
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Allison B. Reiss | -- | 2981 | 2022-06-14 19:34:52 | | | |
| 2 | Steven H. Rauchman | + 8 word(s) | 2989 | 2022-06-14 19:41:02 | | | | |
| 3 | Conner Chen | -8 word(s) | 2981 | 2022-06-15 04:53:08 | | | | |
| 4 | Allison B. Reiss | + 9 word(s) | 2990 | 2022-06-15 23:00:43 | | |
Video Upload Options
Traumatic Brain Injury (TBI) is a major global public health problem. Neurological damage from TBI may be mild, moderate, or severe and occurs both immediately at the time of impact (primary injury) and continues to evolve afterwards (secondary injury). In mild (m)TBI, common symptoms are headaches, dizziness, and fatigue. Visual impairment is especially prevalent. Insomnia, attentional deficits and memory problems often occur. While symptoms resolve spontaneously in many, residual effects may linger for months or years in some mTBI patients. Optimally, the goal of any intervention is a return to baseline uninjured functioning with restoration of the ability to conduct daily activities.
1. Introduction
2. Basics of TBI
2.1. Detemining Severity
2.2. Initial Treatment
2.3. Diagnostic Issues in mTBI
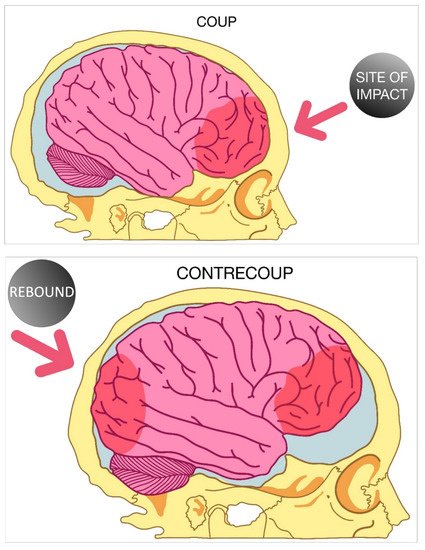
3. Focal and Diffuse Injury
4. Brain Imaging Techniques in TBI
| Classification | Scoring | Key Features |
|---|---|---|
| Marshall (1992) [53] | Diffuse Injury I to Diffuse Injury VI |
Diffuse injury I—No visible intracranial pathology on CT. Progresses up to Diffuse Injury VI with high or mixed density lesion > 25 mL not surgically evacuated. Evaluates perimesencephalic cisterns, midline shift, and presence of a mass lesion. |
| Rotterdam (2006) [54] | 1 to 6 | 4 scored elements: basal cistern compression status; degree of midline shift; epidural hematomas, intraventricular and/or subarachnoid hemorrhage. Differentiates between types of mass lesions, recognizes more favorable prognosis for epidural hematomas. |
| Stockholm (2010) [55] | Traumatic subarachnoid hemorrhage score Range: (0 to 6) |
Builds on Marshall and Rotterdam. Adds separate scoring for traumatic subarachnoid hemorrhage. Magnitude of midline shift used as a continuous variable (not dichotomous) for prediction of favorable or unfavorable outcome. Incorporates diffuse axonal injury. |
| Helsinki (2014) [56] | −3 to 14 | Refined to include type of mass lesion (subdural, intracerebral or epidural hematoma. Intraventricular hemorrhage as a predictor of outcome. Includes suprasellar cisterns status (normal, compressed, obliterated). |
| NeuroImaging Radiological Interpretation System (NIRIS) (2018) [57] | NIRIS 0 to NIRIS 4 |
Score gives management guidance: NIRIS 0—patients typically discharged, NIRIS 1—follow-up neuroimaging and/or hospital admission, NIRIS 2—admission to an advanced care unit, NIRIS 3—neurosurgical intervention, NIRIS 4—high likelihood of fatal outcome from TBI. |
5. Visual Symptoms of TBI
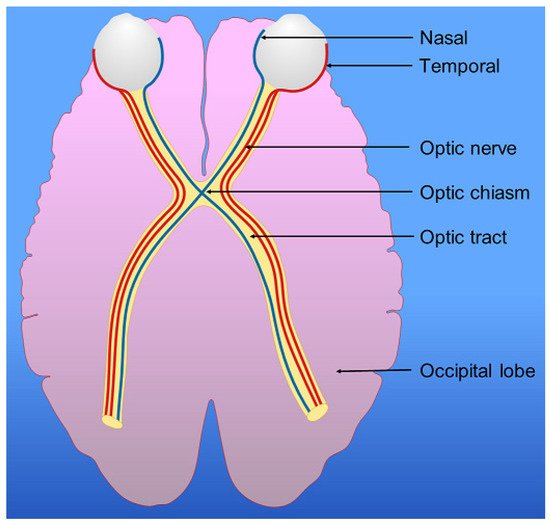
6. Visual Pathway, Parietal Lobes and Vision
7. Conclusions
Large areas of the brain are involved in visual processing and this makes the visual system vulnerable to damage from mTBI. Early and thorough evaluation is important for detection of dysfunction and documentation of recovery or persistence of oculomotor, visual and other symptoms that may interfere with multiple aspects of everyday life. A pro-active approach to treatment involving a rehabilitation program may be preferable to the standard recommendation of rest after mTBI. Avoiding further head trauma is crucial and inter-disciplinary collaboration in research is needed to improve treatment options and maintain functional abilities.
Acknowledgement: Original art in figures by Samantha M. Steiner
References
- Iaccarino, C.; Carretta, A.; Nicolosi, F.; Morselli, C. Epidemiology of severe traumatic brain injury. J. Neurosurg. Sci. 2018, 62, 535–541.
- Centers for Disease Control and Prevention. National Center for Health Statistics: Mortality Data on CDC WONDER. Available online: https://wonder.cdc.gov/mcd.html (accessed on 20 April 2022).
- Savitsky, B.; Givon, A.; Rozenfeld, M.; Radomislensky, I.; Peleg, K. Traumatic brain injury: It is all about definition. Brain Inj. 2016, 30, 1194–1200.
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097.
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16.
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent advances in traumatic brain injury. J. Neurol. 2019, 266, 2878–2889.
- Bonow, R.H.; Barber, J.; Temkin, N.R.; Videtta, W.; Rondina, C.; Petroni, G.; Lujan, S.; Alanis, V.; La Fuente, G.; Lavadenz, A.; et al. The outcome of severe traumatic brain injury in Latin America. World Neurosurg. 2018, 111, e82–e90.
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197.
- McAllister, T.W.; Flashman, L.A.; McDonald, B.C.; Saykin, A.J. Mechanisms of working memory dysfunction after mild and moderate TBI: Evidence from functional MRI and neurogenetics. J. Neurotrauma 2006, 23, 1450–1467.
- Cassidy, J.D.; Cancelliere, C.; Carroll, L.J.; Côté, P.; Hincapié, C.A.; Holm, L.W.; Hartvigsen, J.; Donovan, J.; Boussard, C.N.-D.; Kristman, V.L.; et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: Results of the international collaboration on mild traumatic brain injury prognosis. Arch. Phys. Med. Rehabil. 2014, 95, S132–S151.
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640.
- Feddermann-Demont, N.; Echemendia, R.J.; Schneider, K.J.; Solomon, G.S.; Hayden, K.A.; Turner, M.; Dvořák, J.; Straumann, D.; Tarnutzer, A.A. What domains of clinical function should be assessed after sport-related concussion? A systematic review. Br. J. Sports Med. 2017, 51, 903–918.
- Darshini, J.K.; Afsar, M.; Vandana, V.P.; Shukla, D.; Rajeswaran, J. The Triad of Cognition, Language, and Communication in Traumatic Brain Injury: A Correlational Study. J. Neurosci. Rural Pract. 2021, 12, 666–672.
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238.
- Teasdale, G.; Maas, A.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The Glasgow coma scale at 40 years: Standing the test of time. Lancet Neurol. 2014, 13, 844–854.
- Mena, J.H.; Sanchez, A.I.; Rubiano, A.M.; Peitzman, A.B.; Sperry, J.L.; Gutierrez, M.I.; Puyana, J.C. Effect of the modified Glasgow Coma Scale score criteria for mild traumatic brain injury on mortality prediction: Comparing classic and modified Glasgow Coma Scale score model scores of 13. J. Trauma 2011, 71, 1185–1193.
- Chung, P.; Khan, F. Traumatic Brain Injury (TBI): Overview of Diagnosis and Treatment. J. Neurol. Neurophysiol. 2013, 5, 1.
- Davanzo, J.R.; Sieg, E.P.; Timmons, S.D. Management of Traumatic Brain Injury. Surg. Clin. N. Am. 2017, 97, 1237–1253.
- Abdelmalik, P.A.; Draghic, N.; Ling, G.S.F. Management of moderate and severe traumatic brain injury. Transfusion 2019, 59, 1529–1538.
- Taran, S.; Pelosi, P.; Robba, C. Optimizing oxygen delivery to the injured brain. Curr. Opin. Crit. Care 2022, 28, 145–156.
- Khan, R.; Alromaih, S.; Alshabanat, H.; Alshanqiti, N.; Aldhuwaihy, A.; Almohanna, S.A.; Alqasem, M.; Al-Dorzi, H. The Impact of Hyperoxia Treatment on Neurological Outcomes and Mortality in Moderate to Severe Traumatic Brain Injured Patients. J. Crit. Care Med. (Targu Mures) 2021, 7, 227–236.
- Laskowski, R.A.; Creed, J.A.; Raghupathi, R. Pathophysiology of Mild TBI: Implications for Altered Signaling Pathways. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015.
- Skandsen, T.; Nilsen, T.L.; Einarsen, C.; Normann, I.; McDonagh, D.; Haberg, A.K.; Vik, A. Incidence of Mild Traumatic Brain Injury: A Prospective Hospital, Emergency Room and General Practitioner-Based Study. Front. Neurol. 2019, 10, 638.
- Forrest, R.H.; Henry, J.D.; McGarry, P.J.; Marshall, R.N. Mild traumatic brain injury in New Zealand: Factors influencing post-concussion symptom recovery time in a specialised concussion service. J. Prim. Health Care 2018, 10, 159–166.
- McCrory, P.; Meeuwisse, W.H.; Aubry, M.; Cantu, R.C.; Dvořák, J.; Echemendia, R.J.; Engebretsen, L.; Johnston, K.M.; Kutcher, J.S.; Raftery, M.; et al. Consensus statement on concussion in sport—The 4th International Conference on Concussion in Sport held in Zurich, November 2012. PM R 2013, 5, 255–279.
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282.
- Shepherd, D.; Landon, J.; Kalloor, M.; Barker-Collo, S.; Starkey, N.; Jones, K.; Ameratunga, S.; Theadom, A.; BIONIC Research Group. The association between health-related quality of life and noise or light sensitivity in survivors of a mild traumatic brain injury. Qual. Life Res. 2020, 29, 665–672.
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9.
- Andriessen, T.M.; Jacobs, B.; Vos, P.E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010, 14, 2381–2392.
- McGinn, M.J.; Povlishock, J.T. Pathophysiology of Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 397–407.
- Bigler, E.D. Anterior and middle cranial fossa in traumatic brain injury: Relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology 2007, 21, 515–531.
- Yue, J.K.; Winkler, E.A.; Puffer, R.C.; Deng, H.; Phelps, R.; Wagle, S.; Morrissey, M.R.; Rivera, E.J.; Runyon, S.J.; Vassar, M.J.; et al. The Track-Tbi Investigators Temporal lobe contusions on computed tomography are associated with impaired 6-month functional recovery after mild traumatic brain injury: A TRACK-TBI study. Neurol. Res. 2018, 40, 972–981.
- Cipolotti, L.; MacPherson, S.E.; Gharooni, S.; van-Harskamp, N.; Shallice, T.; Chan, E.; Nachev, P. Cognitive estimation: Performance of patients with focal frontal and posterior lesions. Neuropsychologia 2018, 115, 70–77.
- Korn, A.; Golan, H.; Melamed, I.; Pascual-Marqui, R.; Friedman, A. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J. Clin. Neurophysiol. 2005, 22, 1–9.
- Bar-Klein, G.; Lublinsky, S.; Kamintsky, L.; Noyman, I.; Veksler, R.; Dalipaj, H.; Senatorov, V.V., Jr.; Swissa, E.; Rosenbach, D.; Elazary, N.; et al. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain 2017, 140, 1692–1705.
- Drew, L.B.; Drew, W.E. The contrecoup-coup phenomenon: A new understanding of the mechanism of closed head injury. Neurocrit. Care 2004, 1, 385–390.
- Ratnaike, T.E.; Hastie, H.; Gregson, B.; Mitchell, P. The geometry of brain contusion: Relationship between site of contusion and direction of injury. Br. J. Neurosurg. 2011, 25, 410–413.
- Green, W.; Ciuffreda, K.J.; Thiagarajan, P.; Szymanowicz, D.; Ludlam, D.P.; Kapoor, N. Static and dynamic aspects of accommodation in mild traumatic brain injury: A review. Optometry 2010, 81, 129–136.
- Humble, S.S.; Wilson, L.D.; Wang, L.; Long, D.A.; Smith, M.A.; Siktberg, J.C.; Mirhoseini, M.F.; Bhatia, A.; Pruthi, S.; Day, M.A.; et al. Prognosis of diffuse axonal injury with traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 155–159.
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russel, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130.
- Adams, J.H.; Graham, D.I.; Murray, L.S.; Scott, G. Diffuse Axonal Injury Due to Nonmissile Head Injury in Humans: An Analysis of 45 Cases. Ann. Neurol. 1982, 12, 557–563.
- Mutch, C.A.; Talbott, J.F.; Gean, A. Imaging Evaluation of Acute Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 409–439.
- Lobato, R.D.; Alen, J.F.; Perez-Nuñez, A.; Alday, R.; Gómez, P.A.; Pascual, B.; Lagares, A.; Miranda, P.; Arrese, I.; Kaen, A. Utilidad de la TAC secuencial y la monitorización de la presión intracraneal para detectar nuevo efecto masa intracraneal en pacientes con traumatismo craneal grave y lesión inicial Tipo I-II . Neurocirugia 2005, 16, 217–234.
- Brown, C.V.; Zada, G.; Salim, A.; Inaba, K.; Kasotakis, G.; Hadjizacharia, P.; Demetriades, D.; Rhee, P. Indications for routine repeat head computed tomography (CT) stratified by severity of traumatic brain injury. J. Trauma 2007, 62, 1339–1344; discussion 1344–1345.
- Lindberg, D.M.; Stence, N.V.; Grubenhoff, J.A.; Lewis, T.; Mirsky, D.M.; Miller, A.L.; O’Neill, B.R.; Grice, K.; Mourani, P.M.; Runyan, D.K. Feasibility and Accuracy of Fast MRI Versus CT for Traumatic Brain Injury in Young Children. Pediatrics 2019, 144, e20190419.
- Wilson, T.T.; Merck, L.H.; Zonfrillo, M.R.; Movson, J.S.; Merck, D. Efficacy of Computed Tomography Utilization in the Assessment of Acute Traumatic Brain Injury in Adult and Pediatric Emergency Department Patients. R. I. Med. J. 2019, 102, 33–35.
- Darlan, D.; Prasetya, G.B.; Ismail, A.; Pradana, A.; Fauza, J.; Dariansyah, A.D.; Wardana, G.A.; Apriawan, T.; Bajamal, A.H. Algorithm of Traumatic Brain Injury in Pregnancy (Perspective on Neurosurgery). Asian J. Neurosurg. 2021, 16, 249–257.
- Smith, L.; Milliron, E.; Ho, M.L.; Hu, H.H.; Rusin, J.; Leonard, J.; Sribnick, E.A. Advanced neuroimaging in traumatic brain injury: An overview. Neurosurg. Focus 2019, 47, E17, Erratum in Neurosurg. Focus 2021, 50, E22.
- Chastain, C.A.; Oyoyo, U.E.; Zipperman, M.; Joo, E.; Ashwal, S.; Shutter, L.A.; Tong, K.A. Predicting outcomes of traumatic brain injury by imaging modality and injury distribution. J. Neurotrauma 2009, 26, 1183–1196.
- Audenaert, K.; Jansen, H.M.; Otte, A.; Peremans, K.; Vervaet, M.; Crombez, R.; de Ridder, L.; van Heeringen, C.; Thirot, J.; Dierckx, R.; et al. Imaging of mild traumatic brain injury using 57Co and 99mTc HMPAO SPECT as compared to other diagnostic procedures. Med. Sci. Monit. 2003, 9, MT112–MT117.
- Schweitzer, A.D.; Niogi, S.N.; Whitlow, C.T.; Tsiouris, A.J. Traumatic Brain Injury: Imaging Patterns and Complications. Radiographics 2019, 39, 1571–1595.
- Shetty, V.S.; Reis, M.N.; Aulino, J.M.; Berger, K.L.; Broder, J.; Choudhri, A.F.; Kendi, A.T.; Kessler, M.M.; Kirsch, C.F.; Luttrull, M.D.; et al. ACR Appropriateness Criteria Head Trauma. J. Am. Coll. Radiol. 2016, 13, 668–679.
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Van Berkum Clark, M.; Eisenberg, H.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 1992, 9 (Suppl. S1), S287–S292.
- Maas, A.I.; Hukkelhoven, C.W.; Marshall, L.F.; Steyerberg, E.W. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2006, 57, 1173–1182.
- Nelson, D.W.; Nyström, H.; MacCallum, R.M.; Thornquist, B.; Lilja, A.; Bellander, B.M.; Rudehill, A.; Wanecek, M.; Weitzberg, E. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J. Neurotrauma 2010, 27, 51–64.
- Raj, R.; Siironen, J.; Skrifvars, M.B.; Hernesniemi, J.; Kivisaari, R. Predicting outcome in traumatic brain injury: Development of a novel computerized tomography classification system (Helsinki computerized tomography score). Neurosurgery 2014, 75, 632–646; discussion 646–647.
- Wintermark, M.; Li, Y.; Ding, V.Y.; Xu, Y.; Jiang, B.; Ball, R.L.; Zeineh, M.; Gean, A.; Sanelli, P. Neuroimaging Radiological Interpretation System for Acute Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2665–2672.
- Khaki, D.; Hietanen, V.; Corell, A.; Hergès, H.O.; Ljungqvist, J. Selection of CT variables and prognostic models for outcome prediction in patients with traumatic brain injury. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 94.
- Nedd, K.; Sfakianakis, G.; Ganz, W.; Uricchio, B.; Vernberg, D.; Villanueva, P.; Jabir, A.M.; Bartlett, J.; Keena, J. 99mTc-HMPAO SPECT of the brain in mild to moderate traumatic brain injury patients: Compared with CT—A prospective study. Brain Inj. 1993, 7, 469–479.
- Lin, A.P.; Liao, H.J.; Merugumala, S.K.; Prabhu, S.P.; Meehan, W.P., 3rd; Ross, B.D. Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2012, 6, 208–223.
- Raji, C.A.; Henderson, T.A. PET and Single-Photon Emission Computed Tomography in Brain Concussion. Neuroimaging Clin. N. Am. 2018, 28, 67–82.
- Goffin, K.; van Laere, K. Single-photon emission tomography. Handb. Clin. Neurol. 2016, 135, 241–250.
- Santra, A.; Kumar, R. Brain perfusion single photon emission computed tomography in major psychiatric disorders: From basics to clinical practice. Indian J. Nucl. Med. 2014, 29, 210–221.
- Pavel, D.; Jobe, T.; Devore-Best, S.; Davis, G.; Epstein, P.; Sinha, S.; Kohn, R.; Craita, I.; Liu, P.; Chang, Y. Viewing the functional consequences of traumatic brain injury by using brain SPECT. Brain Cogn. 2006, 60, 211–213.
- Gowda, N.K.; Agrawal, D.; Bal, C.; Chandrashekar, N.; Tripati, M.; Bandopadhyaya, G.P.; Malhotra, A.; Mahapatra, A.K. Technetium Tc-99m ethyl cysteinate dimer brain single-photon emission CT in mild traumatic brain injury: A prospective study. AJNR Am. J. Neuroradiol. 2006, 27, 447–451.
- Sakas, D.E.; Bullock, M.R.; Patterson, J.; Hadley, D.; Wyper, D.J.; Teasdale, G.M. Focal cerebral hyperemia after focal head injury in humans: A benign phenomenon? J. Neurosurg. 1995, 83, 277–284.
- Khalili, H.; Rakhsha, A.; Ghaedian, T.; Niakan, A.; Masoudi, N. Application of Brain Perfusion SPECT in the Evaluation of Response to Zolpidem Therapy in Consciousness Disorder Due to Traumatic Brain Injury. Indian J. Nucl. Med. 2020, 35, 315–320.
- Silverman, I.E.; Galetta, S.L.; Gray, L.G.; Moster, M.; Atlas, S.W.; Maurer, A.H.; Alavi, A. SPECT in patients with cortical visual loss. J. Nucl. Med. 1993, 34, 1447–1451.
- Laatsch, L.; Pavel, D.; Jobe, T.; Lin, Q.; Quintana, J.C. Incorporation of SPECT imaging in a longitudinal cognitive rehabilitation therapy programme. Brain Inj. 1999, 13, 555–570.
- Digre, K.B.; Brennan, K.C. Shedding light on photophobia. J. Neuroophthalmol. 2012, 32, 68–81.
- Merezhinskaya, N.; Mallia, R.K.; Park, D.; Millian-Morell, L.; Barker, F.M., 2nd. Photophobia Associated with Traumatic Brain Injury: A Systematic Review and Meta-analysis. Optom. Vis. Sci. 2021, 98, 891–900.
- Armstrong, R.A. Visual problems associated with traumatic brain injury. Clin. Exp. Optom. 2018, 101, 716–726.
- Mares, C.; Dagher, J.H.; Harissi-Dagher, M. Narrative Review of the Pathophysiology of Headaches and Photosensitivity in Mild Traumatic Brain Injury and Concussion. Can. J. Neurol. Sci. 2019, 46, 14–22.
- DiCesare, C.A.; Kiefer, A.W.; Nalepka, P.; Myer, G.D. Quantification and analysis of saccadic and smooth pursuit eye movements and fixations to detect oculomotor deficits. Behav. Res. Methods 2017, 49, 258–266.
- Reddy, A.; Mani, R.; Selvakumar, A.; Hussaindeen, J.R. Reading eye movements in traumatic brain injury. J. Optom. 2020, 13, 155–162.
- Li, Y.; Singman, E.; McCulley, T.; Wu, C.; Daphalapurkar, N. The Biomechanics of Indirect Traumatic Optic Neuropathy Using a Computational Head Model with a Biofidelic Orbit. Front. Neurol. 2020, 11, 346.
- Jacobs, S.M.; Van Stavern, G.P. Neuro-ophthalmic deficits after head trauma. Curr. Neurol. Neurosci. Rep. 2013, 13, 389.
- Rasiah, P.K.; Geier, B.; Jha, K.A.; Gangaraju, R. Visual deficits after traumatic brain injury. Histol. Histopathol. 2021, 36, 711–724.
- Ellis, M.J.; Ritchie, L.; Cordingley, D.; Essig, M.; Mansouri, B. Traumatic Optic Neuropathy: A Potentially Unrecognized Diagnosis after Sports-Related Concussion. Curr. Sports Med. Rep. 2016, 15, 27–32.
- Saliman, N.H.; Belli, A.; Blanch, R.J. Afferent Visual Manifestations of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2778–2789.
- Chen, H.H.; Lee, M.C.; Tsai, C.H.; Pan, C.H.; Lin, Y.T.; Chen, C.T. Surgical Decompression or Corticosteroid Treatment of Indirect Traumatic Optic Neuropathy: A Randomized Controlled Trial. Ann. Plast. Surg. 2020, 84, S80–S83.
- Yu-Wai-Man, P. Traumatic optic neuropathy-Clinical features and management issues. Taiwan J. Ophthalmol. 2015, 5, 3–8.
- Volpe, N.J.; Levin, L.A. How should patients with indirect traumatic optic neuropathy be treated? J. Neuroophthalmol. 2011, 31, 169–174.
- Whitlock, J.R. Posterior parietal cortex. Curr. Biol. 2017, 27, R691–R695.
- Caminiti, R.; Cafee, M.V.; Battaglia-Mayer, A.; Crowe, D.A.; Georgopoulos, A.P. Understanding the parietal lobe syndrome from a neurophysiological and evolutionary perspective. Eur. J. Neurosci. 2010, 31, 2320–2340.
- Hadjidimitrakis, K.; Bakola, S.; Wong, Y.T.; Hagan, M.A. Mixed Spatial and Movement Representations in the Primate Posterior Parietal Cortex. Front. Neural Circuits 2019, 13, 15.
- Medendorp, W.P.; Heed, T. State estimation in posterior parietal cortex: Distinct poles of environmental and bodily states. Prog. Neurobiol. 2019, 183, 101691.
- Baltaretu, B.R.; Monaco, S.; Velji-Ibrahim, J.; Luabeya, G.N.; Crawford, J.D. Parietal Cortex Integrates Saccade and Object Orientation Signals to Update Grasp Plans. J. Neurosci. 2020, 40, 4525–4535.
- Dziedzic, T.A.; Bala, A.; Marchel, A. Cortical and Subcortical Anatomy of the Parietal Lobe from the Neurosurgical Perspective. Front. Neurol. 2021, 12, 727055.




