Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sahbi Aloui | -- | 478 | 2022-06-10 14:36:19 | | | |
| 2 | Conner Chen | + 577 word(s) | 1055 | 2022-06-13 02:35:13 | | | | |
| 3 | Conner Chen | + 19 word(s) | 1074 | 2022-06-27 08:55:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aloui, S.; , . Influence of Plasticizer´s Polarity on Mechanical Stability. Encyclopedia. Available online: https://encyclopedia.pub/entry/23922 (accessed on 07 February 2026).
Aloui S, . Influence of Plasticizer´s Polarity on Mechanical Stability. Encyclopedia. Available at: https://encyclopedia.pub/entry/23922. Accessed February 07, 2026.
Aloui, Sahbi, . "Influence of Plasticizer´s Polarity on Mechanical Stability" Encyclopedia, https://encyclopedia.pub/entry/23922 (accessed February 07, 2026).
Aloui, S., & , . (2022, June 10). Influence of Plasticizer´s Polarity on Mechanical Stability. In Encyclopedia. https://encyclopedia.pub/entry/23922
Aloui, Sahbi and . "Influence of Plasticizer´s Polarity on Mechanical Stability." Encyclopedia. Web. 10 June, 2022.
Copy Citation
Compared with an styrene butadiene rubber (SBR) sample without plasticizer, the conductivity of mechanically unloaded oil-extended SBR samples decreases by an order of magnitude. The polarity of the plasticizer shows hardly any influence because the plasticizers only affect the distribution of the filler clusters. Under static load, the dielectric properties seem to be oil-dependent.
plasticizer
mechanical stability
1. Introduction
Plasticizers are a widely used additive in rubber compounds [1][2][3][4]. They are particularly important and, as the third-highest ingredient in terms of content level, come in right after rubber and fillers. As processing aids, the plasticizers are added in different concentrations in order to impart rubber products with the desired elastic properties in the operating temperature range [5][6][7][8][9][10].
As a fluid component, the plasticizer migrates in the rubber matrix and its macromolecules are integrated into the polymer chains through intermolecular interactions. Consequently, the intermolecular forces of the polymer chains and the number of free valences in the three-dimensional structure are reduced. The internal space between the polymer chains is thus larger, and the free volume that allows the polymer chains to flow above their glass transition temperature increases [11][12][13][14][15]. This new conformation of the polymer chains, in turn, increases their mobility and enhances the filler distribution in the rubber mixture [16][17][18][19][20][21]. Above a certain percolation threshold, a filler network is formed that reinforces the rubber compounds and provides the necessary mechanical stability [16][17]. This applies to both the carbon-based fillers such as carbon black and silica [18][19][20][21]. Indeed, the plasticizer type strongly affects the mechanical properties of rubber products due to a shift in the glass transition temperature. Consequently, the strain, the mechanical stress, the modulus of elasticity and the damping behavior change [22][23][24].
Furthermore, the dielectric properties of rubber samples filled with electrically conductive filler depend on the structure of its filler network [25][26][27][28][29][30][31][32]. This applies to filler networks made of electrically conductive fillers such as carbon-based carbon black or hybrid filler networks, provided that at least one electrically conductive filler is present [25][26][27][28]. The non-conductive component is mainly used because of its excellent mechanical reinforcement, as is the case with silica used in dynamic systems such as car tires [29][30][31][32]. Aloui et al. have shown that mechanically induced changes in the structure of the electrically conductive filler network have a direct impact on dielectric mechanisms such as charge transport and polarization [33][34]. These, in turn, have consequences for the dielectric constant and the dielectric conductivity of rubber samples [35][36][37][38][39].
The direct relationship between mechanical and dielectric properties makes simultaneous mechanical and dielectric analysis of rubber samples filled with electrically conductive filler an outstanding technique for opening up new horizons in evaluating the microstructure dynamics of rubber materials under mechanical load and hence reproducing authentic situations from operation modes [40][41][42][43]. In addition to quality measurements on test samples, examinations on installed end products can also be guaranteed if sensors are installed to record the current material properties during use and to monitor them in the subsequent step. Mainly the dielectric properties are used as a response to the mechanical load [44].
2. Excursus: Dielectric Relaxation in Elastomers
Dielectric relaxation describes the build-up of the electric polarization of a dielectric medium after application of an external electric field. The characterization of the dielectric relaxation is based on the measurement of the variation of the permittivity as a function of frequency. The permittivity stems from dipole orientation and transport of free charge carriers under the action of an electric field. The measuring method uses capacitance measurements as a function of frequency for a sample placed between two electrodes. An extensive explanation of the phenomenon and the measurement technology can be found in [45].
The permittivity ε∗ is a complex function with the real part ε′ and the imaginary part ε′′, also known as dielectric loss. As is typical for elastomers, not all dipoles have the same relaxation time, but different relaxation times, which exhibit a distribution with a relaxation peak. In order to describe these types of relaxation correctly, there are various empirical models derived from the Debye equation. In the case of symmetrical frequency response, the Cole–Cole approach is mainly used for amorphous dielectrics [46]. According to the Cole–Cole equation,
where εinf is the infinite frequency dielectric permittivity, Δε is the relaxation strength, α is the broadness parameter and τ is the Cole–Cole relaxation time. ω=2πfel is the angular frequency and fel is the electrical frequency. The expressions of ε′ and ε′′ take the following form:
3. Compound Preparation
Four carbon black filled SBR based compounds were prepared at Hansen and Rosenthal KG in Hamburg, Germany. The carbon black N 330 was used at a filler concentration of 60 phr. For a reference sample, no plasticizer was added. The three other samples each contain 20 phr of one plasticizer grade, which differ by polarity. Of course, the good miscibility of the plasticizers in the rubber matrix must be taken into account. Therefore, the following plasticizers are used: The plasticizers used are a paraffinic base oil (SN400), mild extraction solvate (MES) and distillate aromatic extract (DAE). Figure 1 shows the structural formula of the plasticizers with different polarities used [47].
Figure 1. Structural formula of plasticizers SN400, MES and DAE.
Plasticizers SN400, MES and DAE have an aniline point in accordance with DIN ISO 2977 at 101 °C, 84 °C and 43 °C [47]. The aniline point is the temperature at which a homogeneous mixture of equal volumes of aniline and plasticizer separates into 2 phases during the cooling process. The degree of miscibility of aniline with the plasticizer estimates the aromatic content in the plasticizer. The lower the aniline point, the more polar the plasticizer.
The solubility parameter δ is an indicator of the miscibility quality of the various plasticizers within the SBR matrix. SN400, MES and DAE have a solubility parameter δ of 16 MPa1/2, 16.7 Mpa1/2 and 18.5 Mpa1/2. With a value of 17.2 Mpa1/2, the solubility parameter δ for SBR is in the same range as for the plasticizers, implying a good compatibility [47].
The aniline point and the solubility parameter are shown in Figure 2.
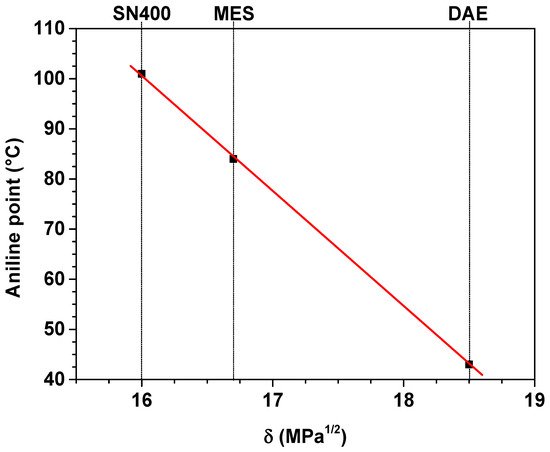
Figure 2. Aniline point and solubility parameter of the plasticizers.
The compound formulation is shown in Table 1.
Table 1. Compound formulation in phr.
| No Oil | SN400 | MES | DAE | |
|---|---|---|---|---|
| SBR 1502 | 100 | 100 | 100 | 100 |
| N 330 | 60 | 60 | 60 | 60 |
| SN400 | - | 20 | - | - |
| MES | - | - | 20 | - |
| DAE | - | - | - | 20 |
| ZnO | 2.5 | 2.5 | 2.5 | 2.5 |
| Stearic acid | 1 | 1 | 1 | 1 |
| TMQ | 1 | 1 | 1 | 1 |
| 6PPD | 1 | 1 | 1 | 1 |
| CBS | 1.8 | 1.8 | 1.8 | 1.8 |
| DDTD | 0.2 | 0.2 | 0.2 | 0.2 |
| Sulphur | 1.5 | 1.5 | 1.5 | 1.5 |
The antioxidants 2,2,4-Trimethyl-1,2-dihydrochinolin (TMQ) and N-(1.3-Dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) were added at a concentration of 1 phr. The samples were sulfur-vulcanized. In addition to sulfur, the vulcanization accelerators N-cyclohexyl-2-benzothiazolesulfenamide (CBS) and Dimethyldiphenylthiuram disulfide (DDTD) were used.
References
- Ni, Y.; Yang, D.; Wei, Q.; Yu, L.; Ai, J.; Zhang, L. Plasticizer-induced enhanced electromechanical performance of natural rubber dielectric elastomer composites. Compos. Sci. Technol. 2020, 195, 108202.
- Okamoto, K.; Toh, M.; Liang, X.; Nakajima, K. Influence of mastication on the microstructure and physical properties of rubber. Rubber Chem. Technol. 2021, 94, 533–548.
- Rahman, M.M.; Oßwald, K.; Reincke, K.; Langer, B. Influence of Bio-Based Plasticizers on the Properties of NBR Materials. Materials 2020, 13, 2095.
- Sokolova, M.D.; Fedorova, A.F.; Pavlova, V.V. Research of Influence of Plasticizers on the Low-Temperature and Mechanical Properties of Rubbers. Mater. Sci. Forum 2019, 945, 459–464.
- Kaliyathan, A.V.; Rane, A.V.; Huskic, M.; Kanny, K.; Kunaver, M.; Kalarikkal, N.; Thomas, S. The effect of adding carbon black to natural rubber/butadiene rubber blends on curing, morphological, and mechanical characteristics. J. Appl. Polym. 2021, 139, 51967.
- Kyei-Manu, W.A.; Herd, C.R.; Chowdhury, M.; Busfield, J.J.C.; Tunnicliffe, L.B. Influence of Colloidal Properties of Carbon Black on Static and Dynamic Mechanical Properties of Natural Rubber. Polymers 2022, 14, 1194.
- Shi, X.; Sun, S.; Zhao, A.; Zhang, H.; Zuo, M.; Song, Y.; Zheng, Q. Influence of carbon black on the Payne effect of filled natural rubber compounds. Compos. Sci. Technol. 2021, 203, 108586.
- Antoev, K.P.; Shadrinov, N.V. Effect of Conductive Carbon Black Concentration on Tyre Regenerate Properties. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1079, 042025.
- Kim, I.J.; Ahn, B.; Kim, D.; Lee, H.J.; Kim, H.J.; Kim, W. Vulcanizate structures and mechanical properties of rubber compounds with silica and carbon black binary filler systems. Rubber Chem. Technol. 2021, 94, 339–354.
- Warasitthinon, N.; Robertson, C.G. Interpretation of the tand peak height for particle-filled rubber and polymer nanocomposites with relevance to tire tread performance balance. Rubber Chem. Technol. 2018, 91, 577–594.
- Hodge, R.M.; Bastow, T.J.; Edward, G.H.; Simon, G.P.; Hill, A.J. Free Volume and the Mechanism of Plasticization in Water-Swollen Poly (vinyl alcohol). Macromolecules 1996, 25, 8137–8143.
- Machin, D.; Rogers, C.E. Free volume theories for penetrant diffusion in polymers. Macromol. Chem. Phys. 1972, 155, 269–281.
- Gomes, A.C.O.; Soares, B.G.; Oliveira, M.G.; Machado, J.C.; Windmöller, D.; Paranhos, C.M. Characterization of crystalline structure and free volume of polyamide 6/nitrile rubber elastomer thermoplastic vulcanizates: Effect of the processing additives. J. Appl. Polym. Sci. 2017, 134, 45576.
- Mohamed, H.F.M.; Taha, H.G.; Alaa, H.B. Electrical conductivity and mechanical properties, free volume, and γ-ray transmission of ethylene propylene diene monomer/butadiene rubber composites. Polym. Compos. 2020, 41, 1405–1417.
- Švajdlenková, H.; Šauša, O.; Maťko, I.; Koch, T.; Gorsche, C. Investigating the Free-Volume Characteristics of Regulated Dimethacrylate Networks Below and Above Glass Transition Temperature. Macromol. Chem. Phys. 2018, 219, 1800119.
- Liu, J.; Li, B.; Jiang, Y.; Zhang, X.; Yu, G.; Sun, C.; Zhao, S. Investigation of filler network percolation in carbon black (CB) filled hydrogenated butadiene-acrylonitrile rubber (HNBR). Polym. Bull. 2022, 79, 87–96.
- Vas, J.V.; Thomas, M.J. Monte Carlo modelling of percolation and conductivity in carbon filled polymer nanocomposites. IET Sci. Meas. 2018, 12, 98–105.
- Nagaraja, S.M.; Henning, S.; Ilisch, S.; Beiner, M. Common Origin of Filler Network Related Contributions to Reinforcement and Dissipation in Rubber Composites. Polymers 2021, 13, 2534.
- Kumar, A.; Dalmiya, M.S.; Goswami, M.; Bansal, V.; Goyal, S.; Nair, S.; Hossain, S.J.; Chattopadhyay, S. Entangled network influenced by carbon black in solution SBR vulcanizates revealed by theory and experiment. Rubber Chem. Technol. 2021, 94, 324–338.
- Wen, S.; Zhang, R.; Xu, Z.; Zheng, L.; Liu, L. Effect of the Topology of Carbon-Based Nanofillers on the Filler Networks and Gas Barrier Properties of Rubber Composites. Materials 2020, 13, 5416.
- Shui, Y.; Huang, L.; Wei, C.; Sun, G.; Chen, J.; Lu, A.; Sun, L.; Liu, D. How the silica determines properties of filled silicone rubber by the formation of filler networking and bound rubber. Compos. Sci. Technol. 2021, 215, 109024.
- Ren, Y.; Zhao, S.; Yao, Q.; Li, Q.; Zhang, X.; Zhang, L. Effects of Plasticizers on the Strain-Induced Crystallization and Mechanical Properties of Natural Rubber and Synthetic Polyisoprene. RSC Adv. 2015, 15, 11317–11324.
- Sharma, P.; Roy, S.; Karimi-Varzaneh, H.A. Impact of Plasticizer Addition on Molecular Properties of Polybutadiene Rubber and its Manifestations to Glass Transition Temperature. Macromol. Theory Simul. 2019, 28, 4.
- Simon, P.P.; Ploehn, H.J. Modeling the effect of plasticizer on the viscoelastic response of crosslinked polymers using the tube-junction model. J. Rheol. 2000, 44, 169.
- Thaptong, P.; Jittham, P.; Sae-oui, P. Effect of conductive carbon black on electrical conductivity and performance of tire tread compounds filled with carbon black/silica hybrid filler. J. Appl. Polym. 2021, 10, 50855.
- Alves, A.M.; Cavalcanti, S.N.; da Silva, M.P.; Freitas, D.M.G.; Agrawal, P.; de Mélo, T.J.A. Electrical, rheological, and mechanical properties copolymer/carbon black composites. J. Vinyl Addit. Technol. 2021, 27, 445–458.
- Utrera-Barrios, S.; Manzanares, R.V.; Araujo-Morera, J.; González, S.; Verdejo, R.; López-Manchado, M.A.; Santana, M.H. Understanding the Molecular Dynamics of Dual Crosslinked Networks by Dielectric Spectroscopy. Polymers 2021, 13, 3234.
- Kropotin, O.V.; Nesov, S.N.; Polonyankin, D.A.; Drozdova, E.A. Structure and phase composition of electrically conductive carbon black. J. Phys. Conf. Ser. 2022, 2182, 012076.
- Salaeh, S.; Kao-ian, P. Conductive epoxidized natural rubber nanocomposite with mechanical and electrical performance boosted by hybrid network structures. Polym. Test. 2022, 108, 107493.
- Qian, M.; Zou, B.; Shi, Y.; Zhang, Y.; Wang, X.; Huang, W.; Zhu, Y. Enhanced mechanical and dielectric properties of natural rubber using sustainable natural hybrid filler. Appl. Surf. Sci. Adv. 2021, 6, 100171.
- Mathias, K.A.; Shivashankar, H.; Shankar, B.S.M.; Kulkarni, S.M. Influence of filler on dielectric properties of silicone rubber particulate composite material. Mater. Today Proc. 2020, 33, 5623–5627.
- Allah, M.M.D.; Ali, Z.M.; Raslan, M.A. Dielectric, thermal and morphological characteristics of Nitrile butadiene rubber under effect filler/hybrid filler. Measures 2019, 131, 13–18.
- Aloui, S.; Lang, A.; Deckmann, H.; Klüppel, M.; Giese, U. Simultaneous characterization of dielectric and dynamic-mechanical properties of elastomeric materials under static and dynamic loads. Polymeters 2021, 215, 123413.
- Aloui, S.; Lang, A.; Deckmann, H.; Klüppel, M.; Giese, U. Corrigendum to ‘Simultaneous characterization of dielectric and dynamic-mechanical properties of elastomeric materials under static and dynamic loads. Polymeters 2021, 223, 123686.
- Abaci, U.; Guney, H.Y.; Yilmazoglu, M. Plasticizer effect on dielectric properties of poly (methyl methacrylate)/titanium dioxide composites. Polym. Compos. 2021, 29, S565–S574.
- Sengwa, R.J.; Dhatarwal, P.; Choudhary, S. Role of preparation methods on the structural and dielectric properties of plasticized polymer blend electrolytes: Correlation between ionic conductivity and dielectric parameters. Electrochim. Acta 2014, 142, 359–370.
- Maya, M.G.; George, S.C.; Jose, T.; Kailas, L.; Thomas, S. Development of a flexible and conductive elastomeric composite based on chloroprene rubber. Polym. Test. 2018, 65, 256–263.
- Chen, J.; Li, H.; Yu, Q.; Hu, Y.; Cui, X.; Zhu, Y.; Jiang, W. Strain sensing behaviors of stretchable conductive polymer composites loaded with different dimensional conductive fillers. Compos. Sci. Technol. 2018, 168, 388–396.
- Tonkov, D.N.; Kobylyatskaya, M.I.; Vasilyeva, E.S.; Semencha, A.V.; Gasumyants, V.E. Conductive properties of flexible polymer composites with different carbon-based fillers. J. Phys. Conf. Ser. 2022, 2227, 012022.
- Figuli, R.; Schwab, L.; Wilhelm, M.; Lacayo-Pineda, J.; Deckmann, H. Combined Dielectric (DEA) and Dynamic Mechanical Thermal Analysis (DMTA) in Compression Mode. KGK-Kautsch. Gummi Kunstst. 2016, 4, 22–27.
- Aloui, S.; Wurpts, W.; Deckmann, H. Methods for simultaneous dynamic-mechanical and dielectric analysis–Part 3: Dielectric investigation of elastomer composites under dynamic deformation. RFP Rubber Fibres Plast. Int. 2020, 4, 197–200.
- Aloui, S.; Wurpts, W.; Deckmann, H. Methods for simultaneous dynamic-mechanical and dielectric analysis–Part 2: Temperature dependence of the dielectric behavior of elastomer composites under static deformation. RFP Rubber Fibres Plast. Int. 2020, 2, 72–75.
- Aloui, S.; Wurpts, W.; Deckmann, H. Methods for simultaneous dynamic-mechanical and dielectric analysis–Part 1: Dielectric investigation of elastomer composites under static deformation. RFP Rubber Fibres Plast. Int. 2020, 1, 26–31.
- Aloui, S.; Deckmann, H. Static seals provide information about their own wear–Monitoring damage development by dynamic-mechanical and dielectric analyzer. KGK-Kautsch. Gummi Kunstst. 2019, 4, 14–19.
- Kremer, F.; Schönhals, A. (Eds.) Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003.
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–351.
- Bergmann, C.; Trimbach, J. Influence of plasticizers on the properties of natural rubber based compounds. KGK-Kautsch. Gummi Kunstst. 2014, 7, 40–49.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
739
Revisions:
3 times
(View History)
Update Date:
27 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




