Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xu, G. New-Onset Acute Kidney Disease Post COVID-19 Vaccination. Encyclopedia. Available online: https://encyclopedia.pub/entry/23105 (accessed on 07 February 2026).
Xu G. New-Onset Acute Kidney Disease Post COVID-19 Vaccination. Encyclopedia. Available at: https://encyclopedia.pub/entry/23105. Accessed February 07, 2026.
Xu, Gaosi. "New-Onset Acute Kidney Disease Post COVID-19 Vaccination" Encyclopedia, https://encyclopedia.pub/entry/23105 (accessed February 07, 2026).
Xu, G. (2022, May 19). New-Onset Acute Kidney Disease Post COVID-19 Vaccination. In Encyclopedia. https://encyclopedia.pub/entry/23105
Xu, Gaosi. "New-Onset Acute Kidney Disease Post COVID-19 Vaccination." Encyclopedia. Web. 19 May, 2022.
Copy Citation
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused an exceptional setback to the global economy and health. Vaccination is one of the most effective interventions to markedly decrease severe illness and death from COVID-19. Acute kidney disease (AKD) is defined as a condition of acute or subacute damage and/or loss of renal function between 7 and 90 days after exposure to an AKI initiating event
acute kidney disease
acute kidney injury
COVID-19
1. Introduction
With the ongoing coronavirus disease 2019 (COVID-19) pandemic and the emergence of new variants of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), the rapid development of effective and safe preventive vaccines is urgently required to control disease outbreaks [1][2]. Over the past 2 years, hundreds of COVID-19 vaccine candidates have been developed, tested, and finally rolled out, including protein-based vaccines (Novavax), inactivated vaccines (Sinovac Life Science), viral vector vaccines (Janssen, Oxford-AstraZeneca), and mRNA vaccines (Pfizer/BioNtech, Moderna, CureVac) (Figure 1) [2][3]. Among them, mRNA-based drugs are new but not unknown [4]. mRNA vaccines deliver transgenic mRNA through lipid nanoparticles, which act as carriers. Once injected, the mRNA is translated into the target protein in vivo, resulting in a strong immune response, and a 2-dose regimen confers 95% protection against COVID-19 [5]. To date, large phase III and IV trials have found these vaccines to have a good safety profile, with few serious reactions [3][6][7][8][9]. Common short-term adverse events include local injection site reactions, fever, fatigue, generalized pain, and headache [6][10].
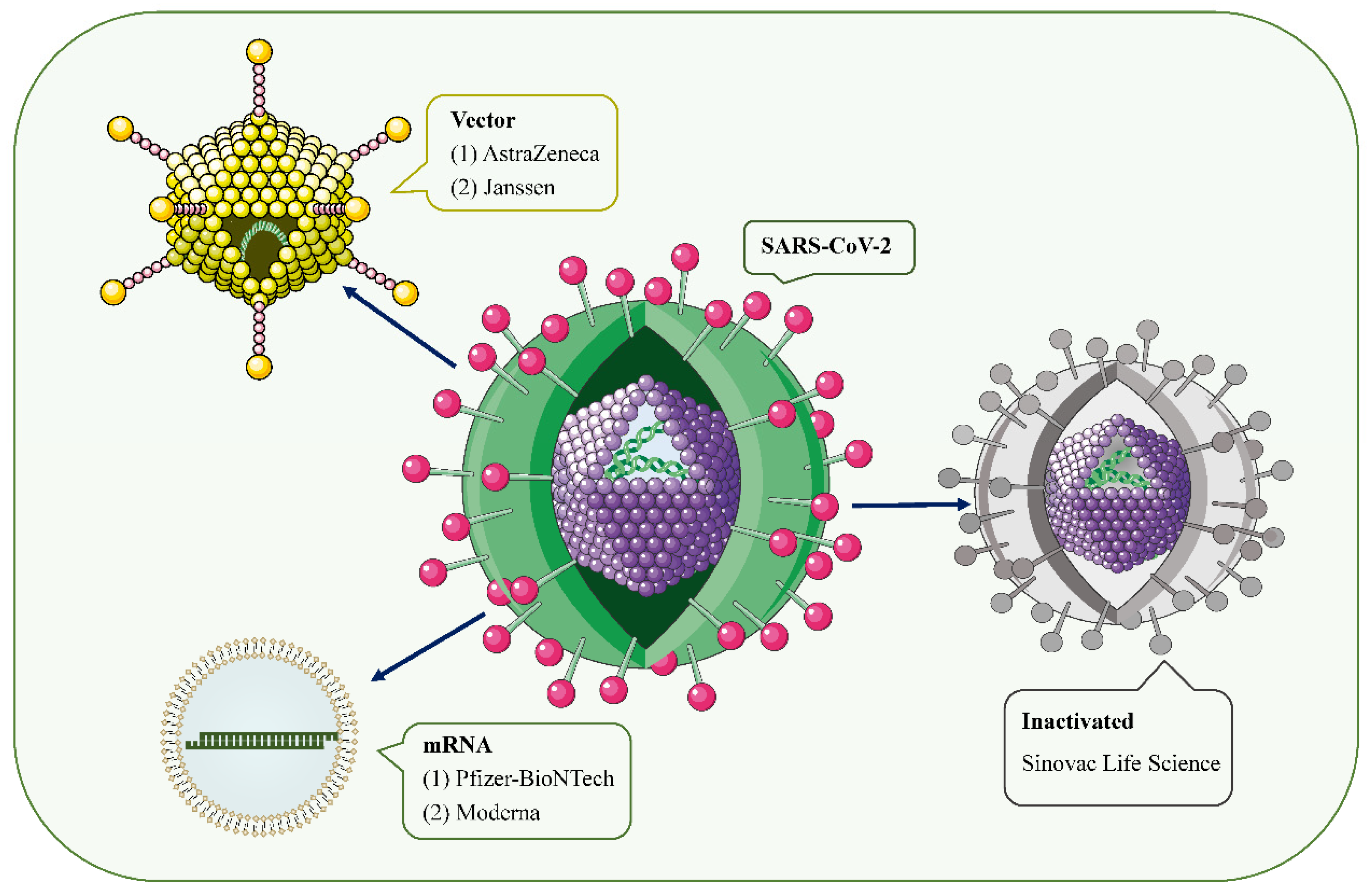 Figure 1. SARS-CoV-2 and the main types of vaccines that may trigger AKD. SARS-CoV-2 is a positive-sense single-stranded RNA virus with a lipid bilayer consisting of the spike S protein and membrane and envelope proteins. mRNA vaccines deliver transgenic mRNA through lipid nanoparticles as carriers. Viral vector vaccines utilize adenovirus and integrate genetic material from SARS-CoV-2 into its genome. Inactivated vaccines involve SARS-CoV-2 that has been killed by physical or chemical means.
Figure 1. SARS-CoV-2 and the main types of vaccines that may trigger AKD. SARS-CoV-2 is a positive-sense single-stranded RNA virus with a lipid bilayer consisting of the spike S protein and membrane and envelope proteins. mRNA vaccines deliver transgenic mRNA through lipid nanoparticles as carriers. Viral vector vaccines utilize adenovirus and integrate genetic material from SARS-CoV-2 into its genome. Inactivated vaccines involve SARS-CoV-2 that has been killed by physical or chemical means.However, since mass vaccination, there have been a few case reports of acute kidney injury (AKI), acute kidney disease (AKD), proteinuria, edema, gross hematuria, and other renal side effects requiring hospitalization after COVID-19 vaccinations [11]. Serum creatinine (Scr) levels and proteinuria recovered within 3 months of treatment in most patients. The vast majority of cases occurred after mRNA vaccine and adenoviral vector injection, and a few cases of glomerulonephritis associated with inactivated virus vaccines have also been reported.
2. Inducing AKD through COVID-19 Vaccine: Hypotheses
2.1. Podocyte Damage
The temporal association between intramuscular vaccination and the development of MCD speculates that a cell-mediated immune response may be a trigger for podocyte injury [12][13]. All 12 patients with MCD reported in the literature were over 60 years of age, developed AKD within 2 weeks of vaccination, and steroids appeared to be effective in achieving rapid remission (Table 1). Typically, following vaccination, the vaccine’s antigens are taken up by dendritic cells and then presented to T cell receptors on naive T cells [14]. This leads to the activation of antigen-specific effector T cells, peaking 7 to 14 days after vaccination [15]. Studies have also confirmed that during viral infection, cellular immune responses can be observed within about 1 week after infection, but T cell activation can occur 2–3 days earlier [16][17]. This answers the question of whether it is reasonable for a COVID-19 vaccine to elicit a cell-mediated response 3–4 days after administration.
Although the exact pathogenesis of MCD remains unclear, podocyte damage caused by circulating factors released by activated T lymphocytes appears to be decisive (Figure 2) [18][19]. During active stages of MCD, T cell subsets are imbalanced, and circulating CD8+ suppresses the prevalence of T cells, which is exacerbated by cytokine-induced damage [20]. Compared with conventional vaccines, mRNA vaccines are expected to provoke higher antibody responses and stronger CD8+ T and CD4+ T cell reactions, including higher chemokine and cytokine production [21][22]. The resulting irregular permeability factors can alter glomerular permeability and lead to marked proteinuria and kidney injury [13].
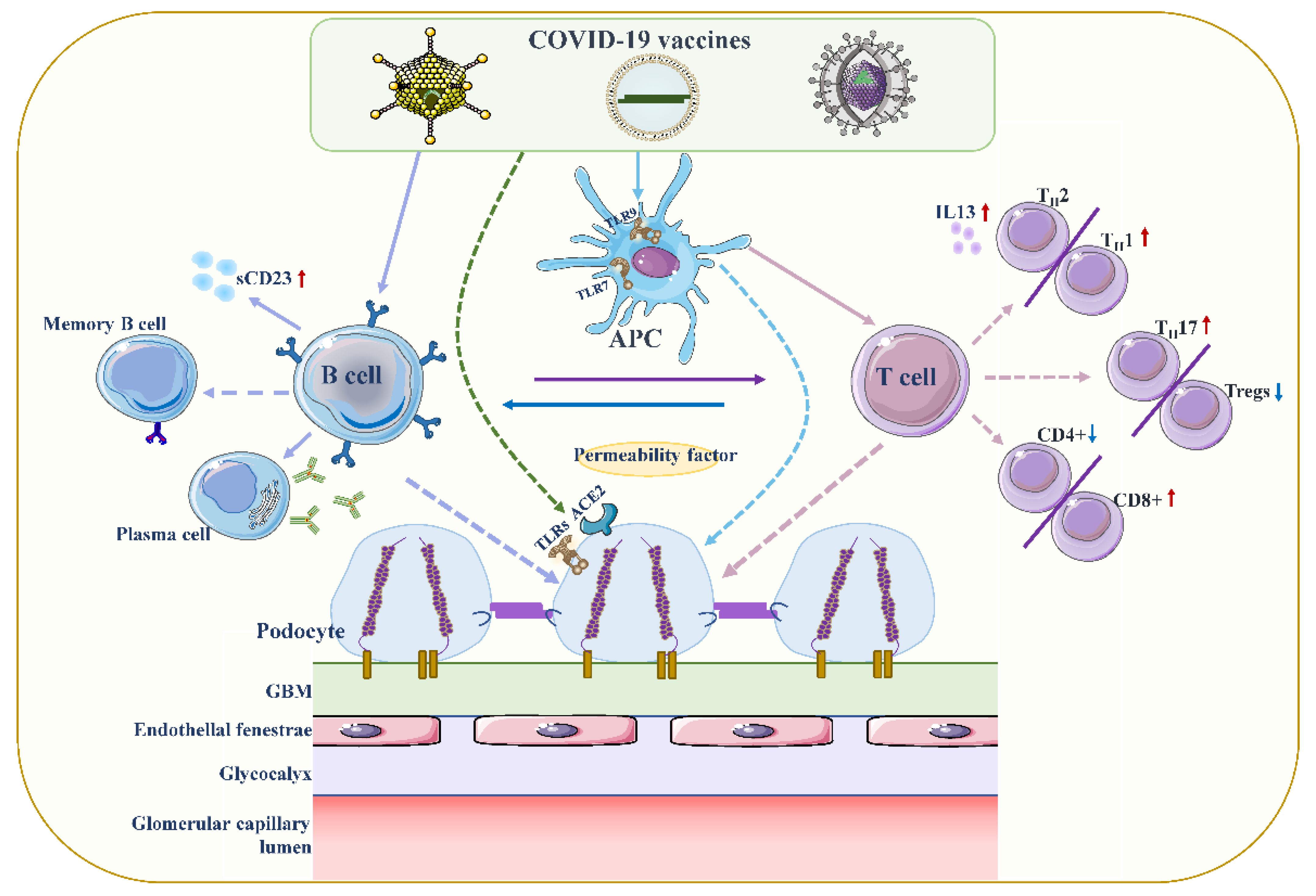 Figure 2. Proposed mechanisms of podocyte injury caused by COVID19 vaccination. Vaccination stimulates antigen-presenting cells (APCs) and B cells, which in turn activate T cells through antigen presentation and cytokine production. A decrease in CD4+ T helper (Th) cells is associated with the prevalence of CD8+ cytotoxic T cells, and an imbalance between Th2 and Th1 cells is associated with an increase in Th2-specific interleukin-13 (IL-13) production, and Th17. In contrast to increased cellular activity, the frequency and function of regulatory T cells (Tregs) decreased. Permeability proteins, such as cytokines and autoantibodies, can directly affect podocytes, leading to loss of foot processes and disruption of the glomerular permeability barrier. In addition, the vaccine can also affect podocytes through specific toll-like receptors (TLRs), and angiotensin conversion enzyme 2 (ACE2). The figure refers to the pathogenesis of minimal change disease by Vivarelli et al [13].
Figure 2. Proposed mechanisms of podocyte injury caused by COVID19 vaccination. Vaccination stimulates antigen-presenting cells (APCs) and B cells, which in turn activate T cells through antigen presentation and cytokine production. A decrease in CD4+ T helper (Th) cells is associated with the prevalence of CD8+ cytotoxic T cells, and an imbalance between Th2 and Th1 cells is associated with an increase in Th2-specific interleukin-13 (IL-13) production, and Th17. In contrast to increased cellular activity, the frequency and function of regulatory T cells (Tregs) decreased. Permeability proteins, such as cytokines and autoantibodies, can directly affect podocytes, leading to loss of foot processes and disruption of the glomerular permeability barrier. In addition, the vaccine can also affect podocytes through specific toll-like receptors (TLRs), and angiotensin conversion enzyme 2 (ACE2). The figure refers to the pathogenesis of minimal change disease by Vivarelli et al [13].Another hypothesis researchers speculate might be relevant is that type 2 helper T cells (Th2) indirectly induce tissue cell damage through hypersensitivity reactions via nucleic acid (NA) sensors. Previous study has demonstrated that T cells sensing their own NAs can trigger and amplify allergic inflammation independent of known NA sensors in innate immunity [23]. Muscle cells presenting viral mRNA-derived products on major histocompatibility complex class I are eliminated by CD8+ T cells, and self-NA released by dead muscle cells may directly induce T cell co-stimulation. This may be followed by Th2 differentiation and Th2-mediated allergic inflammation, causing podocytopathy [24]. Nevertheless, the study by Sahin et al. found that the COVID-19 mRNA vaccine elicited a cytokine response involving Th1 T cell responses [22][25].
Furthermore, SARS-CoV-2 can penetrate proximal tubular cells through ligation with angiotensin conversion enzyme 2 (ACE2) and CD147-spike protein to cause severe AKI, and can also penetrate podocytes through ligation with ACE2, resulting in podocyte dysfunction [26][27]. In addition, SARS-CoV-2 can also unbalance renin-angiotensin-aldosterone system (RAAS) activation, promoting inflammation, glomerular dysfunction, fibrosis, and vasoconstriction [27]. However, whether the vaccine is related to ACE2 and RAAS is unclear.
2.2. Increased Production of Anti-Neutrophil Cytoplasmic Autoantibodies (ANCAs)
Influenza and rabies vaccines based on viral mRNAs have been described to possibly lead to an increase in ANCA, contributing to the development of ANCA-associated vasculitis [28]. Moreover, it was confirmed that the ANCA response was significantly reduced after the treatment of vaccinees with ribonuclease. Scientists have found that in the context of COVID-19, a host response to viral RNA can directly cause ANCA-associated vasculitis (AAV) and an autoimmune response [29][30][31]. COVID-19 mRNA vaccination induced a stronger response of the innate immune system after the second booster compared with primary immunization [32]. The heightened innate immune response observed after the second vaccination with BNT162b2 mRNA vaccine may be an inducer of MPO-ANCA and PR3 autoantibodies [33]. Toll-like receptors (TLRs) can be expressed on leukocyte membranes and play an important role in inflammatory responses, recognizing viral antigens and promoting immune system activation. In AAV, major toll-like receptor 2 (TLR2) and toll-like receptor 9 (TLR9) activation can provoke autoimmunity [34]. Interestingly, Kumar et al. suggested that TLR2 was activated by a robust and specific immune response of immunodominant cytotoxic T-lymphocyte (CTL) to the spike glycoprotein of SARS-CoV2 (also produced by the COVID-19 vaccine) [35]. Messenger RNA vaccines could act as both antigen and adjuvant due to their intrinsic immunostimulatory properties of RNA; thus, they can be recognized by endosomal TLRs and cytosolic inflammasome components [25]. Therefore, the occurrence of AAV in the context of COVID-19 mRNA is highly relevant compared with non-mRNA vaccinations, but further experiments are required to verify the mechanism of the link between autoimmunity and a COVID-19 vaccine.
2.3. Vaccine-Induced Thrombotic Thrombocytopenia (VITT)
Some scholars have speculated that antiphospholipid antibodies (APLs) may be part of the cause of thrombosis after COVID-19 vaccination, by triggering the type I interferon response associated with APLs’ production [36][37]. It binds directly to platelets by inhibiting the anticoagulant pathway of protein C, triggers the coagulation cascade, and appears to be associated with abnormal activation of immune responses involving the complement cascade [36]. Thrombocytopenia and platelet activation have been reported following the administration of adenoviral gene transfer vectors [38]. Thrombocytopenia also occurred after treatment with some anti-sense oligonucleotides [39]. Based on the above background, another hypothesis speculates that the activation of platelets by adenovirus-platelet-leukocyte complexes, mediated by von Willebrand factor (VWF) and P-selectin, may lead to accelerated clearance of platelets in the liver [37][40].
However, the virus in viral vector vaccines is replication-incomparable and the circulating virus disappears 7–14 days after vaccination, so the viral localization to the central nervous system and digestive system causing thrombosis is unlikely [41]. In addition, Greinacher et al. suggested that the rare occurrence of VITT was mediated by platelet factor 4 (PF4)-dependent platelet-activating antibodies, which in turn stimulate platelets via their Fcγ receptors [42][43]. Immune complexes containing PF4 can be recognized by C1q, which binds to the Fc portion of IgG molecules. This results in C3 activation, expansion of the complement response, and production of downstream proinflammatory mediators and effectors, ultimately leading to enhanced thrombus inflammation.
2.4. Direct Induction of Myositis
A previous case reported that a patient who presented with profound left upper arm pain after COVID-19 mRNA vaccination had an increased serum creatine kinase concentration, indicating skeletal muscle damage and inflammation (myositis) [44]. There is also evidence of renal biopsies from post-vaccination patients showing massive rhabdomyolysis-induced myoglobin casting, which may contribute to worsening renal function [33].
References
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577.
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354.
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2021, 28, 202–221.
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773.
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7). an exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362.
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2. an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111.
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001). a phase 4, non-inferiority, single blind, randomised study. Lancet 2022, 399, 521–529.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416.
- Klomjit, N.; Alexander, M.P.; Fervenza, F.C.; Zoghby, Z.; Garg, A.; Hogan, M.C.; Nasr, S.H.; Minshar, M.A.; Zand, L. COVID-19 Vaccination and Glomerulonephritis. Kidney Int. Rep. 2021, 6, 2969–2978.
- D’Agati, V.D.; Kudose, S.; Bomback, A.S.; Adamidis, A.; Tartini, A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021, 100, 461–463.
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345.
- Williams, M.A.; Bevan, M.J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007, 25, 171–192.
- Mudd, P.A.; Minervina, A.A.; Pogorelyy, M.V.; Turner, J.S.; Kim, W.; Kalaidina, E.; Petersen, J.; Schmitz, A.J.; Lei, T.; Haile, A.; et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 2021, 185, 603–613.e15.
- Miao, H.; Hollenbaugh, J.A.; Zand, M.S.; Holden-Wiltse, J.; Mosmann, T.R.; Perelson, A.S.; Wu, H.; Topham, D.J. Quantifying the early immune response and adaptive immune response kinetics in mice infected with influenza A virus. J. Virol. 2010, 84, 6687–6698.
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880.
- Colucci, M.; Corpetti, G.; Emma, F.; Vivarelli, M. Immunology of idiopathic nephrotic syndrome. Pediatr. Nephrol. 2018, 33, 573–584.
- Mathieson, P.W. Immune dysregulation in minimal change nephropathy. Nephrol. Dial. Transplant. 2003, 18 (Suppl. S6), vi26–vi29.
- Le Berre, L.; Herve, C.; Buzelin, F.; Usal, C.; Soulillou, J.P.; Dantal, J. Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int. 2005, 68, 2079–2090.
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279.
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599.
- Imanishi, T.; Ishihara, C.; Badr Mel, S.; Hashimoto-Tane, A.; Kimura, Y.; Kawai, T.; Takeuchi, O.; Ishii, K.J.; Taniguchi, S.; Noda, T.; et al. Nucleic acid sensing by T cells initiates Th2 cell differentiation. Nat. Commun. 2014, 5, 3566.
- Kobayashi, S.; Fugo, K.; Yamazaki, K.; Terawaki, H. Minimal change disease soon after Pfizer-BioNTech COVID-19 vaccination. Clin. Kidney J. 2021, 14, 2606–2607.
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197.
- Gupta, R.K.; Bhargava, R.; Shaukat, A.A.; Albert, E.; Leggat, J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease. a report of 2 cases. BMC Nephrol. 2020, 21, 326.
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intens. Care Med. 2020, 46, 1339–1348.
- Jeffs, L.S.; Nitschke, J.; Tervaert, J.W.; Peh, C.A.; Hurtado, P.R. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin. Rheumatol. 2016, 35, 943–951.
- Uppal, N.N.; Kello, N.; Shah, H.H.; Khanin, Y.; De Oleo, I.R.; Epstein, E.; Sharma, P.; Larsen, C.P.; Bijol, V.; Jhaveri, K.D. De Novo ANCA-Associated Vasculitis With Glomerulonephritis in COVID-19. Kidney Int. Rep. 2020, 5, 2079–2083.
- Vlachoyiannopoulos, P.G.; Magira, E.; Alexopoulos, H.; Jahaj, E.; Theophilopoulou, K.; Kotanidou, A.; Tzioufas, A.G. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann. Rheum Dis. 2020, 79, 1661–1663.
- Izci Duran, T.; Turkmen, E.; Dilek, M.; Sayarlioglu, H.; Arik, N. ANCA-associated vasculitis after COVID-19. Rheumatol. Int. 2021, 41, 1523–1529.
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416.
- Hakroush, S.; Tampe, B. Case Report. ANCA-Associated Vasculitis Presenting With Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front. Immunol. 2021, 12, 762006.
- Summers, S.A.; Steinmetz, O.M.; Gan, P.Y.; Ooi, J.D.; Odobasic, D.; Kitching, A.R.; Holdsworth, S.R. Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis. Arthritis Rheum 2011, 63, 1124–1135.
- Kumar, N.; Admane, N.; Kumari, A.; Sood, D.; Grover, S.; Prajapati, V.K.; Chandra, R.; Grover, A. Cytotoxic T-lymphocyte elicited vaccine against SARS-CoV-2 employing immunoinformatics framework. Sci. Rep. 2021, 11, 7653.
- Talotta, R.; Robertson, E.S. Antiphospholipid antibodies and risk of post-COVID-19 vaccination thrombophilia. The straw that breaks the camel’s back? Cytokine Growth Factor. Rev. 2021, 60, 52–60.
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2021, 165, 386–401.
- Lai, K.Y.; Au, S.Y.; Fong, K.M. Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 385, e11.
- Pasin, F.; Calabrese, A.; Pelagatti, L. Immune thrombocytopenia following COVID-19 mRNA vaccine. casuality or causality? Intern. Emerg. Med. 2022, 17, 295–297.
- Othman, M.; Labelle, A.; Mazzetti, I.; Elbatarny, H.S.; Lillicrap, D. Adenovirus-induced thrombocytopenia. the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 2007, 109, 2832–2839.
- Eichinger, S.; Warkentin, T.E.; Greinacher, A. Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. Reply. N. Engl. J. Med. 2021, 385, e11.
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101.
- Greinacher, A.; Selleng, K.; Mayerle, J.; Palankar, R.; Wesche, J.; Reiche, S.; Aebischer, A.; Warkentin, T.E.; Muenchhoff, M.; Hellmuth, J.C.; et al. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood 2021, 138, 1269–1277.
- Theodorou, D.J.; Theodorou, S.J.; Axiotis, A.; Gianniki, M.; Tsifetaki, N. COVID-19 vaccine-related myositis. QJM 2021, 114, 424–425.
More
Information
Subjects:
Urology & Nephrology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
COVID-19
Revisions:
3 times
(View History)
Update Date:
23 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




