
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anisha Veeren | -- | 1136 | 2022-05-18 03:31:35 | | | |
| 2 | Peter Tang | -5 word(s) | 1131 | 2022-05-18 03:37:50 | | |
Video Upload Options
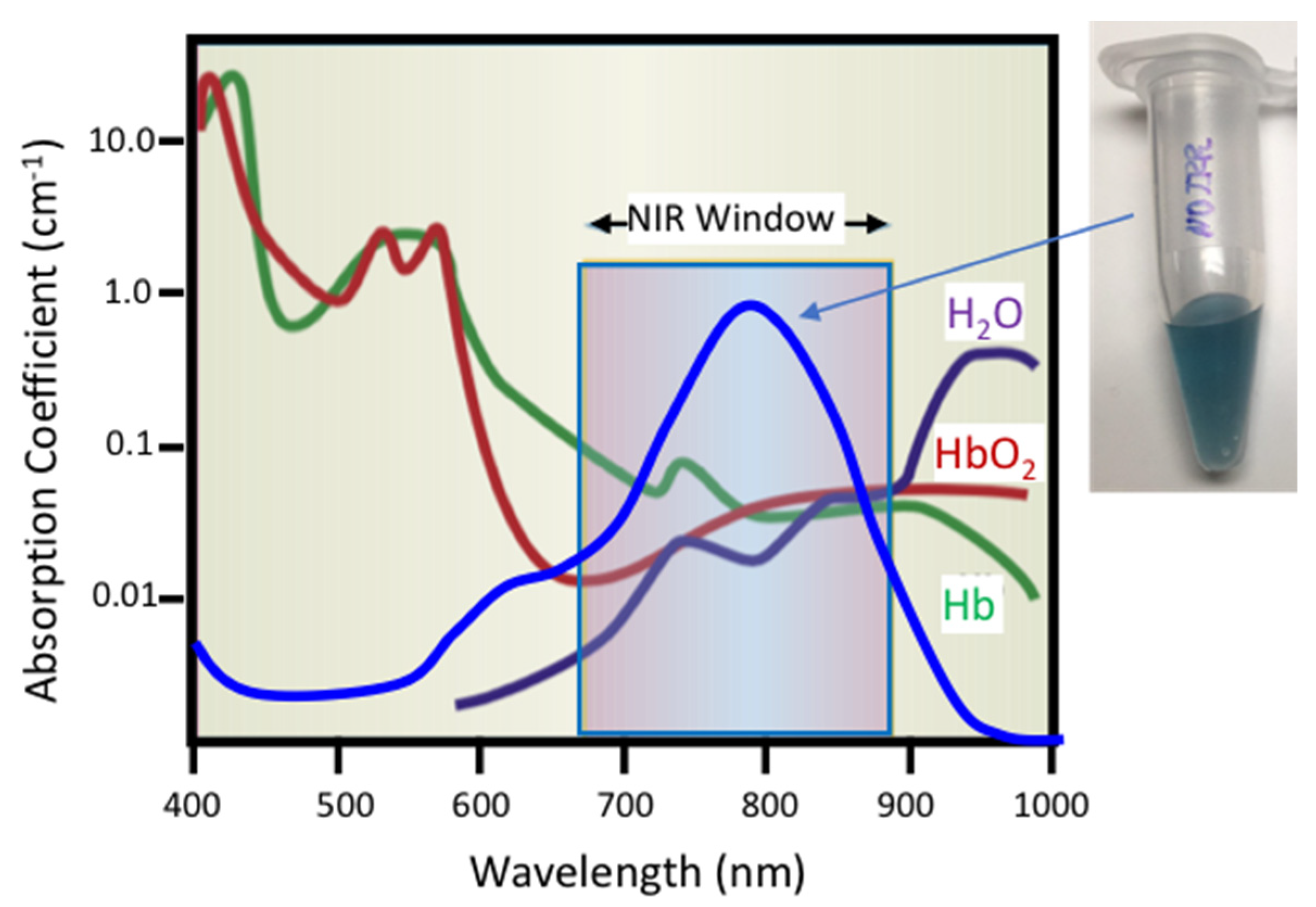
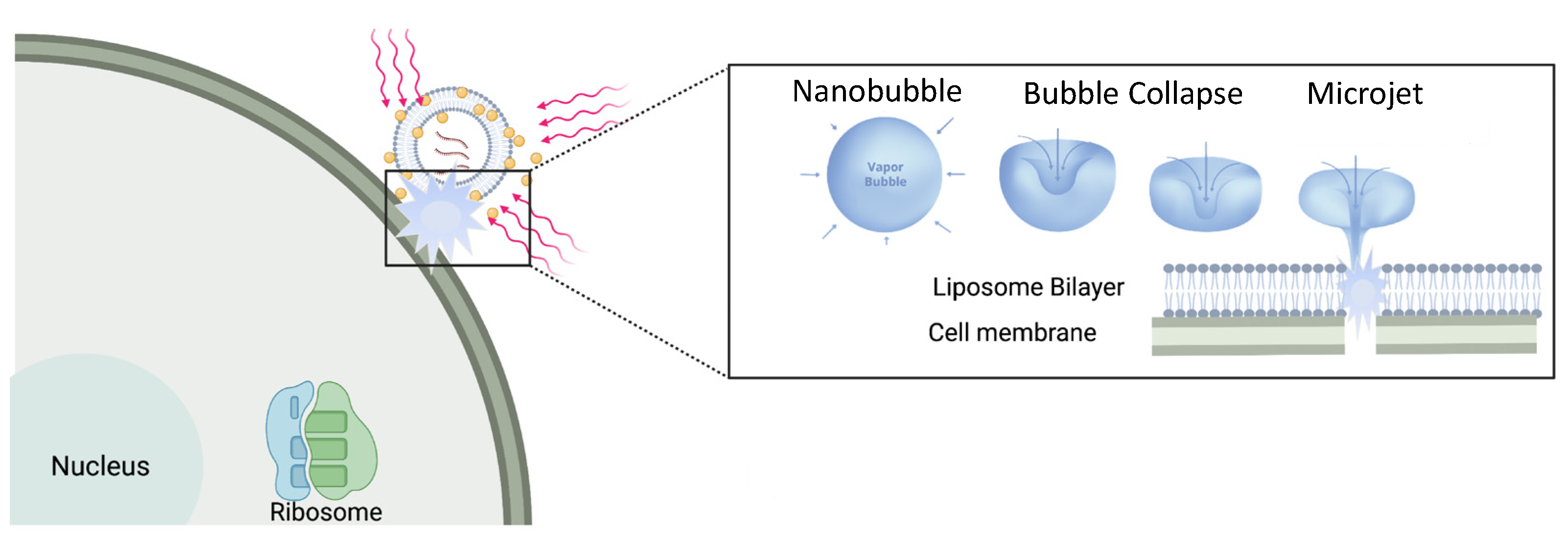
Liposomes can sequester a variety of bioactive water-soluble ions, ligands and enzymes, and oligonucleotides. The bilayer that separates the liposome interior from the exterior solution provides a physical barrier to contents release and degradation. Tethering plasmon-resonant, hollow gold nanoshells to the liposomes, or growing gold nanoparticles directly on the liposome exterior, allows liposome contents to be released by nanosecond or shorter pulses of near-infrared light (NIR). Gold nanoshells or nanoparticles strongly adsorb NIR light; cells, tissues, and physiological media are transparent to NIR, allowing penetration depths of millimeters to centimeters. Nano to picosecond pulses of NIR light rapidly heat the gold nanoshells, inducing the formation of vapor nanobubbles, similar to cavitation bubbles.
1. Introduction
2. Separating Cargo Sequestration from Rapid Release
3. Plasmon-Resonant Gold Nanostructures
References
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27.
- Deamer, D.W. From “Banghasomes” to liposomes: A memoir of Alec Bangham, 1921–2010. FASEB J. 2010, 24, 1308–1310.
- Xing, H.; Hwang, K.; Lu, Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics 2016, 6, 1336–1352.
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966.
- Filipczak, N.; Pan, J.Y.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22.
- Kieler-Ferguson, H.M.; Chan, D.; Sockolosky, J.; Finney, L.; Maxey, E.; Vogt, S.; Szoka, F.C., Jr. Encapsulation, controlled release, and antitumor efficacy of cisplatin delivered in liposomes composed of sterol-modified phospholipids. Eur. J. Pharm. Sci. 2017, 103, 85–93.
- Szoka, F.; Papahadjopoulos, D. Comparative Properties and Methods of Preparation of Lipid Vesicles (Liposomes). Ann Rev Biophys. Bioeng. 1980, 9, 467–508.
- Anderson, L.J.E.; Hansen, E.; Lukianova-Hleb, E.Y.; Hafner, J.H.; Lapotko, D.O. Optically guided controlled release from liposomes with tunable plasmonic nanobubbles. J. Control. Release 2010, 144, 151–158.
- Discher, D.E.; Eisenberg, A. Polymer Vesicles. Science 2002, 297, 967–973.
- Kube, S.; Hersch, N.; Naumovska, E.; Gensch, T.; Hendriks, J.; Franzen, A.; Landvogt, L.; Siebrasse, J.-P.; Kubitscheck, U.; Hoffmann, B.; et al. Fusogenic Liposomes as Nanocarriers for the Delivery of Intracellular Proteins. Langmuir 2017, 33, 1051–1059.
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724.
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control. Release Soc. 2012, 160, 117–134.
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365.
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the Mainstream. Science 2004, 303, 1818–1822.
- Schwendener, R.A. Liposomes in biology and medicine. In Bio-Applications of Nanoparticles; Chan, W.C.W., Ed.; Springer: Berlin, Germany, 2007; pp. 117–128.
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48.
- Wang, N.; Chen, M.N.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150.
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169.
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Drug Deliv. Rev. 2011, 63, 131–135.
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195.
- Willis, M.; Forssen, E. Ligand-targeted liposomes. Adv. Drug Deliv. Rev. 1998, 29, 249–271.
- Maruyama, K. In Vivo Targeting by Liposomes. Biol. Pharm. Bull. 2000, 23, 791–799.
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99.
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528.
- Noble, G.T.; Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. Ligand-targeted liposome design: Challenges and Fundamental Considerations. Trends Biotechnol. 2014, 32, 32–45.
- Agrawal, M.; Ajazuddin; Tripathi, D.K.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 2017, 260, 61–77.
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 48, 107727.
- Allen, T.M.; Hansen, C.B.; Lopes de Menezes, D.E. Pharmacokinetics of long-circulating liposomes. Adv. Drug Deliv. Rev. 1995, 16, 267–284.
- Papahadjopolous, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.L.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C. Sterically stabilized liposomes: Improvements in Pharmacokinetics and Antitumour Therapeutic Efficacy. Proc. Nat. Acad. Sci. USA 1991, 88, 11460–11464.
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953.
- Abraham, S.A.; Waterhouse, D.N.; Mayer, L.D.; Cullis, P.R.; Madden, T.D.; Bally, M.B. The Liposomal Formulation of Doxorubicin. Methods Enzymol. 2005, 391, 71–97.
- Forbes, N.; Shin, J.E.; Ogunyankin, M.; Zasadzinski, J.A. Inside-outside self-assembly of light-activated fast-release liposomes. Phys. Chem. Chem. Phys. 2015, 17, 15569–15578.
- Forbes, N.; Pallaoro, A.; Reich, N.O.; Zasadzinski, J.A. Rapid, reversible release from thermosensitive liposomes triggered by near infrared light. Part. Part. Syst. Charact. 2014, 31, 1158–1167.
- White, S.C.; Lorigan, P.; Margison, G.P.; Margison, J.M.; Martin, F.; Thatcher, N.; Anderson, H.; Ranson, M. Phase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancer. Br. J. Cancer 2006, 95, 822–828.
- Ponce, A.M.; Vujaskovic, Z.; Yuan, F.; Needham, D.; Dewhirst, M.W. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperth. 2006, 22, 205–213.
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.-Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming Limitations in Nanoparticle Drug Delivery: Triggered, Intravascular Release to Improve Drug Penetration into Tumors. Cancer Res. 2012, 72, 5566–5575.
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125.
- Ruoslahti, E. Vascular zip codes in angiogenesis and metastasis. Biochem. Soc. Trans. 2004, 32, 397–402.
- Ruoslahti, E. Peptides as Targeting Elements and Tissue Penetration Devices for Nanoparticles. Adv. Mater. 2012, 24, 3747–3756.
- Noble, C.O.; Kirpotin, D.B.; Hayes, M.E.; Mamot, C.; Hong, K.; Park, J.W.; Benz, C.C.; Marks, J.D.; Drummond, D.C. Development of ligand-targeted liposomes for cancer therapy. Expert Opin. Ther. Targets 2004, 8, 335–353.
- Oishi, M.; Kataoka, K.; Nagasaki, Y. pH-responsive three-layered PEGylated polyplex micelle based on a lactosylated ABC triblock copolymer as a targetable and endosome disruptive nonviral gene vector. Bioconjugate Chem. 2006, 17, 677–688.
- Jørgensen, K.; Davidsen, J.; Mouritsen, O.G. Biophysical mechanisms of phospholipase A2 activation and their use in liposome-based drug delivery. FEBS Lett. 2002, 531, 23–27.
- Davidsen, J.; Jørgensen, K.; Andresen, T.L.; Mouritsen, O.G. Secreted phospholipase A2 as a new enzymatic trigger mechanism for localised liposomal drug release and absorption in diseased tissue. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1609, 95–101.
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243.
- Ogunyankin, M.O.; Shin, J.E.; Lapotko, D.O.; Ferry, V.; Zasadzinski, J.A. Optimizing the NIR Fluence Threshold for Nanobubble Generation by Controlled Synthesis of 10–40 nm Hollow Gold Nanoshells. Adv. Funct. Mater. 2018, 28, 1705272.
- Li, X.; Che, Z.; Mazhar, K.; Price, T.J.; Qin, Z. Ultrafast Near-Infrared Light-Triggered Intracellular Uncaging to Probe Cell Signaling. Adv. Funct. Mater. 2017, 27, 1605778.
- Xiong, H.; Li, X.; Kang, P.; Perish, J.; Neuhaus, F.; PLoSki, J.E.; Kroener, S.; Ogunyankin, M.O.; Shin, J.E.; Zasadzinski, J.A.; et al. Near-Infrared Light Triggered-Release in Deep Brain Regions Using Ultra-photosensitive Nanovesicles. Angew. Chem. Int. Ed. 2020, 59, 8608–8615.
- Shin, J.E.; Ogunyankin, M.O.; Zasadzinski, J.A. Near Infrared-Triggered Liposome Cages for Rapid, Localized Small Molecule Delivery. Sci. Rep. 2020, 10, 1706–1711.
- Troutman, T.S.; Leung, S.J.; Romanowski, M. Light-Induced Content Release from Plasmon-Resonant Liposomes. Adv. Mater. 2009, 21, 2334–2338.
- Troutman, T.S.; Barton, J.K.; Romanowski, M. Biodegradable Plasmon Resonant Nanoshells. Adv. Mater. 2008, 20, 2604–2608.
- Wu, G.; Mikhailovsky, A.; Khant, H.A.; Fu, C.; Chiu, W.; Zasadzinski, J.A. Remotely Triggered Liposome Release by Near-Infrared Light Absorption via Hollow Gold Nanoshells. J. Am. Chem. Soc. 2008, 130, 8175–8177.
- Wu, G.H.; Mikhailovsky, A.; Khant, H.A.; Zasadzinski, J.A. Synthesis, characterization an optical response of gold nanoshells used to trigger release from liposomes. In Methods in Enzymology; Nejat, D., Ed.; Academic Press: Burlington, VT, USA, 2009; Volume 464, pp. 279–307.
- Forbes, N. Photothermally activated drug release from liposomes coupled to hollow gold nanoshells. SPIE Proc. Plasmon. Biol. Med. VIII 2011, 7911, 24–30.
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317.
- Prevo, B.G.; Esakoff, S.A.; Mikhailovsky, A.; Zasadzinski, J.A. Scalable Routes to Gold Nanoshells with Tunable Sizes and Response to Near-Infrared Pulsed-Laser Irradiation. Small 2008, 4, 1183–1195.
- Paliwal, S.; Mitragotri, S. Ultrasound-induced cavitation: Applications in Drug and Gene Delivery. Expert Opin. Drug Deliv. 2006, 3, 713–726.
- Pecha, R.; Gompf, B. Microimplosions: Cavitation Collapse and Shock Wave Emission on a Nanosecond Time Scale. Phys. Rev. Lett. 2000, 84, 1328–1330.
- Braun, G.B.; Pallaoro, A.; Wu, G.; Missirlis, D.; Zasadzinski, J.A.; Tirrell, M.; Reich, N.O. Laser-Activated Gene Silencing via Gold Nanoshell−siRNA Conjugates. ACS Nano 2009, 3, 2007–2015.
- Huang, X.; Hu, Q.; Braun, G.B.; Pallaoro, A.; Morales, D.P.; Zasadzinski, J.; Clegg, D.O.; Reich, N.O. Light-activated RNA interference in human embryonic stem cells. Biomaterials 2015, 63, 70–79.
- Huang, X.; Pallaoro, A.; Braun, G.B.; Morales, D.P.; Ogunyankin, M.O.; Zasadzinski, J.; Reich, N.O. Modular Plasmonic Nanocarriers for Efficient and Targeted Delivery of Cancer-Therapeutic siRNA. Nano Lett. 2014, 14, 2046–2051.
- Morales, D.P.; Braun, G.B.; Pallaoro, A.; Chen, R.; Huang, X.; Zasadzinski, J.A.; Reich, N.O. Targeted Intracellular Delivery of Proteins with Spatial and Temporal Control. Mol. Pharm. 2015, 12, 600–609.
- Lukianova-Hleb, E.Y.; Mutonga, M.B.G.; Lapotko, D.O. Cell-Specific Multifunctional Processing of Heterogeneous Cell Systems in a Single Laser Pulse Treatment. ACS Nano 2012, 6, 10973–10981.
- Lukianova-Hleb, E.Y.; Yvon, E.S.; Shpall, E.J.; Lapotko, D.O. All-in-one processing of heterogeneous human cell grafts for gene and cell therapy. Mol. Ther.-Methods Clin. Dev. 2016, 3, 16012.
- Lukianova-Hleb, E.Y.; Ren, X.; Sawant, R.R.; Wu, X.; Torchilin, V.P.; Lapotko, D.O. On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nat. Med. 2014, 20, 778–784.
- Lukianova-Hleb, E.Y.; Ren, X.Y.; Sawant, R.R.; Gillenwater, A.M.; Kupferman, M.; Hanna, E.Y.; Zasadzinski, J.A.; Wu, X.W.; Torchilin, V.P.; Lapotko, D.O. Plasmonic nanobubble theranostics for intra-operative and preventive treatment of head and neck squamous cell carcinoma. In Proceedings of the Conference on Photonic Therapeutics and Diagnostics X, San Francisco, CA, USA, 1–2 February 2014; Volume 8926.
- Morales, D.P.; Morgan, E.N.; McAdams, M.; Chron, A.B.; Shin, J.E.; Zasadzinski, J.A.; Reich, N.O. Light-Triggered Genome Editing: Cre Recombinase Mediated Gene Editing with Near-Infrared Light. Small 2018, 14, 1800543.




