Liposomes can sequester a variety of bioactive water-soluble ions, ligands and enzymes, and oligonucleotides. The bilayer that separates the liposome interior from the exterior solution provides a physical barrier to contents release and degradation. Tethering plasmon-resonant, hollow gold nanoshells to the liposomes, or growing gold nanoparticles directly on the liposome exterior, allows liposome contents to be released by nanosecond or shorter pulses of near-infrared light (NIR). Gold nanoshells or nanoparticles strongly adsorb NIR light; cells, tissues, and physiological media are transparent to NIR, allowing penetration depths of millimeters to centimeters. Nano to picosecond pulses of NIR light rapidly heat the gold nanoshells, inducing the formation of vapor nanobubbles, similar to cavitation bubbles.
1. Introduction
Following their discovery by Bangham in 1965 [
1,
2], liposomes have been one of the most thoroughly investigated nanocarriers for drug delivery [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. Many promising drugs are discarded due to difficulties in maintaining a safe biodistribution in the concentration range necessary for efficacy [
14]. Liposomes and other lipid-based drug carriers sequester toxic drugs within a lipid bilayer to alter drug biodistribution, thereby enhancing efficacy while minimizing damage to healthy tissue and organs [
14]. Liposomes are capable of concentrating and stabilizing small molecule therapeutic compounds and larger oligonucleotides for delivery in vivo and in vitro [
12,
15,
16,
17,
18]. Liposomes based on naturally occurring lipids are inherently biocompatible and hence can overcome many of the obstacles to cellular and tissue uptake. Small liposomes (<200 nm) are passively targeted to tumors and sites of inflammation by the enhanced permeation and retention (EPR) effect [
18,
19]. Liposomes can also be actively targeted by treating their surface with antibodies to ligands overexpressed on tumor cells [
20,
21,
22,
23,
24,
25,
26]. Hydrophilic cargoes can be encapsulated within the aqueous liposome core, while hydrophobic molecules can be trapped within the hydrophobic bilayer interior [
27].
2. Separating Cargo Sequestration from Rapid Release
Despite these advantages, it is difficult for a single bilayer to simultaneously prevent drug release and trigger rapid release at the preferred site of action [
28]. It remains a challenge to initiate a biological or chemical response in cells or tissues with spatial and temporal control. Conventional liposomes often release small molecule drugs prematurely in the circulation due to degradation of the liposome bilayer or are taken up in the liver and spleen by interactions with the reticuloendothelial system (RES). “Stealth” coatings of polyethylene glycol and other polymer lipids can prolong the circulation time [
29,
30]. However, optimizing the liposome cargo retention in the circulation to maximize drug accumulation at the disease site may slow drug release from the liposome at the site of interest and reduce therapeutic activity. This is the case for clinical doxorubicin liposomes (Doxil) [
31,
32,
33] and for clinically tested cisplatin liposomes [
34]; drug release is so slow that the critical drug concentration is not easily achieved. As a result, multiple strategies for enhancing local drug release at the site of interest have included the incorporation of membrane destabilizing agents that respond to stimuli such as general hyperthermia [
35,
36,
37], receptor targets [
38,
39,
40], pH changes [
41], or tumor-specific enzymes [
42,
43]. These strategies promote release but can compromise long-term liposome stability and decrease drug retention in the circulation. Specific ligands with high affinities to receptor overexpressed on disease cells can in turn lead to “binding-site barriers” where high concentrations of bound nanocarriers can prevent drug penetration into tissue [
44].
3. Plasmon-Resonant Gold Nanostructures
To address the challenge of spatially and temporally controlled fast release, modified liposome nanocarriers have been developed in which the liposome functions primarily as the cargo container. Rapid spatial- and temporal-controlled contents release is addressed by tethered gold nanostructures triggered by an externally applied pulsed NIR laser. Such liposomes are also well-suited for in vitro, in vivo, and ex vivo use where spatial- and temporal-controlled delivery of cargo ranges from small molecules to complex proteins or oligonucleotides [
45,
46,
47,
48]. Plasmon-resonant hollow gold nanoshells (HGN) or simple gold nanoparticles [
46,
47,
49,
50] can be chemically tethered to conventional polyethylene glycol stabilized liposomes of any membrane composition to absorb and concentrate near-infrared light (NIR) [
32,
33,
48,
51,
52,
53]. A hollow shell structure of gold, silver, or alloys can strongly absorb NIR light at a characteristic localized surface plasmon resonance (LSPR) wavelength (
Figure 1) that can be tuned by controlling the nanoparticle size and shell thickness [
45]. As shown schematically in
Figure 1, the absorption spectra of common physiological molecules such as water, oxygenated hemoglobin (HbO
2), and hemoglobin (Hb) show a distinct minima in what has been called the “NIR window” [
54]. As a result, NIR light can penetrate deeply within cell suspensions, tissue, and other physiological media. Unstructured solid silver or gold nanoparticles of 10–100 nm size absorb in the same wavelength range as hemoglobin (400–540 nm) and cannot be readily addressed in physiological fluids.
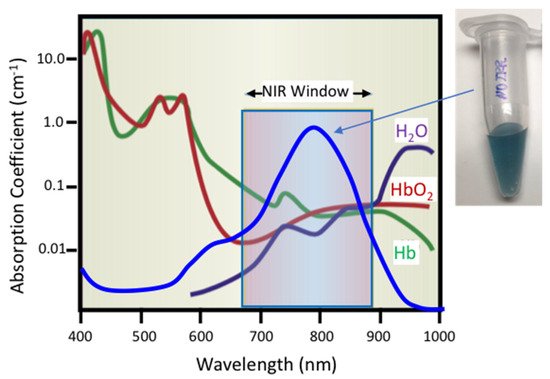
Figure 1. Schematic representation of optical adsorption of water (H
2O), oxygenated hemoglobin (HbO
2), and hemoglobin (Hb) showing the near-infrared window (NIR) from 650–900 nm over which physiological materials do not adsorb significant fractions of incident light, adapted from [
54], published by Nature, 2001. Hollow gold nanoshells (HGN) have a surface plasmon resonance determined by the HGN wall thickness and diameter that can be tuned to peak in the NIR Window. This causes HGN suspensions to appear dark blue to purple.
By manipulating the shape and organization of gold nanostructures, the surface plasmon-resonance can be moved to the NIR window. Light absorption from high power nanosecond or shorter pulses cause the gold nanostructures to undergo a rapid increase in temperature before any heat can be dissipated to the surrounding fluids [
45,
55]. Following this rapid heating of the nanoparticle, the hot nanoparticles begin to dissipate their thermal energy to the surrounding solution in microseconds. The high temperature gradients around the gold nanoparticles vaporize a minute amount of the surrounding water leading to the formation of unstable nanobubbles as the heat is dissipated (30 nm diameter particles thermally equilibrate with their surroundings in fractions of a microsecond). These nanobubbles rapidly expand and contract and give rise to microjets that have similar mechanical and thermal effects as ultrasound-induced cavitation (
Figure 2) [
51,
56,
57]. Nanobubble generation requires a threshold energy; any additional energy above threshold makes the nanobubbles grow larger. Once formed, the mechanical forces generated by the expansion and collapse of the nanobubbles can lyse endosomes, liposomes, or cells, providing rapid contents release [
51,
58,
59,
60,
61], cell poration [
62,
63], or even cell death [
62,
63,
64,
65]. It is these mechanical cavitation forces that lead to endosome, liposome, and cell membrane rupture and contents release rather than the change in temperature. As a result, complex proteins and oligonucleotides, as well as small molecules, can be delivered without damage following NIR light triggering of the gold nanostructures [
47,
48,
58,
61,
66].
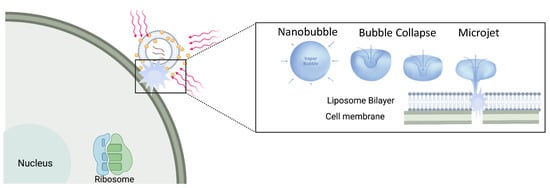
Figure 2. Plasmon-resonant gold nanoparticles tethered to liposomes can be triggered by short pulses of NIR laser light (red lines) that heat the nanoparticles. At a threshold light fluence, heat dissipating from the nanoparticles boils a minute amount of water to form unstable vapor nanobubbles that rapidly expand and contract. As the nanobubbles collapse, liquid–vapor microjets form that can perforate cell and liposome membranes. This allows the liposome contents to be rapidly released and cells to be perforated.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14040701