| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ELENA OBRADOR | + 2351 word(s) | 2351 | 2020-11-03 07:56:26 | | | |
| 2 | Nicole Yin | -1 word(s) | 2350 | 2022-02-23 05:18:18 | | |
Video Upload Options
The development of protective agents against harmful radiations has been a subject of investigation for decades. However, effective (ideal) radioprotectors and radiomitigators remain an unsolved problem. Because ionizing radiation-induced cellular damage is primarily attributed to free radicals, radical scavengers are promising as potential radioprotectors. Early development of such agents focused on thiol synthetic compounds, e.g., amifostine (2-(3-aminopropylamino) ethylsulfanylphosphonic acid), approved as a radioprotector by the Food and Drug Administration (FDA, USA) but for limited clinical indications and not for nonclinical uses. To date, no new chemical entity has been approved by the FDA as a radiation countermeasure for acute radiation syndrome (ARS). All FDA-approved radiation countermeasures (filgrastim, a recombinant DNA form of the naturally occurring granulocyte colony-stimulating factor, G-CSF; pegfilgrastim, a PEGylated form of the recombinant human G-CSF; sargramostim, a recombinant granulocyte macrophage colony-stimulating factor, GM-CSF) are classified as radiomitigators. No radioprotector that can be administered prior to exposure has been approved for ARS. This differentiates radioprotectors (reduce direct damage caused by radiation) and radiomitigators (minimize toxicity even after radiation has been delivered). Molecules under development with the aim of reaching clinical practice and other nonclinical applications are discussed. Assays to evaluate the biological effects of ionizing radiations are also analyzed.
Ionizing radiation is the energy released by atoms in the form of electromagnetic waves (e.g., X or gamma rays) or particle radiation (alpha, beta, electrons, protons, neutrons, mesons, prions, and heavy ions) with sufficient energy to ionize atoms or molecules.
1. Introduction
Ionizing radiation emission can occur as a consequence of the decay process of unstable nuclei or due to nuclear de-excitation in devices such as nuclear reactors, X-ray machines, and cyclotrons. Radioactivity is defined as spontaneous disintegration of atoms. The excess energy emitted in this process is considered as a type of ionizing radiation. Unstable elements that disintegrate in this process and emit ionizing radiation are called radionuclides. The activity of a radionuclide is expressed in becquerels (one Bq is one disintegration per second)[1].
The absorption of radiation-derived energy by biological materials may cause excitation or ionization. Sufficient energy can cause the ejection of one or more orbital electrons from an atom or molecule, a process known as ionization, and such radiation is called ionizing radiation[2].
2. Interaction of Ionizing Radiation with Living Matter
2.1. The Effects Are Determined by the Radiation Type and Its Penetration Capacity
Distribution of ionization and excitation along the track of an ionizing particle will vary according to the type of radiation. A useful comparative term to describe the deposition of energy by different types of radiation is linear energy transfer (LET) or the amount of energy that a specific ionizing particle transfers to the material traversed per unit distance. Thus, LET directly affects the relative biological effectiveness (RBE) of a specific radiation type[2]. The RBE is defined as the ratio between an absorbed standard dose of radiation (typically X) and the absorbed dose of any other type of radiation that causes the same amount of biological damage. In many cases, the biological effect of radiation increases in proportion to the increase in LET. Radiations commonly used to assess RBE are low-LET X or γ, for which RBE is 1.0. However, when evaluating some biological effects caused by high-LET radiation (such as fast neutrons), the RBE can vary widely, from about 3 to more than 100 depending on the cellular or tissue effect considered. For example, higher RBEs for neutron radiation are associated with high LET effects, which are directly linked with protons released by collisions of these neutrons with hydrogen nuclei[3]. Consequently, doses should be evaluated in terms of absorbed dose (in Grays, Gy), and, when high-LET radiations are involved, the absorbed dose must be correlated with an appropriate RBE. The RBE is correlated with the amount of the radiation dose absorbed, expressed in Gy (1 Gy is 1 joule of radiation energy absorbed per kg of matter). In parallel, the Sievert (Sv), defined as the corresponding biological effect of the deposit of one joule of radiation energy in 1 kg of human tissue (www.icrp.org), is used to evaluate the biological effect of low doses of ionizing radiation representing the risk of external radiation from sources outside the body, as well as those representing the risk of internal irradiation due to accidentally inhaled or ingested radioactive substances. The Sv helps to value the stochastic health risk, which represents the probability of radiation-induced cancer and genetic damages. The following considerations describe the most common types of radiation:
Alpha radiation happens when an atom goes through radioactive decay, emanating a particle composed of two protons and two neutrons (e.g., a helium-4 atom’s nucleus). α particles interact heavily with matter because of their charge and mass. They, however, travel merely a few centimeters through the air. Thus, they cannot enter into the external layer of dead skin cells. However, a substance emitting α can be very deleterious for the cell, in cases where it is ingested through food or air[4].
Beta radiation may be either an electron or a positron. Because of having lower mass, this radiation can travel a few meters in the air, but some dense pieces of plastic or a pile of paper can block it. This type can enter into the skin a few centimeters deep. However, its main threat lies in internal emissions caused by ingested material[5].
Gamma radiation entails an emission of a photon of energy from an unstable nucleus. γ radiation is capable of traveling much longer distances through the air because it has no mass or charge; in every 150 m, it loses approximately half its energy. γ radiation can be blocked by a thick or dense enough material, e.g., lead or depleted uranium. X-rays behave in an analogues manner to γ radiation; however, compared to γ radiation, their wavelength is longer and (usually) their energy is lower. X-rays originate from energy changes in an electron, such as moving from a higher energy level to a lower one, which causes the release of excess energy[6].
Neutron radiation is composed of free neutrons, resulting from nuclear fusion, which could be spontaneous or induced. They are capable of traveling hundreds to thousands of meters in the air; a hydrogen-rich material, such as concrete or water, can block them. Neutrons have no charge and cannot ionize an atom directly. Thus, when they are absorbed into a stable atom, they commonly cause indirect ionization. This makes them unstable and consequently emit other types of ionizing radiation[7]. After neutrons strike the hydrogen nuclei, proton radiation (fast protons) is produced. These protons that have high energy, are charged, and interact with the electrons in matter are considered ionizing particles[8].
Proton and carbon ion therapy are two types of hadron therapy which have been increasingly used in recent years for cancer treatment. Proton therapy uses a beam of protons to irradiate tissues, most often as a type of cancer therapy. Its main advantage is that the dose is deposited over a narrow range of depth, which results in minimal entry, exit, or scattered radiation dose to healthy nearby tissues[9]. Carbon ions exhibit a characteristic energy distribution in depth, known as the “Bragg peak,” where low levels of energy are deposited in tissues proximal to the target, and the majority of energy is released in the target. Its main advantage is that it may allow dose escalation to tumors while reducing radiation dose to adjacent normal tissues[10].
2.2. Physical, Biological, and Chemical Regulatory Factors
Many different physical, biological, and chemical regulatory factors influence the effects of radiation. Physical regulation implies that the kinetic energy of radiation is transferred to atoms or molecules, thus leading to their excitation and ionization. This is influenced by the time, dose and dose rate, fractionation regimen, volume of tissue irradiated, temperature, and type of radiation. Biological regulators relate to the type of cell/tissue/organ, its sensitivity, bystander effects, age, and physiological mechanisms of reparation. Chemical regulators include radiosensitizers, radioprotectors, radiomitigators, and therapeutic agents[11][12]. The effect of a specific type of radiation in living matter will depend on a combination of these factors. Consequently, the development of a therapy to prevent or treat damage from radiation must take into account the relative influence of different regulatory factors.
2.3. Exposure to Ionizing Radiation: Adverse Effects
Most adverse effects of exposure to ionizing radiation can be assigned to two types of categories. The first is deterministic or predictable (in a time range known a posteriori of the event) and due to harmful tissue/organ damage following high doses of radiation; as a function of the time interval between irradiation and its observable effect, deterministic effects may be classified as early or late effects. The second is stochastic (random), i.e., cell mutation-associated pathologies (mainly cancer) and heritable effects following moderate and possibly low doses[13]. Thus, it seems that following the well-established radiobiological concept of no radiation dose can be considered completely safe is judicious[14].
Acute effects are generated due to the death of considerable number of cells in tissues that have rapid turnover rates (e.g., the bone marrow, epidermis, and mucosae of the upper and lower intestinal tract). The effects are usually revealed in a timespan of days to even weeks after the irradiation[15]. This response is usually associated with inflammation, which might be directly produced by the radiation exposure or secondary to cell loss[16][17]. The local release of proinflammatory factors (e.g., IL-1, TNF-α, COX-2, NO) can trigger the proliferation of damaging radicals, e.g., reactive oxygen species (ROS), on top of the radicals directly produced by the ionizing radiation[18].
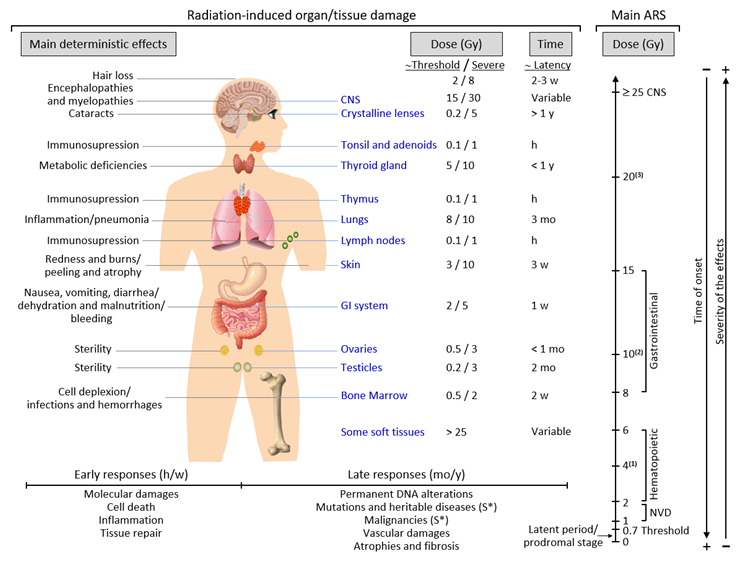
Late responses tend to occur in tissues with slow cell turnover. They are normally persistent and progressive, located in organs with infrequent parenchymal cell division (such as the kidney and liver) or those that do not divide (for example, the central nervous system (CNS) and muscles)[19]. Their nature and timing are dependent upon the involved tissue and could manifest as a decrease in organ function—for instance, radiation-induced nephropathy—thus causing hypertension, high creatinine, elevated blood nitrogen levels, and functional loss[20]. Another common example is the development of tissue fibrosis (increased collagen synthesis and deposition) that happens in a range of tissues (including subcutaneous tissue, muscle, lung, and the gastrointestinal tract), sometimes a few years after irradiation[21]. It seems that fibrosis is associated with the abnormal and chronic expression of proinflammatory cytokines. The immune system (i.e., macrophages and mast cells) contributes to the fibrotic reaction[22]. Moreover, connective tissue damage and/or an impairment in the vasculature of an organ potentially cause a progressive impairment of organ circulation. Under these circumstances, secondary cell death may occur due to nutrient deprivation[23]. Stochastic effects likely derive from an injury to a single cell or a small number of cells. Cancer induction is the most important somatic late effect of low-dose radiation exposure. Figure 1 schematically describes the main consequences of exposure to ionizing radiation in organs and tissues.
2.4. Acute and Chronic Radiation Syndromes
Acute radiation syndrome (ARS) involves a number of health effects caused by exposure to elevated amounts of ionizing radiation (total dose >0.7 Gy) in a limited timespan[24]. The symptomatology might begin less than one hour after the dose is received and last for months[25]. The prodromal stage is accompanied by general symptoms such as headaches, vertigo, muscle weakness, and abnormal sensations of taste or smell. Any exposure to 1–2 Gy leads to NVD (i.e., nausea, vomiting, diarrhea) as part of ARS-related prodromal stage. However, exposure to 2–6 Gy produces hematopoietic syndrome, which affects the bone marrow, spleen, and thymus. An exposure to 8–15 Gy could produce gastrointestinal syndrome, while exposure to >25 Gys can provoke CNS syndrome[25]. NVD might be associated with flu-like symptoms of fever and/or faintness. Such symptoms, however, tend to attenuate rapidly, and the patient might mistakenly have a sense of being recovered because of degeneration and repair of proliferative tissues. These symptoms represent a latent stage. On the basis of the received dose, the latent stage could continue for a few hours or even up to a few days. Later in time, and as the dose received increases, more severe damage may occur, affecting the skin (reddening, blistering, and/or ulceration), lungs (inflammation), bone marrow (leukopenia, thrombopenia, and increased sedimentation rate), gastrointestinal tract (inflammation and/or bleeding), cardiovascular system (arrhythmia, fall of blood pressure), and CNS (increased irritability, insomnia, fear, and symptoms derived from damage affecting neuromotor functions). This damage may eventually cause death. ARS is generally a rare event, although it may affect a large number of people in the case of an accident such as that in Chernobyl. ARS treatment may require blood transfusions, antibiotics, colony-stimulating factors, or even stem-cell transplants[25][26].
Chronic radiation syndrome (CRS) involves radiation-induced health effects that may require years to develop after exposure. The threshold for CRS is around 0.7 and 1.5 Gy, at dose rates >0.1 Gy/year, and cumulative doses exceeding 2–3 Gy over 2–3 years[27]. Its latency can comprise a period of 1–5 years and has been defined as a systemic response of the body to chronic total body exposure in humans. The early symptomatology of CRS can involve alterations in vegetative functions and, eventually, changes in tactile and olfactory sensitivity. At a more advanced stage, gastrointestinal toxicity (transmural injury of the bowel wall might later cause progressive vasculitis, thrombosis, and, finally, variable grades of ischemia and necrosis), atrophy of the skin and muscle, and eye cataracts are common. Genetic damage-related cancer, i.e., different solid cancers or leukemia, may also develop either at an early stage or later in time[28][29].
Figure 1. Consequences of exposure to ionizing radiation in organs and tissues are time- and dose-dependent. The main radiation-induced biological effects are displayed, while indicating the differences between the threshold doses and those that cause a severe effect. Time abbreviations: h, hours; w, weeks; mo, months; y, years. (S*) indicates a main stochastic effect. Acute radiation syndrome (ARS) may be difficult to differentiate from chronic radiation syndrome (CRS) since the dose threshold for the CRS is approximately 0.7–1 Gy (see the text for more details). (1) Possible death (approximately in 2 mo) due to bone marrow depletion. (2) Destruction of the intestinal lining, intestinal bleeding, possible death (1–2 w). (3) Cognitive impairment, convulsion, possible death (hours).
References
- Zakariya, N.I.; Kahn, M.T.E.; Benefits and Biological Effects of Ionizing Radiation. Sch. Acad. J. Biosci. SAJB 2014, 2, 583–591.
- Jongho Jeon; Review of Therapeutic Applications of Radiolabeled Functional Nanomaterials. International Journal of Molecular Sciences 2019, 20, 2323, 10.3390/ijms20092323.
- Brita Singers Sørensen; Jens Overgaard; Niels Bassler; In vitro RBE-LET dependence for multiple particle types. Acta Oncologica 2011, 50, 757-762, 10.3109/0284186x.2011.582518.
- Robin M. De Kruijff; Hubert T. Wolterbeek; Antonia G. Denkova; A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters?. Pharmaceuticals 2015, 8, 321-336, 10.3390/ph8020321.
- J F Kirwan; P H Constable; I E Murdoch; P T Khaw; Beta irradiation: new uses for an old treatment: a review. Eye 2003, 17, 207-215, 10.1038/sj.eye.6700306.
- Julie A. Reisz; Nidhi Bansal; Jiang Qian; Weiling Zhao; Cristina M. Furdui; Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxidants & Redox Signaling 2014, 21, 260-292, 10.1089/ars.2013.5489.
- Masao S. Sasaki; Satoru Endo; Masaharu Hoshi; Taisei Nomura; Neutron relative biological effectiveness in Hiroshima and Nagasaki atomic bomb survivors: a critical review. Journal of Radiation Research 2016, 57, 583-595, 10.1093/jrr/rrw079.
- Armin Lühr; Cläre von Neubeck; Jörg Pawelke; Annekatrin Seidlitz; Claudia Peitzsch; Soren M Bentzen; Thomas Bortfeld; Jürgen Debus; Eric Deutsch; Johannes A. Langendijk; et al.Jay S. LoefflerRadhe MohanMichael ScholzBrita Singers SørensenDamien C. WeberMichael BaumannMechthild Krause “Radiobiology of Proton Therapy”: Results of an international expert workshop. Radiotherapy and Oncology 2018, 128, 56-67, 10.1016/j.radonc.2018.05.018.
- L. De Marzi; A. Patriarca; N. Scher; J. Thariat; M. Vidal; Exploiting the full potential of proton therapy: An update on the specifics and innovations towards spatial or temporal optimisation of dose delivery. Cancer/Radiothérapie 2020, 24, 691-698, 10.1016/j.canrad.2020.06.003.
- Timothy D. Malouff; Anita Mahajan; Sunil Krishnan; Chris Beltran; Danushka S. Seneviratne; Daniel Michael Trifiletti; Carbon Ion Therapy: A Modern Review of an Emerging Technology. Frontiers in Oncology 2020, 10, 82, 10.3389/fonc.2020.00082.
- Francis Dumont; Antoine Le Roux; Pierre Bischoff; Radiation countermeasure agents: an update. Expert Opinion on Therapeutic Patents 2009, 20, 73-101, 10.1517/13543770903490429.
- Vijay K. Singh; Thomas M. Seed; A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. International Journal of Radiation Biology 2017, 93, 851-869, 10.1080/09553002.2017.1332438.
- P. Rajaraman; M. Hauptmann; Simon Bouffler; A. Wojcik; Human individual radiation sensitivity and prospects for prediction. Annals of the ICRP 2018, 47, 126-141, 10.1177/0146645318764091.
- NIRS. Available online: https://www.nirs.org/ (accessed on 25 April 2020)
- William H. McBride; Dörthe Schaue; Radiation‐induced tissue damage and response. The Journal of Pathology 2020, 250, 647-655, 10.1002/path.5389.
- Sally A Lorimore; Philip J Coates; Gillian E Scobie; Gordon Milne; Eric G Wright; Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects?. Oncogene 2001, 20, 7085-7095, 10.1038/sj.onc.1204903.
- Yu-Qing Li; Paul Chen; Vipan Jain; Raymond M. Reilly; C. Shun Wong; Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord.. Radiation Research 2004, 161, 143-152, 10.1667/rr3117.
- Federica Maria Di Maggio; Luigi Minafra; Giusi Irma Forte; Francesco Paolo Cammarata; Domenico Lio; Cristina Messa; Maria Carla Gilardi; Valentina Bravatà; Portrait of inflammatory response to ionizing radiation treatment. Journal of Inflammation 2015, 12, 1-11, 10.1186/s12950-015-0058-3.
- Emiliya Domina; Alex Philchenkov; Anna Dubrovska; Individual Response to Ionizing Radiation and Personalized Radiotherapy. Critical Reviews™ in Oncogenesis 2018, 23, 69-92, 10.1615/critrevoncog.2018026308.
- Søren M. Bentzen; Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nature Reviews Cancer 2006, 6, 702-713, 10.1038/nrc1950.
- Jeffrey M. Straub; Jacob New; Chase D. Hamilton; Chris Lominska; Yelizaveta Shnayder; Sufi M. Thomas; Radiation-induced fibrosis: mechanisms and implications for therapy. Journal of Cancer Research and Clinical Oncology 2015, 141, 1985-1994, 10.1007/s00432-015-1974-6.
- Anup Kainthola; Teena Haritwal; Mrinialini Tiwari; Noopur Gupta; Suhel Parvez; Manisha Tiwari; Hrideysh Prakash; Paban K. Agrawala; Immunological Aspect of Radiation-Induced Pneumonitis, Current Treatment Strategies, and Future Prospects. Frontiers in Immunology 2017, 8, 506, 10.3389/fimmu.2017.00506.
- Olivier Guipaud; Cyprien Jaillet; Karen Clement-Colmou; Agnès François; Stéphane Supiot; Fabien Milliat; The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. The British Journal of Radiology 2018, 91, 20170762, 10.1259/bjr.20170762.
- Andrei I. Vorobiev; Acute radiation disease and biological dosimetry in 1993. Stem Cells 2009, 15, 269-274, 10.1002/stem.5530150736.
- L Stenke; K Lindberg; M Lagergren Lindberg; R Lewensohn; Jack Valentin; R Powles; N Dainiak; COORDINATION OF MANAGEMENT OF THE ACUTE RADIATION SYNDROME. Radiation Protection Dosimetry 2018, 182, 80-84, 10.1093/rpd/ncy144.
- Harald Dörr; Viktor Meineke; Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC Medicine 2011, 9, 126-126, 10.1186/1741-7015-9-126.
- Ignacia Braga-Tanaka Iii; Satoshi Tanaka; Atsushi Kohda; Daisaku Takai; Shingo Nakamura; Tetsuya Ono; Kimio Tanaka; Jun-Ichiro Komura; Experimental studies on the biological effects of chronic low dose-rate radiation exposure in mice: overview of the studies at the Institute for Environmental Sciences. International Journal of Radiation Biology 2018, 94, 423-433, 10.1080/09553002.2018.1451048.
- Yoo-Kang Kwak; Sea-Won Lee; Chul Seung Kay; Hee Hyun Park; Intensity-modulated radiotherapy reduces gastrointestinal toxicity in pelvic radiation therapy with moderate dose. PLOS ONE 2017, 12, e0183339-e0183339, 10.1371/journal.pone.0183339.
- Mitsuaki Ojima; Tokuhisa Hirouchi; Reo Etani; Kentaro Ariyoshi; Yohei Fujishima; Michiaki Kai; Dose-Rate-Dependent PU.1 Inactivation to Develop Acute Myeloid Leukemia in Mice Through Persistent Stem Cell Proliferation After Acute or Chronic Gamma Irradiation. Radiation Research 2019, 192, 612-620, 10.1667/rr15359.1.