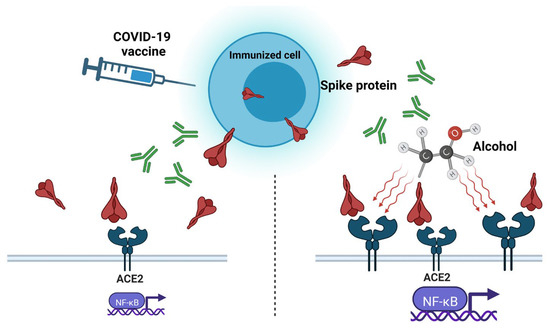
Vaccines based on inactivated pathogens have been used for over a hundred years as a protective agent against bacteria and viruses. Inactivated viral vaccines are first cultivated on a substrate (primary and continuous cell lines, tissues, fertilized eggs, and even whole organisms) to produce large amounts of antigens [
66]. The multiplied virus in the substrate is purified, concentrated, and inactivated by various chemical agents (ascorbic acid, hydrogen peroxide, etc.) or by using physical methods (heat, ultraviolet exposure, gamma irradiation, etc.). In recent decades, only formaldehyde and β-Propiolactone have been used as inactivated agents for human viral vaccines [
67]. Adjuvants are important components of many inactivated vaccines due to their ability to induce more robust and long-lasting specific immune responses [
68]. Aluminum salts, such as aluminum hydroxide, phosphate, and potassium sulfate, have been widely used in vaccines for a long time [
69].
Developed in China, the inactivated whole-virus vaccine Sinopharm (Beijing, China) BBIBP-CorV, containing an aluminum hydroxide adjuvant, has been approved by the WHO for emergency use, and it has been distributed in more than 40 countries [
70]. Another Chinese vaccine approved by the WHO is CoronaVac (Sinovac (Beijing, China)), an inactivated SARS-CoV-2 aluminum-hydroxide-adjuvanted vaccine created from African green monkey kidney cells (Vero cells) that have been inoculated with SARS-CoV-2 [
71,
72]. In both BBIBP-CorV and CoronaVac clinical trials, alcohol addiction was one of the exclusion criteria [
73]. No serious adverse reactions to vaccines, which could be aggravated by alcohol consumption, have been reported. A clinical study carried out by Jingwen Ai et al., demonstrated the safety of inactivated whole-virion SARS-CoV-2 vaccines in patients with alcoholic liver disease; however, those patients demonstrated a lower immunologic response to the vaccines than healthy patients [
74].
6. Viral-Vector-Based COVID-19 Vaccines and Alcohol
On 29 January 2020, the European Commission granted conditional marketing authorization for the Oxford/AstraZeneca COVID-19 vaccine (Covishield, Vaxzevria (Oxford, UK)), a monovalent vaccine composed of a single recombinant, replication-deficient chimpanzee adenovirus (ChAdOx1) vector encoding the S glycoprotein of SARS-CoV-2. However, five countries in the European Union have since placed age limitations on the vaccine, which has given rise to a certain distrust in it [
79]. One of the rare but most severe side effects of this vaccine is a syndrome named vaccine-associated immune thrombosis and thrombocytopenia (VITT) [
80]. Usually, the administration of a viral-vector-based COVID-19 vaccine induces the production of antibodies to the SARS-CoV-2 S protein. In very rare cases, VITT antibodies are generated that can bind to platelet factor 4 (PF4) and construct immune complexes that lead to a coagulation cascade and reduce the number of platelets [
81]. As of April 2021, there had been 222 registered cases of VITT in Europe [
80]. The Victorian Department of Health (Australia) equated this to eight cases of thrombopenia per million doses for the AstraZeneca vaccine [
82]. Based on several reported cases, young women, especially those taking hormonal contraceptives, are at the highest risk of developing this vaccine-related adverse reaction [
80]. Consequently, the AstraZeneca vaccine has not been authorized for use in the U.S. There have been no reports of Oxford/AstraZeneca-vaccine-related thrombosis and thrombocytopenia complications after alcohol consumption. However, binge alcohol consumption can lead to endothelial dysfunction, which, in combination with stasis and hypercoagulability, could increase venous thromboembolism (VTE) formation [
83]. Liver dysfunction, caused by chronic alcohol intoxication, decreases the synthesis of anticoagulant thrombotic factors [
84]. Nonetheless, there are also studies suggesting that low or moderate alcohol consumption could decrease the risk of deep venous thrombosis and pulmonary embolism in older people [
85]. The ethanol treatment of human whole blood led to a decrease in PF4 release in response to a-thrombin [
86]. According to Abolmaali’s study, AstraZeneca is the vaccine most reported to be associated with Guillain–Barré syndrome [
87]. Summarizing the above facts, we can say that young people who drink alcohol, as well as those who chronically drink alcohol, have an increased risk of complications after immunization with the Oxford/AstraZeneca vaccine (
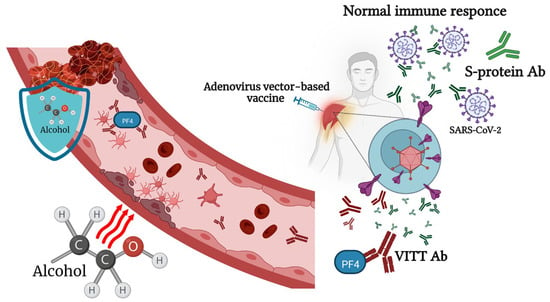
Figure 2).
Figure 2. Alcohol consumption affects the immune response to adenovirus-vector-based vaccines and vaccine-associated immune thrombosis and thrombocytopenia (VITT). Physiologically relevant concentrations of alcohol lead to endothelial dysfunction, which, in combination with stasis and hypercoagulability, could increase venous thromboembolism (VTE) formation. At the same time, small or moderate doses of alcohol have an inhibitory effect on secondary platelet aggregation responses.
The next vaccine, approved by both the FDA and EUA for emergency use in February 2021, was the adenovirus-vector-based vaccine JNJ-78435735 developed by Johnson and Johnson (New Brunswick, NJ, USA) [
57] along with Beth Israel Deaconess Medical Center (Boston, MA, USA) [
88]. The clear advantage of this vaccine over other vector-based vaccines is that it is a single-shot vaccine. However, doctors faced a problem similar to that of the AstraZeneca vaccine—cases of a condition characterized by low platelets and thrombosis, including cerebral venous sinus thrombosis [
89]. All cases of VITT occurred among women: 13 cases in 18–49-year-old women and 2 cases among women aged 50 years and older [
90]. Following an emergency meeting that was held in December 2021, the use of mRNA COVID-19 vaccines was recommended over the Janssen COVID-19 vaccine [
91]. The vaccine label information does not warn against alcohol use, but we can consider that alcohol abuse can increase the risk of VITT in young women.
The Gamaleya National Research Center for Epidemiology and Microbiology (Moscow, Russia) was the first to announce the creation of Gam-COVID-Vac (Sputnik V (Moscow, Russia)), a recombinant adenovirus-based vaccine [
92]. Even though Sputnik V has not yet been approved by the WHO, it has been approved in 70 countries with a combined population of more than 4 billion people [
93]. Sputnik V consists of two doses containing different components of the SARS-CoV-2 glycoprotein S gene, Ad26 and Ad5, administered separately 21 days apart [
94]. The latter Ad’s immune complexes activate the dendritic T-cell axis [
95]. A large proportion of this population, especially Africans, have high anti-Ad5 antibody titers from previous infections [
96]. In an experiment on DO11.10 transgenic mice, it was shown that alcohol diminishes the capacity of dendritic cells to secrete interleukins IL-12 and IL-6 and reduces the ability to maintain the secretion of cytokines IL-17A and IFN-c but increases IL-13 expression [
97]. Thompson et al., reported that ethanol promotes a reduced immune stimulatory capacity of female DC by reducing IL-12 production [
98]. Thus, alcohol consumption after the second dose of the Sputnik V vaccine may significantly compromise its effectiveness, especially in some population categories. A Ministry of Health official representative warned that anyone being vaccinated against COVID-19 with Russia’s Sputnik V vaccine should give up alcohol for almost two months [
99]. Interestingly, the Phase 3 trial on patients who received the Sputnik V vaccine showed only one patient with vein thrombosis unlike the above adenovirus-vector-based vaccines [
93].
7. mRNA-Based Vaccines and Alcohol Consumption
The first report of protein production following reporter gene mRNA in mice was published by Wolff at al. in 1990 [100]. During that period, pharmaceutical companies did not consider mRNA a prospective technology because of doubts about its stability and its low efficacy [101]. Despite mRNA vaccines representing only 11% of all the developed COVID-19 vaccines, two mRNA vaccines, mRNA-1273 and BNT162b, were the first vaccines approved by the FDA and EUA for COVID-19 [102]. Both new mRNA vaccines, BNT162b2, manufactured by Pfizer/BioNTech, and mRNA-1273, produced by Moderna, contain molecules of RNA, modified with pseudo-uridine and encapsulated in a lipid nanoparticle vehicle. The Pfizer–BioNTech and Moderna vaccine constructs do not contain an S-protein S1/S2 furin cleavage site. Ribonucleic acid is endowed to be rapidly translated into nonactive SARS-CoV-2 S proteins in a stable closed structure in order to induce the immune response without causing cell damage due to its interaction with the ACE2 receptor [103]. However, these two vaccines were the most feared among people at the initial stage of vaccination due to the lack of data on their long-term side effects.
The S protein encoded by the vaccine is stabilized in its pre-fusion form; thus, it is possible that, if it enters the bloodstream and is distributed systemically throughout the human body, it may contribute to adverse effects [
104]. Ndeupen et al., reported that the mRNA platform’s lipid nanoparticle (LNP) component used in preclinical vaccine studies causes a highly inflammatory response in mice. LNPs administrated intra-dermally, intramuscularly, or intranasally at a dose of 10 μg/mouse led to severe neutrophil infiltration, the activation of inflammatory pathways, and cytokine and chemokine production [
105]. Such a reaction, in combination with the spike effect, can increase the negative consequences of vaccination in the body.
Among Japanese healthcare workers who were vaccinated with the BNT162b2 mRNA vaccine, alcohol consumption, along with other factors, was identified as a factor predicting lower IgG antibody titers after vaccination [
106]. Wang et al., in their study of vaccinated patients with substance use disorders (SUDs), including alcohol disorders, demonstrated that patients with SUDs remain vulnerable to COVID-19 breakthrough infection, even after full vaccination. The risk was higher in patients who received the Pfizer-BioNTech vaccine than in those who received the Moderna vaccine [
19].
Several cases of myocarditis have been reported following the administration of COVID-19 mRNA vaccines [
107]. After the self-controlled case series, studies found that myocarditis after vaccination is higher in men younger than 40 years old, particularly after the second dose of the mRNA-1273 vaccine [
108]. Excessive alcohol consumption can cause non-ischemic dilated cardiomyopathy and chronic heart disease, characterized by dilation and the impaired contraction of myocardial ventricles [
109]. Of all alcohol-related myocardiopathy cases, 30% were myocarditis with a lymphocytic infiltrate in association with myocyte degeneration or focal necrosis [
110]. Most people who heavily drink alcohol do not have any symptoms in the earlier stages of the disease, and many never develop clinical heart failure [
111]. A case of vasospastic angina (VSA) caused by alcohol consumption following Pfizer/BioNTech vaccination has been reported [
112]. Thus, a patient who chronically drinks alcohol, unaware of the presence of heart problems, could exacerbate them with an injection of the mRNA COVID-19 vaccine. Mark J. Mulligan et al., reported that up to 50% of patients demonstrated a decrease in lymphocytes after the first dose of the BNT162b1 vaccine [
113], which, combined with the negative effect of alcohol on these cells, can have severe consequences for the immune system.
There is no data suggesting that other alcohol-associated chronic illnesses reduce the effectiveness of mRNA vaccines. Patients with compensated and decompensated cirrhosis demonstrated a 100% reduction in COVID-19-related hospitalization or death following the first dose of either the BNT162b2 or the mRNA-1273 vaccines [
114].
8. Conclusions
To date, 24 COVID-19 vaccines have been approved by various institutions in different countries, with more than 100 vaccines undergoing clinical trials and more than 270 currently in pre-clinical development [
115]. Besides the well-known adverse effects associated with antiviral vaccines, cases of severe pathologies and syndromes have been rarely observed among people who have received COVID-19 vaccines. Moreover,
The Lancet reported that 1.3% of the cases processed by the Vaccine Adverse Event Reporting System (VAERS) in the USA were deaths [
116]. Considering the risk of severe COVID-19 and the widespread distribution of vaccinations within a short time span, we can safely say that all these cases are insignificant compared to the benefits of vaccines. At the same time, the currently predominant Omicron strain variants have a reduced risk of severe disease, which, in turn, reduces the advantages of vaccination relative to the disadvantages. It was reported that the repeated use of vaccine boosters induced humoral and cellular tolerance against the Delta and Omicron variants [
117]. There is no direct evidence in the literature indicating that moderate alcohol consumption has any effect on the health of vaccinated patients. However, there are several health conditions associated with alcohol abuse for which vaccination poses additional risks.
At present, the “spike effect” of vaccines and its amplification by alcohol exposure is of most interest. More research is needed to understand the full mechanism of the alcohol-enhanced “spike effect” and to develop appropriate countermeasures to block it. It should also be considered that the chronic and excessive consumption of alcoholic beverages leads to a weakening of the immune system and, as a result, a lower effectiveness of vaccination.