
- COVID-19 Vaccine Tracker. 18 August 2022. Available online: https://www.covid-19vaccinetracker.org/#Top-of-Page (accessed on 19 December 2022).
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022, 22, 802–812.
- Gao, F.X.; Wu, R.X.; Shen, M.Y.; Huang, J.J.; Li, T.T.; Hu, C.; Luo, F.; Song, S.; Mu, S.; Hao, Y.; et al. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. iScience 2022, 25, 105479.




