The c由严重急性呼吸系统综合征冠状病毒2(SARS-Coronavirus disease 2019 (V-2)引起的2019年冠状病毒病(COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused an exceptional setback to the global economy and health. Vaccination is one of the most effective interventions to markedly decrease severe illness and death from C)大流行对全球经济和健康造成了异常的挫折。疫苗接种是显著减少COVID-19. Acute kidney disease (AKD) is defined as a condition of acute or subacute damage and/or loss of renal function between 7 and 90 days after exposure to an AKI initiating event重症和死亡的最有效干预措施之一。
1. Introduction
With the ongoing coronavirus disease 2019 (COVID-19) pandemic and the emergence of new variants of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), the rapid development of effective and safe preventive vaccines is urgently required to control disease outbreaks
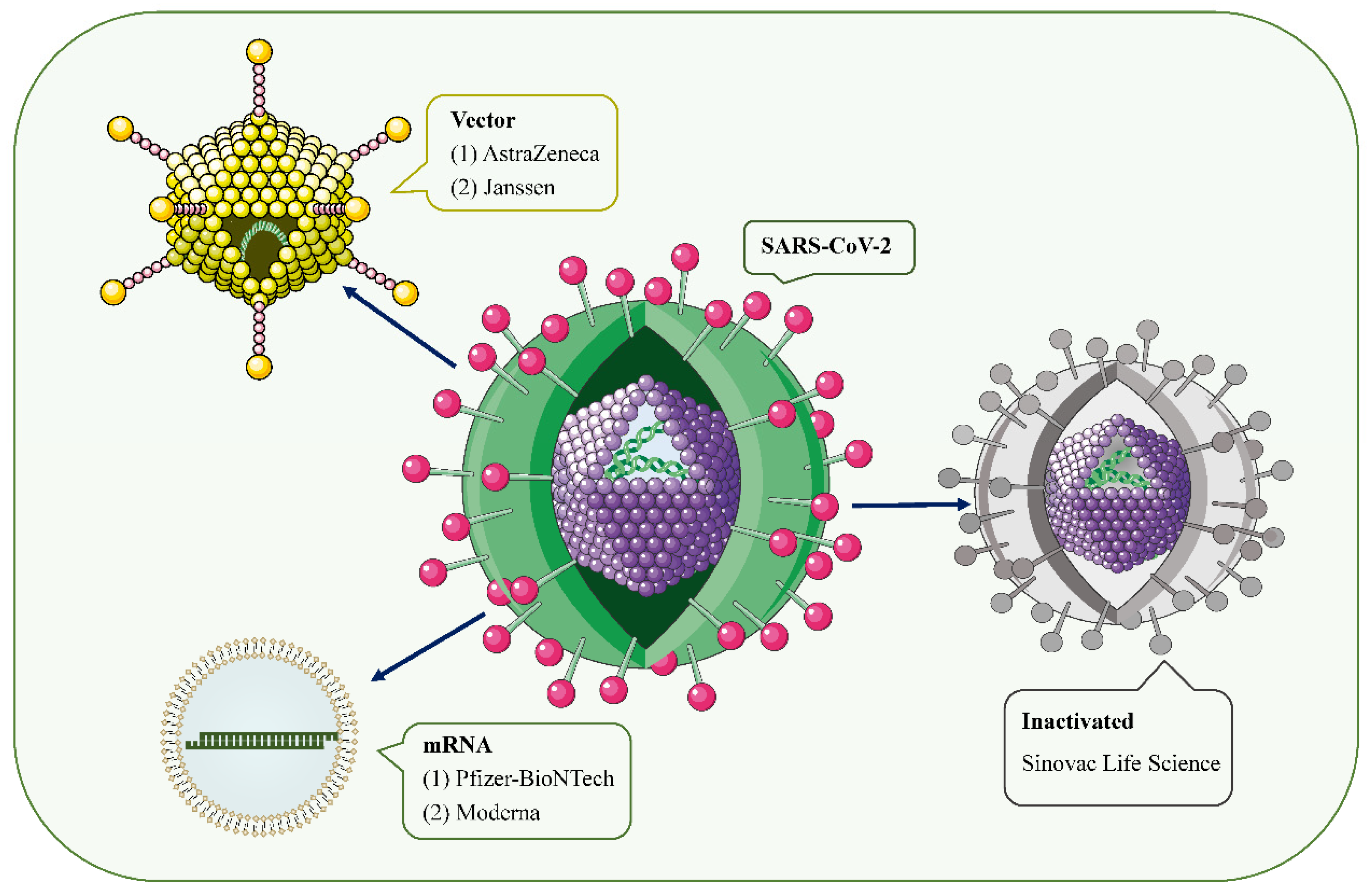
[1][2][1,2]. Over the past 2 years, hundreds of COVID-19 vaccine candidates have been developed, tested, and finally rolled out, including protein-based vaccines (Novavax), inactivated vaccines (Sinovac Life Science), viral vector vaccines (Janssen, Oxford-AstraZeneca), and mRNA vaccines (Pfizer/BioNtech, Moderna, CureVac) (
Figure 1)
[2][3][2,3]. Among them, mRNA-based drugs are new but not unknown
[4]. mRNA vaccines deliver transgenic mRNA through lipid nanoparticles, which act as carriers. Once injected, the mRNA is translated into the target protein in vivo, resulting in a strong immune response, and a 2-dose regimen confers 95% protection against COVID-19
[5]. To date, large phase III and IV trials have found these vaccines to have a good safety profile, with few serious reactions
[3][6][7][8][9][3,6,7,8,9]. Common short-term adverse events include local injection site reactions, fever, fatigue, generalized pain, and headache
[6][10][6,10].
Figure 1. SARS-CoV-2 and the main types of vaccines that may trigger AKD. SARS-CoV-2 is a positive-sense single-stranded RNA virus with a lipid bilayer consisting of the spike S protein and membrane and envelope proteins. mRNA vaccines deliver transgenic mRNA through lipid nanoparticles as carriers. Viral vector vaccines utilize adenovirus and integrate genetic material from SARS-CoV-2 into its genome. Inactivated vaccines involve SARS-CoV-2 that has been killed by physical or chemical means.
However, since mass vaccination, there have been a few case reports of acute kidney injury (AKI), acute kidney disease (AKD), proteinuria, edema, gross hematuria, and other renal side effects requiring hospitalization after COVID-19 vaccinations
[11]. Serum creatinine (Scr) levels and proteinuria recovered within 3 months of treatment in most patients. The vast majority of cases occurred after mRNA vaccine and adenoviral vector injection, and a few cases of glomerulonephritis associated with inactivated virus vaccines have also been reported.
2. Inducing AKD through COVID-19 Vaccine: Hypotheses
2.1. Podocyte Damage
The temporal association between intramuscular vaccination and the development of MCD speculates that a cell-mediated immune response may be a trigger for podocyte injury
[12][13][20,53]. All 12 patients with MCD reported in the literature were over 60 years of age, developed AKD within 2 weeks of vaccination, and steroids appeared to be effective in achieving rapid remission (
Table 1). Typically, following vaccination, the vaccine’s antigens are taken up by dendritic cells and then presented to T cell receptors on naive T cells
[14][54]. This leads to the activation of antigen-specific effector T cells, peaking 7 to 14 days after vaccination
[15][55]. Studies have also confirmed that during viral infection, cellular immune responses can be observed within about 1 week after infection, but T cell activation can occur 2–3 days earlier
[16][17][56,57]. This answers the question of whether it is reasonable for a COVID-19 vaccine to elicit a cell-mediated response 3–4 days after administration.
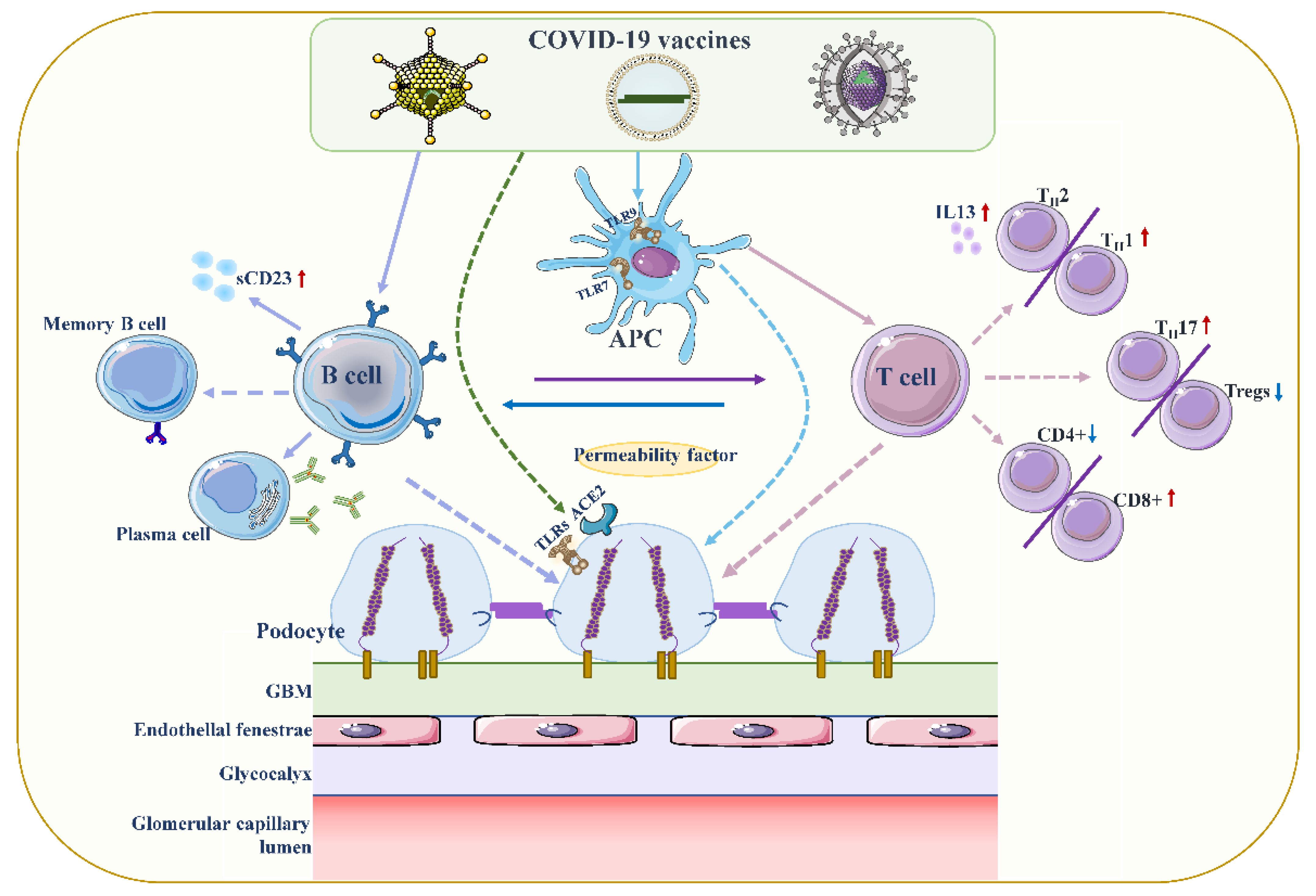
Although the exact pathogenesis of MCD remains unclear, podocyte damage caused by circulating factors released by activated T lymphocytes appears to be decisive (
Figure 2)
[18][19][58,59]. During active stages of MCD, T cell subsets are imbalanced, and circulating CD8+ suppresses the prevalence of T cells, which is exacerbated by cytokine-induced damage
[20][60]. Compared with conventional vaccines, mRNA vaccines are expected to provoke higher antibody responses and stronger CD8+ T and CD4+ T cell reactions, including higher chemokine and cytokine production
[21][22][61,62]. The resulting irregular permeability factors can alter glomerular permeability and lead to marked proteinuria and kidney injury
[13][53].
Figure 2. Proposed mechanisms of podocyte injury caused by COVID19 vaccination. Vaccination stimulates antigen-presenting cells (APCs) and B cells, which in turn activate T cells through antigen presentation and cytokine production. A decrease in CD4+ T helper (Th) cells is associated with the prevalence of CD8+ cytotoxic T cells, and an imbalance between Th2 and Th1 cells is associated with an increase in Th2-specific interleukin-13 (IL-13) production, and Th17. In contrast to increased cellular activity, the frequency and function of regulatory T cells (Tregs) decreased. Permeability proteins, such as cytokines and autoantibodies, can directly affect podocytes, leading to loss of foot processes and disruption of the glomerular permeability barrier. In addition, the vaccine can also affect podocytes through specific toll-like receptors (TLRs), and angiotensin conversion enzyme 2 (ACE2). The figure refers to the pathogenesis of minimal change disease by Vivarelli et al
[13][53].
Another hypothesis
rwe
searchers speculate might be relevant is that type 2 helper T cells (Th2) indirectly induce tissue cell damage through hypersensitivity reactions via nucleic acid (NA) sensors. Previous study has demonstrated that T cells sensing their own NAs can trigger and amplify allergic inflammation independent of known NA sensors in innate immunity
[23][63]. Muscle cells presenting viral mRNA-derived products on major histocompatibility complex class I are eliminated by CD8+ T cells, and self-NA released by dead muscle cells may directly induce T cell co-stimulation. This may be followed by Th2 differentiation and Th2-mediated allergic inflammation, causing podocytopathy
[24][23]. Nevertheless, the study by Sahin et al. found that the COVID-19 mRNA vaccine elicited a cytokine response involving Th1 T cell responses
[22][25][62,64].
Furthermore, SARS-CoV-2 can penetrate proximal tubular cells through ligation with angiotensin conversion enzyme 2 (ACE2) and CD147-spike protein to cause severe AKI, and can also penetrate podocytes through ligation with ACE2, resulting in podocyte dysfunction
[26][27][65,66]. In addition, SARS-CoV-2 can also unbalance renin-angiotensin-aldosterone system (RAAS) activation, promoting inflammation, glomerular dysfunction, fibrosis, and vasoconstriction
[27][66]. However, whether the vaccine is related to ACE2 and RAAS is unclear.
2.2. Increased Production of Anti-Neutrophil Cytoplasmic Autoantibodies (ANCAs)
Influenza and rabies vaccines based on viral 基于病毒性mRNA
s have been described to possibly lead to an increase in ANCA, contributing to the development of ANCA-associated vasculitis [28]. Moreover, it was confirmed that the 的流感和狂犬病疫苗已被描述可能导致ANCA升高,从而导致ANCA相关血管炎的发展[67]。此外,证实在用核糖核酸酶治疗疫苗后,ANCA
response was significantly reduced after the treatment of vaccinees with ribonuclease. Scientists have found that in the context of 反应显着降低。科学家发现,在COVID-19
, a host response to viral RNA can directly cause ANCA-associated vasculitis (AAV) and an autoimmune response [29][30][31]. 的背景下,宿主对病毒RNA的反应可直接引起ANCA相关血管炎(AAV)和自身免疫反应[68,69,70]。与初次免疫相比,COVID-19 mRNA
vaccination induced a stronger response of the innate immune system after the second booster compared with primary immunization [32]. The heightened innate immune response observed after the second vaccination with 疫苗接种在第二次加强免疫后诱导先天免疫系统的反应更强[71]。第二次接种BNT162b2 mRNA
vaccine may be an inducer of 疫苗后观察到的先天免疫应答增强可能是MPO-ANCA
and PR3 autoantibodies [33]. 和PR3自身抗体的诱导剂[34]。Toll
-like receptors (样受体(TLR
s) can be expressed on leukocyte membranes and play an important role in inflammatory responses, recognizing viral antigens and promoting immune system activation. In AAV, major toll-like receptor 2 (TLR2) and toll-like receptor 9 (TLR9) activation can provoke autoimmunity [34]. Interestingly, )可以在白细胞膜上表达,并在炎症反应,识别病毒抗原和促进免疫系统激活中起重要作用。在AAV中,主要收费样受体2(TLR2)和收费样受体9(TLR9)激活可引起自身免疫[72]。有趣的是,Kumar
et al. suggested that 等人提出,TLR2
was activated by a robust and specific immune response of immunodominant cytotoxic T-lymphocyte (CTL) to the spike glycoprotein of 是由免疫显性细胞毒性T淋巴细胞(CTL)对SARS-CoV2
(also produced by the (也由COVID-19
vaccine) [35]. Messenger 疫苗产生的)的刺突糖蛋白的强健和特异性免疫反应激活的[73]。信使RNA
vaccines could act as both antigen and adjuvant due to their intrinsic immunostimulatory properties of 疫苗由于其RNA
; thus, they can be recognized by endosomal TLRs and cytosolic infla的内在免疫刺激特性,可以同时作为抗原和佐剂;因此,它们可以通过内体TLR和胞质炎症小体成分来识别[64]。因此,与非m
masome components [25]. Therefore, the occurrence of RNA
AV in the context of 疫苗接种相比,在COVID-19 mRNA
is highly relevant compared with non-mRNA vaccinations, but further experiments are required to verify the mechanism of the link between autoimmunity and a 背景下AAV的发生具有高度相关性,但需要进一步的实验来验证自身免疫与COVID-19
vaccine.疫苗之间联系的机制。
2.3. Vaccine-Induced Thrombotic Thrombocytopenia (VITT)
疫苗诱导的血栓性血小板减少症(VITT)
Some scholars have speculated that antiphospholipid antibodies (一些学者推测,抗磷脂抗体(APL
s) may be part of the cause of thrombosis after )可能是COVID-19
vaccination, by triggering the type I interferon response associated with APLs’ production [36][37]. 疫苗接种后血栓形成的部分原因,因为它会触发与APLs产生相关的I
t binds directly to platelets by inhibiting the anticoagulant pathway of protein 型干扰素反应[74,75]。它通过抑制蛋白C
, triggers the coagulation cascade, and appears to be associated with abnormal activation of immune responses involving the complement cascade [36]. Thrombocytopenia and platelet activation have been reported following the administration of adenoviral gene transfer vectors [38]. Thrombocytopenia also occurred after treatment with some anti-sense oligonucleotides [39]. Based on the above background, another hypothesis speculates that the activation of platelets by adenovirus-platelet-leukocyte complexes, mediated by von Willebrand factor (的抗凝血途径直接与血小板结合,触发凝血级联反应,并且似乎与涉及补体级联反应的免疫应答的异常激活有关[74]。据报道,在给予腺病毒基因转移载体后,血小板减少和血小板活化[76]。血小板减少症也发生在一些反义寡核苷酸治疗后[77]。基于上述背景,另一种假设推测,由血管性血友病因子(VWF
) and )和P-
selectin, may lead to accelerated clearance of platelets in the liver [37][40].选择素介导的腺病毒-血小板-白细胞复合物活化血小板可能导致肝脏血小板清除加速[75,78]。
However, the virus in viral vector vaccines is replication然而,病毒载体疫苗中的病毒是不可复制的,并且循环病毒在接种疫苗后7-
incomparable and the circulating virus disappears 7–14 days after vaccination, so the viral localization to the central nervous system and digestive system causing thrombosis is unlikely [41]. In addition, 14日消失,因此病毒定位到中枢神经系统和消化系统以引起血栓形成的可能性不大[79]。此外,Greinacher
et al. suggested that the rare occurrence of 等人提出,VITT
was mediated by platelet factor 4 (PF4)-dependent platelet-activating antibodies, which in turn stimulate platelets via their Fcγ receptors [42][43]. Immune complexes containing 的罕见发生是由血小板因子4(PF4)依赖性血小板活化抗体介导的,而血小板激活抗体又通过其Fcγ受体刺激血小板[80,81]。含有PF4
can be recognized by 的免疫复合物可以被C1q识别,C1q
, which binds to the Fc portion of IgG molecules. This results in C3 activation, expansion of the complement response, and production of downstream proinflammatory mediators and effectors, ultimately leading to enhanced thrombus inflammation.与IgG分子的Fc部分结合。这导致C3活化,补体反应的扩大以及下游促炎介质和效应子的产生,最终导致血栓炎症增强。
2.4. Direct Induction of Myositis
2.4. 直接诱导肌炎
A previous case reported that a patient who presented with profound left upper arm pain after 一例既往病例报告称,一例患者在接种COVID-19 mRNA
vaccination had an increased serum creatine kinase concentration, indicating skeletal muscle damage and inflammation (myositis) [44]. There is also evidence of renal biopsies from post-vaccination patients showing massive rhabdomyolysis-induced myoglobin casting, which may contribute to worsening renal function [33].疫苗后出现严重左上臂疼痛,血清肌酸激酶浓度升高,提示骨骼肌损伤和炎症(肌炎)[82]。还有证据表明,疫苗接种后患者的肾活检显示大量横纹肌溶解诱导的肌红蛋白投射,这可能导致肾功能恶化[34]。