Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Urology & Nephrology
由严重急性呼吸系统综合征冠状病毒2(SARS-CoV-2)引起的2019年冠状病毒病(COVID-19)大流行对全球经济和健康造成了异常的挫折。疫苗接种是显著减少COVID-19重症和死亡的最有效干预措施之一。
- acute kidney disease
- acute kidney injury
- COVID-19
1. Introduction
With the ongoing coronavirus disease 2019 (COVID-19) pandemic and the emergence of new variants of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), the rapid development of effective and safe preventive vaccines is urgently required to control disease outbreaks [1,2]. Over the past 2 years, hundreds of COVID-19 vaccine candidates have been developed, tested, and finally rolled out, including protein-based vaccines (Novavax), inactivated vaccines (Sinovac Life Science), viral vector vaccines (Janssen, Oxford-AstraZeneca), and mRNA vaccines (Pfizer/BioNtech, Moderna, CureVac) (Figure 1) [2,3]. Among them, mRNA-based drugs are new but not unknown [4]. mRNA vaccines deliver transgenic mRNA through lipid nanoparticles, which act as carriers. Once injected, the mRNA is translated into the target protein in vivo, resulting in a strong immune response, and a 2-dose regimen confers 95% protection against COVID-19 [5]. To date, large phase III and IV trials have found these vaccines to have a good safety profile, with few serious reactions [3,6,7,8,9]. Common short-term adverse events include local injection site reactions, fever, fatigue, generalized pain, and headache [6,10].
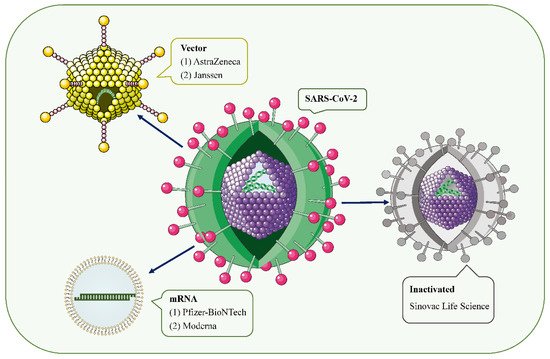
Figure 1. SARS-CoV-2 and the main types of vaccines that may trigger AKD. SARS-CoV-2 is a positive-sense single-stranded RNA virus with a lipid bilayer consisting of the spike S protein and membrane and envelope proteins. mRNA vaccines deliver transgenic mRNA through lipid nanoparticles as carriers. Viral vector vaccines utilize adenovirus and integrate genetic material from SARS-CoV-2 into its genome. Inactivated vaccines involve SARS-CoV-2 that has been killed by physical or chemical means.
However, since mass vaccination, there have been a few case reports of acute kidney injury (AKI), acute kidney disease (AKD), proteinuria, edema, gross hematuria, and other renal side effects requiring hospitalization after COVID-19 vaccinations [11]. Serum creatinine (Scr) levels and proteinuria recovered within 3 months of treatment in most patients. The vast majority of cases occurred after mRNA vaccine and adenoviral vector injection, and a few cases of glomerulonephritis associated with inactivated virus vaccines have also been reported.
2. Inducing AKD through COVID-19 Vaccine: Hypotheses
2.1. Podocyte Damage
The temporal association between intramuscular vaccination and the development of MCD speculates that a cell-mediated immune response may be a trigger for podocyte injury [20,53]. All 12 patients with MCD reported in the literature were over 60 years of age, developed AKD within 2 weeks of vaccination, and steroids appeared to be effective in achieving rapid remission (Table 1). Typically, following vaccination, the vaccine’s antigens are taken up by dendritic cells and then presented to T cell receptors on naive T cells [54]. This leads to the activation of antigen-specific effector T cells, peaking 7 to 14 days after vaccination [55]. Studies have also confirmed that during viral infection, cellular immune responses can be observed within about 1 week after infection, but T cell activation can occur 2–3 days earlier [56,57]. This answers the question of whether it is reasonable for a COVID-19 vaccine to elicit a cell-mediated response 3–4 days after administration.
Although the exact pathogenesis of MCD remains unclear, podocyte damage caused by circulating factors released by activated T lymphocytes appears to be decisive (Figure 2) [58,59]. During active stages of MCD, T cell subsets are imbalanced, and circulating CD8+ suppresses the prevalence of T cells, which is exacerbated by cytokine-induced damage [60]. Compared with conventional vaccines, mRNA vaccines are expected to provoke higher antibody responses and stronger CD8+ T and CD4+ T cell reactions, including higher chemokine and cytokine production [61,62]. The resulting irregular permeability factors can alter glomerular permeability and lead to marked proteinuria and kidney injury [53].
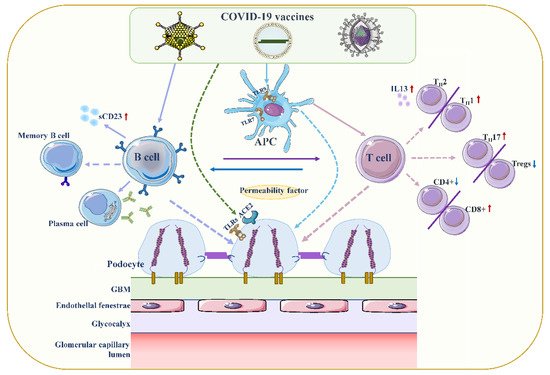
Figure 2. Proposed mechanisms of podocyte injury caused by COVID19 vaccination. Vaccination stimulates antigen-presenting cells (APCs) and B cells, which in turn activate T cells through antigen presentation and cytokine production. A decrease in CD4+ T helper (Th) cells is associated with the prevalence of CD8+ cytotoxic T cells, and an imbalance between Th2 and Th1 cells is associated with an increase in Th2-specific interleukin-13 (IL-13) production, and Th17. In contrast to increased cellular activity, the frequency and function of regulatory T cells (Tregs) decreased. Permeability proteins, such as cytokines and autoantibodies, can directly affect podocytes, leading to loss of foot processes and disruption of the glomerular permeability barrier. In addition, the vaccine can also affect podocytes through specific toll-like receptors (TLRs), and angiotensin conversion enzyme 2 (ACE2). The figure refers to the pathogenesis of minimal change disease by Vivarelli et al [53].
Another hypothesis we speculate might be relevant is that type 2 helper T cells (Th2) indirectly induce tissue cell damage through hypersensitivity reactions via nucleic acid (NA) sensors. Previous study has demonstrated that T cells sensing their own NAs can trigger and amplify allergic inflammation independent of known NA sensors in innate immunity [63]. Muscle cells presenting viral mRNA-derived products on major histocompatibility complex class I are eliminated by CD8+ T cells, and self-NA released by dead muscle cells may directly induce T cell co-stimulation. This may be followed by Th2 differentiation and Th2-mediated allergic inflammation, causing podocytopathy [23]. Nevertheless, the study by Sahin et al. found that the COVID-19 mRNA vaccine elicited a cytokine response involving Th1 T cell responses [62,64].
Furthermore, SARS-CoV-2 can penetrate proximal tubular cells through ligation with angiotensin conversion enzyme 2 (ACE2) and CD147-spike protein to cause severe AKI, and can also penetrate podocytes through ligation with ACE2, resulting in podocyte dysfunction [65,66]. In addition, SARS-CoV-2 can also unbalance renin-angiotensin-aldosterone system (RAAS) activation, promoting inflammation, glomerular dysfunction, fibrosis, and vasoconstriction [66]. However, whether the vaccine is related to ACE2 and RAAS is unclear.
2.2. Increased Production of Anti-Neutrophil Cytoplasmic Autoantibodies (ANCAs)
基于病毒性mRNA的流感和狂犬病疫苗已被描述可能导致ANCA升高,从而导致ANCA相关血管炎的发展[67]。此外,证实在用核糖核酸酶治疗疫苗后,ANCA反应显着降低。科学家发现,在COVID-19的背景下,宿主对病毒RNA的反应可直接引起ANCA相关血管炎(AAV)和自身免疫反应[68,69,70]。与初次免疫相比,COVID-19 mRNA疫苗接种在第二次加强免疫后诱导先天免疫系统的反应更强[71]。第二次接种BNT162b2 mRNA疫苗后观察到的先天免疫应答增强可能是MPO-ANCA和PR3自身抗体的诱导剂[34]。Toll样受体(TLR)可以在白细胞膜上表达,并在炎症反应,识别病毒抗原和促进免疫系统激活中起重要作用。在AAV中,主要收费样受体2(TLR2)和收费样受体9(TLR9)激活可引起自身免疫[72]。有趣的是,Kumar等人提出,TLR2是由免疫显性细胞毒性T淋巴细胞(CTL)对SARS-CoV2(也由COVID-19疫苗产生的)的刺突糖蛋白的强健和特异性免疫反应激活的[73]。信使RNA疫苗由于其RNA的内在免疫刺激特性,可以同时作为抗原和佐剂;因此,它们可以通过内体TLR和胞质炎症小体成分来识别[64]。因此,与非mRNA疫苗接种相比,在COVID-19 mRNA背景下AAV的发生具有高度相关性,但需要进一步的实验来验证自身免疫与COVID-19疫苗之间联系的机制。
疫苗诱导的血栓性血小板减少症(VITT)
一些学者推测,抗磷脂抗体(APL)可能是COVID-19疫苗接种后血栓形成的部分原因,因为它会触发与APLs产生相关的I型干扰素反应[74,75]。它通过抑制蛋白C的抗凝血途径直接与血小板结合,触发凝血级联反应,并且似乎与涉及补体级联反应的免疫应答的异常激活有关[74]。据报道,在给予腺病毒基因转移载体后,血小板减少和血小板活化[76]。血小板减少症也发生在一些反义寡核苷酸治疗后[77]。基于上述背景,另一种假设推测,由血管性血友病因子(VWF)和P-选择素介导的腺病毒-血小板-白细胞复合物活化血小板可能导致肝脏血小板清除加速[75,78]。
然而,病毒载体疫苗中的病毒是不可复制的,并且循环病毒在接种疫苗后7-14日消失,因此病毒定位到中枢神经系统和消化系统以引起血栓形成的可能性不大[79]。此外,Greinacher等人提出,VITT的罕见发生是由血小板因子4(PF4)依赖性血小板活化抗体介导的,而血小板激活抗体又通过其Fcγ受体刺激血小板[80,81]。含有PF4的免疫复合物可以被C1q识别,C1q与IgG分子的Fc部分结合。这导致C3活化,补体反应的扩大以及下游促炎介质和效应子的产生,最终导致血栓炎症增强。
2.4. 直接诱导肌炎
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10050742
This entry is offline, you can click here to edit this entry!
