Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yanyan Jiang | -- | 1644 | 2023-01-16 15:20:59 | | | |
| 2 | Dean Liu | -10 word(s) | 1634 | 2023-01-17 04:28:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yang, Y.; Gao, F.; Wang, Y.; Li, H.; Zhang, J.; Sun, Z.; Jiang, Y. Fluorescent Organic Small Molecule Probes. Encyclopedia. Available online: https://encyclopedia.pub/entry/40235 (accessed on 07 February 2026).
Yang Y, Gao F, Wang Y, Li H, Zhang J, Sun Z, et al. Fluorescent Organic Small Molecule Probes. Encyclopedia. Available at: https://encyclopedia.pub/entry/40235. Accessed February 07, 2026.
Yang, Yufei, Fucheng Gao, Yandong Wang, Hui Li, Jie Zhang, Zhiwei Sun, Yanyan Jiang. "Fluorescent Organic Small Molecule Probes" Encyclopedia, https://encyclopedia.pub/entry/40235 (accessed February 07, 2026).
Yang, Y., Gao, F., Wang, Y., Li, H., Zhang, J., Sun, Z., & Jiang, Y. (2023, January 16). Fluorescent Organic Small Molecule Probes. In Encyclopedia. https://encyclopedia.pub/entry/40235
Yang, Yufei, et al. "Fluorescent Organic Small Molecule Probes." Encyclopedia. Web. 16 January, 2023.
Copy Citation
Fluorescence imaging technology provides a visual tool for medicine, showing great potential in the fields of molecular biology, cellular immunology and oncology. In recent years, organic fluorescent probes have attracted much attention in the bioanalytical field. Among various organic fluorescent probes, fluorescent organic small molecule probes (FOSMPs) have become a research hotspot due to their excellent physicochemical properties, such as good photostability, high spatial and temporal resolution, as well as excellent biocompatibility. FOSMPs have proved to be suitable for in vivo bioimaging and detection.
fluorescent organic small molecules
bioimaging
detection
recognition mechanisms
fluorescent organic nanoparticles
1. Introduction
The key substances inside the human body, including metal ions [1], biological small molecules [2][3], reactive oxygen species [4][5][6] and reactive nitrogen [7][8], are closely related to biological events, regulating physiological functions and metabolism. For instance, metal ions (e.g., Fe3+, Na+, Al3+ and Mg2+) play important regulatory roles in metabolism and osmolality [9][10][11]. Biological small molecules, such as homocysteine (Hcy), cysteine (Cys) and glutathione (GSH) participate in metabolism and redox reactions [12][13][14]. The content of reactive oxygen species and reactive nitrogen reflects many pathological states, especially the development of cancer [15][16][17][18]. Therefore, monitoring the levels of these substances is helpful for understanding human health status.
With the development of in vivo imaging technology, more and more tracer techniques have been applied in biomedical research. Traditional tracing methods are usually required to remove tissues from the body, which cannot realize the real-time dynamic monitoring of the target substances. Currently, the commonly used in vivo tracer techniques include radionuclide imaging, magnetic resonance imaging (MRI), optical imaging and so on [19]. Radionuclide imaging can achieve quantitative and localization analysis of cells, but it has the shortcomings of low spatial resolution and the need of expensive equipment [20][21]. MRI with high spatial and temporal resolution enables monitoring of changes in cellular function, but this technique faces the problem of long imaging time [22]. In vivo luminescence imaging is a non-invasive technology, including bioluminescence imaging (BLI) and fluorescence imaging, mainly used to study gene expression and cell activity. Compared with BLI, the signal of fluorescence imaging is stronger and the detection accuracy is higher [23][24][25]. At present, in vivo fluorescence imaging has become a hotspot in biomedical research due to its advantages of low toxicity, high spatiotemporal resolution and utilization of an inexpensive instrument [26][27][28]. For example, fluorescent dye indocyanine green (ICG) imaging has been used in a variety of abdominal surgery applications, such as lymph node localization, ureteral detection and tumor identification [29]. In recent years, some fluorescent probes with high specificity have also been developed and used for in vivo imaging [30][31][32].
To date, many types of fluorescent probes, such as fluorescent organics [33][34][35][36], fluorescent proteins [37], inorganic nanoparticles [38][39] and semiconductor polymer nanoparticles [40][41] have been developed and widely used in bioimaging. Fluorescent proteins can be generated by cells themselves through genetic engineering, which is convenient for in vivo imaging. However, they are hard to metabolize in the body. Inorganic nanoparticles have good spectral properties and light stability, but the biological toxicity limits their application in bioimaging. Semiconductor polymer nanoparticles have high fluorescence brightness, but slow metabolism causes them to accumulate in the liver. Comparatively, fluorescent organic small molecule probes (FOSMPs) attracted more attention because of their controllable synthesis, stable luminescence, good biocompatibility, sensitive response and high signal-to-noise ratio [42][43]. When applied to imaging, the photochemical properties of FOSMPs are generally more stable than other types of probes. Additionally, small molecules also help to achieve higher fluorophore density and spatial resolution. Therefore, a large number of FOSMPs have been designed and applied to detect substances in various matrix [44][45][46][47][48][49][50][51]. Fluorescent organic nanoparticles (FONPs), which were designed with FOSMPs as fluorophores, show the ability to build a multifunctional biosensing platform through surface functionalization and drug encapsulation [52]. A summary of the progress on the applications of FOSMPs in bioimaging and detection is of significance for the development of new diagnostic tools. There are no special reviews focusing on this field.
2. FOSMPs for Bioimaging and Detection
Fluorescence is the phenomenon that electrons return from the first singlet state to the ground state with concomitant energy release in the form of light. The characteristics of the fluorescent probes such as excitation and emission wavelength, intensity, lifetime and polarization are easily influenced by the environment conditions, thereby providing sensitive signals for the tracking and monitoring of analytes [53][54][55][56][57][58]. The structure of FOSMPs is mainly composed of three parts: the recognition group, the fluorophore, and the linking group (linker) (Figure 1). The recognition group endows the fluorescent probe with detection selectivity. The fluorophore provides the signal response when the probe recognizes the target. The linker connects the recognition group and the fluorophore and is not necessary for the fluorescent probe.
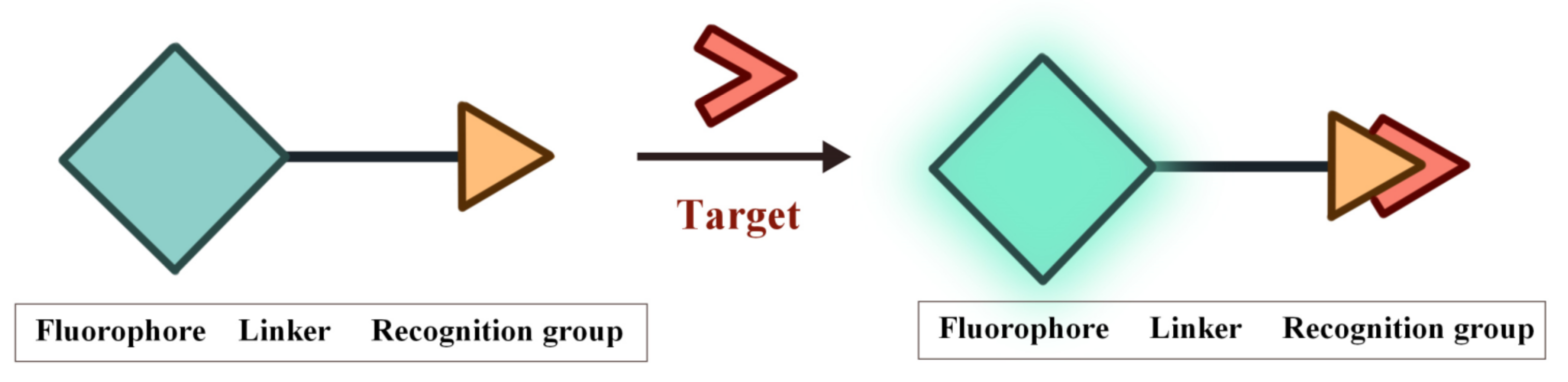
Figure 1. The structure of FOSMPs and its recognition of the target.
As shown in Figure 2, according to the interaction manner between FOSMPs and targets, the recognition mechanisms can be divided into five types: photo-induced electron transfer (PET), intramolecular charge transfer (ICT), fluorescence resonance energy transfer (FRET), excited state intramolecular proton transfer (ESIPT) and aggregation-induced emission (AIE). Based on these recognition mechanisms, various FOSMPs for bioimaging and detection have been developed, as listed in Table 1. In the following, the applications of FOSMPs in bioimaging and analysis based on these recognition mechanisms are reviewed.
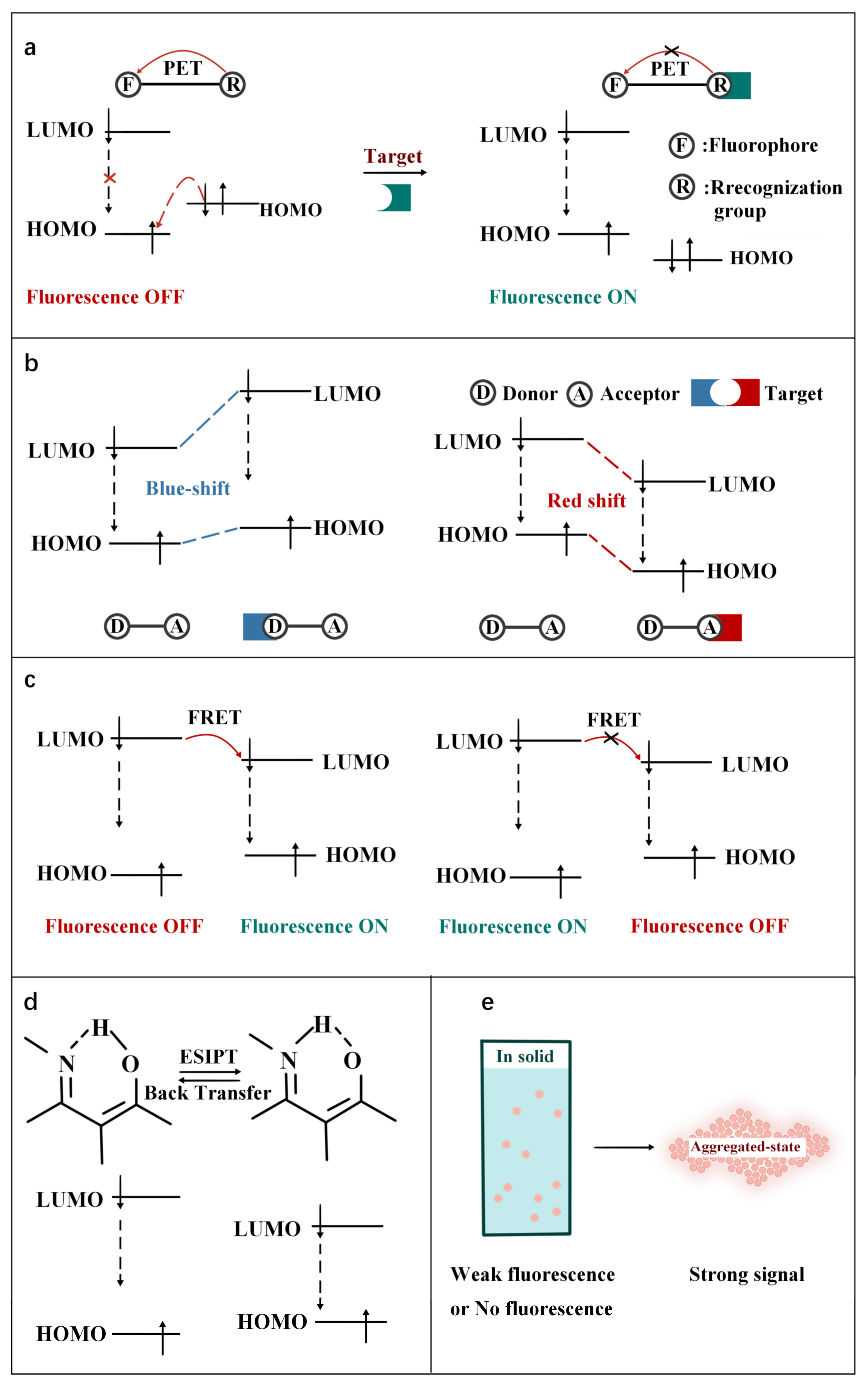
Figure 2. Schematic illustration of the principles of (a) PET, (b) ICT, (c) FRET, (d) ESIPT and (e) AIE.
Table 1. Fluorescence characteristic, recognition mechanisms, and applications of FOSMPs.
| Probe | λex/λem (nm) | Mechanism | Analyte | Solvent System | Linear Detection Range | Limit of Detection (LOD) | Target | Ref. |
|---|---|---|---|---|---|---|---|---|
| Probe | 500/525 | PET | Cys | CH3CN-Water-HEPES buffer | 0–100 μM | 3.7 × 10−2 μM | A375 cells | [59] |
| NP-S | 455/551 | PET | H2S | PBS buffer-EtOH | 30–300 μM | 0.376 μM | Mouse liver slices | [60] |
| Tyro1, Tyro2 | 455/560 | PET | Tyrosinase | Potassium phosphate buffer | - | - | B16F10 cells | [61] |
| NFP-G, NFP-A | 440/541 | PET | Formaldehyde (FA) | DMSO-PBS buffer | 0–30 μM, 0–15 μM | 1.2 μM, 0.18 μM | HepG-2 cells | [62] |
| Naphthalimide chromophore | 400/502 | ICT | CN− | HEPES buffer -CH3OH | 0–15 μM | 0.066 μM | HepG2 cells | [63] |
| DCM-β-gal | 535/685 | ICT | β-galactosidase (β-gal) | PBS buffer-DMSO | 0–12 U/L | 0.17 U/L | 293T cells | [64] |
| DCDHF-Glu | 510/613 | ICT | γ-Glutamyltranspeptidase (GGT) | PBS buffer | 0–40 U/L | 0.0379 U/L | HepG2 cells, LO2 cells | [65] |
| SHC | 370/540 | ICT | hNQO1 | PBS buffer | 0–0.8 μM | 0.0146 μM | HT-29 cells, MDA-MB-468 cells | [66] |
| AI | 370/495 | ICT | Hypochlorous acid (HOCl) |
DMSO-PBS buffer | 0–50 μM | 0.84 μM | HeLa cells, Nude mice | [67] |
| P-ONOO− | 365/480 | ICT | ONOO− | DMSO-PBS buffer | 0.429–3.0 µM | 0.0104 µM | HeLa cells | [68] |
| SR400, SR550 | 400/525, 550/675 | FRET | H2S | PBS buffer | - | - | HEK293 cells | [69] |
| CF | 415/517 | FRET | HNO | PBS buffer | 0–100 μM | 1.4 μM | HeLa cells | [70] |
| FIP-1 | 515/556 | FRET | Fe(II) | HEPES buffer | - | - | HEK293 cells, MDAMB-231 cells | [71] |
| PNCy3Cy5 | 530/660 | FRET | OONO– | Phosphate buffer-DMF | 0–0.7 μM | 6.5 × 10−4 μM | RAW264.7 | [72] |
| FTR-βgal | 450/540 | FRET | β-gal | PBS buffer-EtOH | 0–5 U/L | 4.11 × 10−8 U/L | Hek293 cells | [73] |
| PPA | 400/511 | ESIPT | Palladium | CH3CN-PBS buffer | 0–180 μM | 0.028 μM | A549 cells | [74] |
| Py-GSH | 488/545 | ESIPT | GGT | PBS buffer | 1–30 U/L | 1 × 10−2 U/L | SKOV3 cells, HOSEpiC cells, Tumor-bearing mice, Human specimens |
[75] |
| NIR-TS | 550/836 | ESIPT | SO2 | Water | 0.5–40 μM | 0.067 μM | HeLa cells, Mice | [76] |
| TPE-Gal | 344/512 | AIE | β-gal | PBS buffer | 8 × 10−4–4.8 × 10−3 U/L | 3.3 × 10−4 U/L | HeLa cells, OVCAR-3 cells | [77] |
| TT | 320/440–550 | AIE | H2O2 | DMSO-Water | - | - | RAW264.7 cells, HLF cells | [78] |
| QP-DNP | 482/582 | AIE | Hydrazine | DMSO-HEPES buffer | 0–0.8 μM | 0.055 μM | HeLa cells, Kunming mouse | [79] |
| AIE-Lyso-1 | 356/532 | AIE/ESIPT | Esterase | DMSO–Water | 100–500 U/L | 2.4 U/L | MCF-7 cells | [80] |
| Probe 1 | 314/446 | PET/ICT | Cys | PBS buffer | 0.2–2.0 μM | 1.4 × 10−3 μM | HeLa cells | [81] |
| NCQ | 423/490, 544 423/490423/490, 624 |
Triple-emission | Cys/Hcy, GSH/H2S, thiopheno(PhSH) |
PBS buffer-acetonitrile | 0–10 μM/10 μM, 0–6 μM/8 μM, 0–70 μM |
0.57 μM/0.65 μM, 0.49 μM/0.52 μM, 0.34 μM |
HeLa cells | [82] |
3. FOSMPs-Based FONPs for Bioimaging and Detection
Compared with FOSMPs, FONPs have larger surface area, better biodegradability and greater resistance to photobleaching [83][84]. FONPs-based multifunctional biodetection platforms can be constructed through drug encapsulation and surface modification of targeting groups. Researchers briefly describes the preparation and applications of FOSMPs-based FONPs in bioimaging and detection.
Zhang et al. [85] reported a strategy to prepare FONPs by encapsulating the fluorophore C18-R in synthetic copolymer matrices. First, the copolymer aqueous solution was added to the C18-Rd THF solution under sonication. In the process of removing THF, the hydrophobic segment of the copolymer wrapped C18-R through hydrophobic interaction to form a “core-shell” structure, and finally the C18-R-PEG FONPs were obtained. CLSM images and cell uptake experiments proved that the C18-R-PEG FONPs has good biocompatibility and can be used for bioimaging.
Enhanced fluorescence emission can be obtained by loading high concentrations of fluorophores into FONPs since AIE can occur at high concentrations of fluorophores. Zhang et al. [86] prepared near-infrared emitting AIE dots with a particle size of ~20 nm and a fluorescence quantum efficiency of 20% by using the amphiphilic polymer poly (styrene co maleic anhydride) (PSMA) as the co-encapsulation matrix and a novel small-molecule fluorophore (2Z,2′Z)-3,3′-(2,5-di(piperidin-1-yl)-1,4-phenylene)bis(2-phenylacrylonitrile) (DPPBPA) as the core. The final product SA dots were obtained by modifying streptavidin on its surface to achieve specific binding to target cells. SA dots have been successfully used for fluorescence imaging of MCF-7 cell line benefit from their uniform size, stable luminescence and excellent biocompatibility.
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(polyethylene glycol) (DSPE-PEG2000) is a typical package matrix that is widely used in the design of FONPs due to its good biocompatibility [87][88][89][90]. Transcription-AIE (Tat-AIE) dots prepared using DSPE-PEG2000 as the matrix was first reported as cell-tracing probes in 2013, showing brighter fluorescence, better fluorescence stability and cell-tracking ability than commercial quantum dot-based probes. Li et al. [88] encapsulated the fluorophore TPETPAFN by using a mixture of DSPE-PEG2000 and DSPE-PEG2000-NH2. Then, Tat-AIE dots were obtained by coupling AIE dots with cell penetrating peptide HIV-1 Tat. TPETPAFN has poor water solubility but is easily soluble in THF solution. The fluorescence of TPETPAFN was turned on when the THF/water volume ratio was 1:1 and the fluorescence intensity increased exponentially with the increase of the ratio of water. The fluorescence intensity of TPETPAFN showed a 70-fold enhancement at a 90% water volume fraction. The hydrodynamic size and quantum yield of the as-prepared NIR-emitting Tat-AIE dots were ~30 nm and 24%, respectively. Compared to commercial Qtracker® 655, Tat-AIE dots displayed 10-fold stronger fluorescence intensity and better long-term tracing ability. Tat-AIE dots could trace MCF-7 cells for 10–12 generations and trace C6 cells for 21 days in vivo.
References
- Hao, J.; Yan, B. A water-stable lanthanide-functionalized MOF as a highly selective and sensitive fluorescent probe for Cd2+. Chem. Commun. 2015, 51, 7737–7740.
- Donia, M.S.; Fischbach, M.A. Small molecules from the human microbiota. Science 2015, 349, 1254766.
- Fukuto, J.M.; Carrington, S.J.; Tantillo, D.J.; Harrison, J.G.; Ignarro, L.J.; Freeman, B.A.; Chen, A.; Wink, D.A. Small Molecule Signaling Agents: The Integrated Chemistry and Biochemistry of Nitrogen Oxides, Oxides of Carbon, Dioxygen, Hydrogen Sulfide, and Their Derived Species. Chem. Res. Toxicol. 2012, 25, 769–793.
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.; Harrison, D.G.; Bhatnagar, A.; Amer, H.A.C.B. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 119, E39–E75.
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-Molecule Fluorescent Probes for Imaging and Detection of Reactive Oxygen, Nitrogen, and Sulfur Species in Biological Systems. Anal. Chem. 2018, 90, 533–555.
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257.
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.B.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a Source of Reactive Oxygen and Nitrogen Species: From Molecular Mechanisms to Human Health. Antioxid. Redox Sign. 2013, 18, 2029–2074.
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Bba.-Mol. Basis Dis. 2012, 1822, 1363–1373.
- Mehta, V.N.; Desai, M.L.; Basu, H.; Kumar Singhal, R.; Kailasa, S.K. Recent developments on fluorescent hybrid nanomaterials for metal ions sensing and bioimaging applications: A review. J. Mol. Liq. 2021, 333, 115950.
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe(II) Redox Chemistry in the Environment. Chem. Rev. 2021, 121, 8161–8233.
- Kim, S.; Noh, J.Y.; Kim, K.Y.; Kim, J.H.; Kang, H.K.; Nam, S.; Kim, S.H.; Park, S.; Kim, C.; Kim, J. Salicylimine-Based Fluorescent Chemosensor for Aluminum Ions and Application to Bioimaging. Inorg. Chem. 2012, 51, 3597–3602.
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78.
- Skovierova, H.; Vidomanova, E.; Mahmood, S.; Sopkova, J.; Drgova, A.; Cervenova, T.; Halasova, E.; Lehotsky, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733.
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radical Bio. Med. 2015, 80, 148–157.
- Dao, N.V.; Ercole, F.; Urquhart, M.C.; Kaminskas, L.M.; Nowell, C.J.; Davis, T.P.; Sloan, E.K.; Whittaker, M.R.; Quinn, J.F. Trisulfide linked cholesteryl PEG conjugate attenuates intracellular ROS and collagen-1 production in a breast cancer co-culture model. Biomater. Sci. UK 2021, 9, 835–846.
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016.
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001.
- Van-Nghia, N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent developments of BODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coordin. Chem. Rev. 2021, 439, 213936.
- Ogawa, M.; Takakura, H. In Vivo Molecular Imaging for Biomedical Analysis and Therapies. Anal. Sci. 2018, 34, 273–281.
- Peng, F. Recent Advances in Cancer Imaging with (CuCl2)-Cu-64 PET/CT. Nucl. Med. Mol. Imaging. 2022, 56, 80–85.
- Yan, Y.; Wang, H.; Zhao, Y. Radiolabeled peptide probe for tumor imaging. Chinese Chem. Lett. 2022, 33, 3361–3370.
- Seetharam Bhat, K.R.; Samavedi, S.; Moschovas, M.C.; Onol, F.F.; Roof, S.; Rogers, T.; Patel, V.R.; Sivaraman, A. Magnetic resonance imaging-guided prostate biopsy—A review of literature. Asian J. Urol. 2021, 8, 105–116.
- Kitada, N.; Maki, S.; Kim, S. Visible Light Bioluminescence Imaging Platform for Animal Cell Imaging. Methods Mol. Biol. 2022, 2524, 37–51.
- Komatsu, H.; Kobayashi, E.; Gonzalez, N.; Rawson, J.; Ortiz, J.; Donohue, C.; Ku, H.T.; Kandeel, F.; Mullen, Y. Critical Considerations in Bioluminescence Imaging of Transplanted Islets Dynamic Signal Change in Early Posttransplant Phase and Signal Absorption by Tissues. Pancreas 2022, 51, 234–242.
- Sato, K. Bioluminescence Imaging for Evaluation of Antitumor Effect In Vitro and In Vivo in Mice Xenografted Tumor Models. Methods Mol. Biol. 2022, 2524, 307–315.
- Pan, X.; Gao, A.; Lin, Z. Fluorescence imaging of tumor immune contexture in immune checkpoint blockade therapy. Int. Immunopharmacol. 2022, 106, 108617.
- Wu, X.; Li, H.; Lee, E.; Yoon, J. Sensors for In Situ Real-Time Fluorescence Imaging of Enzymes. Chem 2020, 6, 2893–2901.
- Zhu, H.; Hamachi, I. Fluorescence imaging of drug target proteins using chemical probes. J. Pharm. Sci. 2020, 10, 426–433.
- van Manen, L.; Handgraaf, H.J.M.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018, 118, 283–300.
- Wang, J.; Li, C.; Jiang, G. Progress in Fluorescent Probes for Aminopeptidase N. Chem. Eur. J. 2018, 81, 972–980.
- Xu, Y.; Liu, R.; Xu, K.; Dai, Z. Fluorescent Probes for Intraoperative Navigation. Prog. Chem. 2021, 33, 52–65.
- Yagishita, A.; Ueno, T.; Tsuchihara, K.; Urano, Y. Amino BODIPY-Based Blue Fluorescent Probes for Aldehyde Dehydrogenase 1-Expressing Cells. Bioconjugate Chem. 2021, 32, 234–238.
- Han, H.; Zhong, Y.; He, C.; Fu, L.; Huang, Q.; Kuang, Y.; Yi, X.; Zeng, W.; Zhong, H.; Yang, M. Recent advances in organic fluorescent probes for tumor related enzyme detection. Dyes Pigm. 2022, 204, 110386.
- Pak, Y.L.; Swamy, K.; Yoon, J. Recent Progress in Fluorescent Imaging Probes. Sensors 2015, 15, 24374–24396.
- Shaya, J.; Corridon, P.R.; Al-Omari, B.; Aoudi, A.; Shunnar, A.; Mohideen, M.I.H.; Qurashi, A.; Michel, B.Y.; Burger, A. Design, photophysical properties, and applications of fluorene-based fluorophores in two-photon fluorescence bioimaging: A review. J. Photoch. Photobio. C. 2022, 52, 100529.
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521.
- Balleza, E.; Kim, J.M.; Cluzel, P. Systematic characterization of maturation time of fluorescent proteins in living cells. Nat. Methods 2018, 15, 47.
- Han, Y.; Wang, X.; Li, S. Biocompatible Europium Doped Hydroxyapatite Nanoparticles as a Biological Fluorescent Probe. Curr. Nanosci. 2010, 6, 178–183.
- Shore, A. A review on fluorescent inorganic nanoparticles for optical sensing applications (vol 6, pg 21624, 2016). RSC Adv. 2019, 9, 16565.
- Wu, C.; Chiu, D.T. Highly Fluorescent Semiconducting Polymer Dots for Biology and Medicine. Angew. Chem. Int. Edit. 2013, 52, 3086–3109.
- Zhang, J.; Huang, Y.; Wang, D.; Pollard, A.C.; Chen, Z.G.; Egap, E. Triblock near-infrared fluorescent polymer semiconductor nanoparticles for targeted imaging. J. Mater. Chem. C. 2017, 5, 5685–5692.
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984.
- Gu, B.; Zhang, Q. Recent Advances on Functionalized Upconversion Nanoparticles for Detection of Small Molecules and Ions in Biosystems. Adv. Sci. 2018, 5, 1700609.
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K. A review of the applications of Schiff bases as optical chemical sensors. TrAC, Trends Anal. Chem. 2019, 116, 74–91.
- Immanuel David, C.; Prabakaran, G.; Nandhakumar, R. Recent approaches of 2HN derived fluorophores on recognition of Al3+ ions: A review for future outlook. Microchem. J. 2021, 169, 106590.
- Zoubi, W.A. Biological Activities of Schiff Bases and Their Complexes: A Review of Recent Works. Int. J. Org. Chem. 2013, 3, 24.
- Han, X.; Wang, Y.; Huang, Y.; Wang, X.; Choo, J.; Chen, L. Fluorescent probes for biomolecule detection under environmental stress. J. Hazard. Mater. 2022, 431, 128527.
- Zhang, Y.; Hao, Y.; Ma, X.; Chen, S.; Xu, M. A dicyanoisophorone-based highly sensitive and selective near-infrared fluorescent probe for sensing thiophenol in water samples and living cells. Environ. Pollut. 2020, 265, 114958.
- Lai, X.; Qiu, G.; Ye, Q.; Wang, R.; Liu, J. A reaction-based fluorescent probe for detecting o-phenylenediamine in water and lateritic soil samples. J. Photochem. Photobiol. A. 2020, 386, 112101.
- Suratsawadee, A.; Wangmo, L.; Ratvijitvech, T.; Siripinyanond, A. A spoilage indicator card based on distance-based color change of paper impregnated with acid-base indicator for freshness monitoring of shrimp. Microchem. J. 2022, 175, 107110.
- Zhong, K.; Zhou, S.; Yan, X.; Li, X.; Hou, S.; Cheng, L.; Gao, X.; Li, Y.; Tang, L. A simple H2S fluorescent probe with long wavelength emission: Application in water, wine, living cells and detection of H2S gas. Dyes Pigment. 2020, 174, 108049.
- Choudhury, P.; Das, P.K. Progress and trends in self-assembly driven fluorescent organic nanoparticles: A brief overview. J. Indian Chem. Soc. 2021, 98, 100123.
- Li, J.; Yim, D.; Jang, W.; Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 2017, 46, 2437–2458.
- Liu, Z.; Zhou, X.; Miao, Y.; Hu, Y.; Kwon, N.; Wu, X.; Yoon, J. A Reversible Fluorescent Probe for Real-Time Quantitative Monitoring of Cellular Glutathione. Angew. Chem. Int. Edit. 2017, 56, 5812–5816.
- Niu, L.; Chen, Y.; Zheng, H.; Wu, L.; Tung, C.; Yang, Q. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44, 6143–6160.
- Park, S.; Kwon, N.; Lee, J.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179.
- Wang, Y.; Zhang, L.; Han, X.; Zhang, L.; Wang, X.; Chen, L. Fluorescent probe for mercury ion imaging analysis: Strategies and applications. Chem. Eng. J. 2021, 406, 127166.
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-Based Fluorescent Probes for Biothiols. Chem.-Eur. J. 2020, 26, 4172–4192.
- Fan, W.; Huang, X.; Shi, X.; Wang, Z.; Lu, Z.; Fan, C.; Bo, Q. A simple fluorescent probe for sensing cysteine over homocysteine and glutathione based on PET. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 918–923.
- Xu, K.; He, L.; Yang, Y.; Lin, W. A PET-based turn-on fluorescent probe for sensitive detection of thiols and H2S and its bioimaging application in living cells, tissues and zebrafish. New J. Chem. 2019, 43, 2865–2869.
- Kumar, P.; Biswas, S.; Koner, A.L. Fast tyrosinase detection in early-stage melanoma with nanomolar sensitivity using a naphthalimide-based fluorescent read-out probe. New J. Chem. 2020, 44, 10771–10775.
- Zhou, L.; Cui, J.; Yu, Z.; Zou, D.; Zhang, W.; Qian, J. A β-d-galactose-guided fluorescent probe for selectively bioimaging endogenous formaldehyde in living HepG-2 cells. Sens. Actuators B Chem. 2021, 332, 129494.
- Xiong, K.; Huo, F.; Yin, C.; Yang, Y.; Chao, J.; Zhang, Y.; Xu, M. A off–on green fluorescent chemosensor for cyanide based on a hybrid coumarin–hemicyanine dye and its bioimaging. Sens. Actuators B Chem. 2015, 220, 822–828.
- Gu, K.; Xu, Y.; Li, H.; Guo, Z.; Zhu, S.; Zhu, S.; Shi, P.; James, T.D.; Tian, H.; Zhu, W. Real-Time Tracking and In Vivo Visualization of β-Galactosidase Activity in Colorectal Tumor with a Ratiometric Near-Infrared Fluorescent Probe. J. Am. Chem. Soc. 2016, 138, 5334–5340.
- Liu, F.; Wang, Z.; Wang, W.; Luo, J.; Kong, L. Red-Emitting Fluorescent Probe for Detection of γ-Glutamyltranspeptidase and Its Application of Real-Time Imaging under Oxidative Stress in Cells and in Vivo. Anal. Chem. 2018, 90, 7467–7473.
- Cho, M.K.; Lim, C.S.; Sarkar, A.R.; Lee, H.W.; Choi, H.J.; Noh, C.; Shin, S.J.; Kim, H.M. A two-photon ratiometric probe for detection of hNQO1 enzyme activity in human colon tissue. Sens. Actuators Chem. 2018, 272, 203–210.
- Shi, Y.; Huo, F.; Yin, C. Malononitrile as the ‘double-edged sword’ of passivation-activation regulating two ICT to highly sensitive and accurate ratiometric fluorescent detection for hypochlorous acid in biological system. Sens. Actuators Chem. 2020, 325, 128793.
- Yu, H.; Fang, Y.; Wang, J.; Zhang, Q.; Chen, S.; Wang, K.; Hu, Z. Enhancing probe’s sensitivity for peroxynitrite through alkoxy modification of dicyanovinylchromene. Anal. Bioanal. Chem. 2022, 414, 6779–6789.
- Wei, L.; Yi, L.; Song, F.; Wei, C.; Wang, B.; Xi, Z. FRET ratiometric probes reveal the chiral-sensitive cysteine-dependent H2S production and regulation in living cells. Sci. Rep. UK 2014, 4, 4521.
- Zhang, H.; Liu, R.; Tan, Y.; Xie, W.H.; Lei, H.; Cheung, H.; Sun, H. A FRET-based Ratiometric Fluorescent Probe for Nitroxyl Detection in Living Cells. ACS Appl. Mater. Inter. 2015, 7, 5438–5443.
- Aron, A.T.; Loehr, M.O.; Bogena, J.; Chang, C.J. An Endoperoxide Reactivity-Based FRET Probe for Ratiometric Fluorescence Imaging of Labile Iron Pools in Living Cells. J. Am. Chem. Soc. 2016, 138, 14338–14346.
- Jia, X.; Chen, Q.; Yang, Y.; Tang, Y.; Wang, R.; Xu, Y.; Zhu, W.; Qian, X. FRET-Based Mito-Specific Fluorescent Probe for Ratiometric Detection and Imaging of Endogenous Peroxynitrite: Dyad of Cy3 and Cy5. J. Am. Chem. Soc. 2016, 138, 10778–10781.
- Wei, X.; Hu, X.; Zhang, L.; Li, J.; Wang, J.; Wang, P.; Song, Z.; Zhang, J.; Yan, M.; Yu, J. Highly selective and sensitive FRET based ratiometric two-photon fluorescent probe for endogenous β-galactosidase detection in living cells and tissues. Microchem. J. 2020, 157, 105046.
- Shen, Y.; Zhang, X.; Wu, Y.; Zhang, Y.; Liu, X.; Chen, Y.; Li, H.; Zhong, Y. A lysosome targetable fluorescent probe for palladium species detection base on an ESIPT phthalimide derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 66–71.
- Zhou, X.; Liu, Y.; Liu, Q.; Yan, L.; Xue, M.; Yuan, W.; Shi, M.; Feng, W.; Xu, C.; Li, F. Point-of-care Ratiometric Fluorescence Imaging of Tissue for the Diagnosis of Ovarian Cancer. Theranostics 2019, 9, 4597–4607.
- Ren, H.; Huo, F.; Wu, X.; Liu, X.; Yin, C. An ESIPT-induced NIR fluorescent probe to visualize mitochondrial sulfur dioxide during oxidative stress in vivo. Chem. Commun. 2021, 57, 655–658.
- Jiang, G.; Zeng, G.; Zhu, W.; Li, Y.; Dong, X.; Zhang, G.; Fan, X.; Wang, J.; Wu, Y.; Tang, B.Z. A selective and light-up fluorescent probe for β-galactosidase activity detection and imaging in living cells based on an AIE tetraphenylethylene derivative. Chem. Commun. 2017, 53, 4505–4508.
- Cheng, Y.; Dai, J.; Sun, C.; Liu, R.; Zhai, T.; Lou, X.; Xia, F. An Intracellular H2O2-Responsive AIEgen for the Peroxidase-Mediated Selective Imaging and Inhibition of Inflammatory Cells. Angew. Chem. Int. Ed. 2018, 57, 3123–3127.
- Muthusamy, S.; Yin, S.; Rajalakshmi, K.; Meng, S.; Zhu, D.; Xie, M.; Xie, J.; Lodi, R.S.; Xu, Y. Development of a quinoline-derived turn-on fluorescent probe for real time detection of hydrazine and its applications in environment and bioimaging. Dyes Pigm. 2022, 206, 110618.
- Gao, M.; Hu, Q.; Feng, G.; Tang, B.Z.; Liu, B. A fluorescent light-up probe with “AIE + ESIPT” characteristics for specific detection of lysosomal esterase. J. Mater. Chem. B. 2014, 2, 3438–3442.
- Chen, X.; Xu, H.; Ma, S.; Tong, H.; Lou, K.; Wang, W. A simple two-photon turn-on fluorescent probe for the selective detection of cysteine based on a dual PeT/ICT mechanism. RSC Adv. 2018, 8, 13388–13392.
- Yang, L.; Su, Y.; Geng, Y.; Zhang, Y.; Ren, X.; He, L.; Song, X. A Triple-Emission Fluorescent Probe for Discriminatory Detection of Cysteine/Homocysteine, Glutathione/Hydrogen Sulfide, and Thiophenol in Living Cells. ACS Sens. 2018, 3, 1863–1869.
- Ning, Z.; Wu, S.; Liu, G.; Ji, Y.; Jia, L.; Niu, X.; Ma, R.; Zhang, Y.; Xing, G. Water-soluble AIE-Active Fluorescent Organic Nanoparticles: Design, Preparation and Application for Specific Detection of Cysteine over Homocysteine and Glutathione in Living Cells. Chem.-Asian J. 2019, 14, 2220–2224.
- Yan, L.; Zhang, Y.; Xu, B.; Tian, W. Fluorescent nanoparticles based on AIE fluorogens for bioimaging. Nanoscale 2016, 8, 2471–2487.
- Zhang, X.; Zhang, X.; Yang, B.; Zhang, Y.; Liu, M.; Liu, W.; Chen, Y.; Wei, Y. Fabrication of water-dispersible and biocompatible red fluorescent organic nanoparticles via PEGylation of aggregate induced emission enhancement dye and their cell imaging applications. Colloids Surf. B. 2014, 113, 435–441.
- Zhang, Y.; Chang, K.; Xu, B.; Chen, J.; Yan, L.; Ma, S.; Wu, C.; Tian, W. Highly efficient near-infrared organic dots based on novel AEE fluorogen for specific cancer cell imaging. RSC Adv. 2015, 5, 36837–36844.
- Zhao, Q.; Li, K.; Chen, S.; Qin, A.; Ding, D.; Zhang, S.; Liu, Y.; Liu, B.; Sun, J.Z.; Tang, B.Z. Aggregation-induced red-NIR emission organic nanoparticles as effective and photostable fluorescent probes for bioimaging. J. Mater. Chem. 2012, 22, 15128–15135.
- Li, K.; Qin, W.; Ding, D.; Tomczak, N.; Geng, J.; Liu, R.; Liu, J.; Zhang, X.; Liu, H.; Liu, B. Photostable fluorescent organic dots with aggregation-induced emission (AIE dots) for noninvasive long-term cell tracing. Sci. Rep. UK. 2013, 24, 635–643.
- Geng, J.; Zhu, Z.; Qin, W.; Ma, L.; Hu, Y.; Gurzadyan, G.G.; Tang, B.Z.; Liu, B. Near-infrared fluorescence amplified organic nanoparticles with aggregation-induced emission characteristics for in vivo imaging. Nanoscale 2014, 6, 939–945.
- Feng, G.; Tay, C.Y.; Chui, Q.X.; Liu, R.; Tomczak, N.; Liu, J.; Tang, B.Z.; Leong, D.T.; Liu, B. Ultrabright organic dots with aggregation-induced emission characteristics for cell tracking. Biomaterials 2014, 35, 8669–8677.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
17 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




