Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Consolato Sergi | -- | 1934 | 2022-04-22 19:17:52 | | | |
| 2 | Conner Chen | + 2 word(s) | 1936 | 2022-04-24 02:34:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sergi, C.; , . ‘Teratoid’ Hepatoblastoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/22186 (accessed on 07 February 2026).
Sergi C, . ‘Teratoid’ Hepatoblastoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/22186. Accessed February 07, 2026.
Sergi, Consolato, . "‘Teratoid’ Hepatoblastoma" Encyclopedia, https://encyclopedia.pub/entry/22186 (accessed February 07, 2026).
Sergi, C., & , . (2022, April 22). ‘Teratoid’ Hepatoblastoma. In Encyclopedia. https://encyclopedia.pub/entry/22186
Sergi, Consolato and . "‘Teratoid’ Hepatoblastoma." Encyclopedia. Web. 22 April, 2022.
Copy Citation
Liver neoplasms are quite rare in childhood. They often involve 6.7 cases per 10 million children aged 18 years or younger. Hepatoblastoma (HB) is the most frequent tumor, but this neoplasm’s rarity points essentially to the difficulty of performing biologic studies and large-scale therapeutic trials. On the pathological ground, HB is separated into an entirely epithelial neoplasm or a mixed neoplasm with epithelial and mesenchymal components. This last category has been further subdivided into harboring teratoid features or not. The ‘teratoid’ HB includes a mixture of components with heterologous origin.
liver
cancer
1. Roots of the ‘Teratoid’ Concept
The concept and, specifically, the term ‘teratoid’ hepatoblastoma or hepatoblastoma with ‘teratoid’ features was first advanced by Manivel et al. more than three decades ago [1], even if an analogous lesion had been previously described in 1965 [2]. The identification of deviating tissue, such as osteoid or even frank bone, is a well-recognized occurrence in the mesenchymal HB, and these divergencies in the differentiation are more often detected in the post-chemotherapy liver specimens (hepatectomy). The presence of a combination of heterologous elements, including endoderm, neuroectoderm, neural crest elements (e.g., ganglion cells and melanocytes), or melanin-containing cells, correctly characterizes the mixed epithelial designation and mesenchymal tumor as ‘teratoid’ HB [3][4][5]. Thus, the term ‘teratoid’ should be reserved for mixed HBs harboring tissues originating from all three germ layers. These elements include cartilage, muscle (skeletal), keratinizing multistratified squamous epithelium, intestinal epithelium, bronchial epithelium, and melanin pigment [6]. In four-fifths of mixed tumors, it has been detected that the mesenchymal component is represented by “fibrous” tissue of immature or primitive type, cartilage, and/or osteoid. At the same time, the remaining 20% are mixed HB with teratoid features that may include additional tissue phenotypes, such as intestinal-type glandular elements, melanin pigment, classic skeletal muscle, neural tissue, or melanin pigment, which can be enhanced using DOPA (3,4-dihydroxyphenylalanine) oxidase, ferrous iron, Fontana-Masson, and Fontana-Masson picrosirius methods. The most important criteria for the teratoid component remain the neuroepithelium, melanin, and, more recently, the yolk-sac-like component and neuroendocrine components. The mesenchymal components include muscle, osteoid, and cartilage, which are most often observed mainly in ‘teratoid’ neoplasms. The teratoid component or mesenchymal components are diagnosed with biopsies. They appear more prominent after chemotherapy due to the response and shrinkage of epithelial elements and non- or low-responsive components of mixed HB. The response to chemotherapy in these neoplasms of the liver is quite variable, with some tumors displaying complete resolution and some persistence post-therapy. It is important to emphasize that it is the epithelial component that determines the aggressiveness of the HB, with the teratoid component only rarely being demonstrated in metastases.
2. Clinical-Radiological Features
Primary malignant neoplasms of the liver are sporadic neoplasms, contributing to about 1% of all childhood malignancies. The most common types are HBs, hepatocellular carcinomas (HCCs), and sarcomas, of which some are most often seen before the age of three and some after this age. Some are associated with specific genetic variations, such as the fibrolamellar carcinoma—also called the fibrolamellar variant of HCC [7][8]. In 90% of HBs, this tumor occurs in the first and second infancy (first five years of life), and 5% of these neoplasms are congenital. There is a perceptible trend in the last three decades (1990’s–2020) of increased incidence of HB in infants born prematurely or with a very low birth weight [9][10][11]. Environmental considerations may need further studies in this direction. There are several genetic constellations and syndromes associated with HB, including familial adenomatous polyposis (FAP), Beckwith–Wiedemann syndrome (BWS), trisomy 13, 18, and 21, and partial trisomy 9p [9][10][11][12][13][14][15][16][17]. From the clinical standpoint, four-fifths of patients present with a single mass, while one-fifth present with multiple nodules. Although rare, the metastatic spreading ability of HB has been described in both humans and animals [18][19][20][21][22], and human spreading intumescences occur most frequently in the respiratory tract (lungs) and, rarely, in the brain, choroid, iris, and skin [23]. Radiologically, HB of mixed epithelial and mesenchymal types is usually an inhomogeneous mass detected by magnetic resonance tomography (MRT) (Figure 1). Most often, the tumor presents with two main components, and the coronal view shows that the neoplasm may be located predominantly outside the liver. Equally, the transversal view repeatedly demonstrated that one part has a cystic appearance, whereas another appears more solid. It is advantageous to perform the T1 sequence carried out after a contrast application because it shows an inhomogeneous contrast enhancement with one section of the tumor enhancing more than other sections.
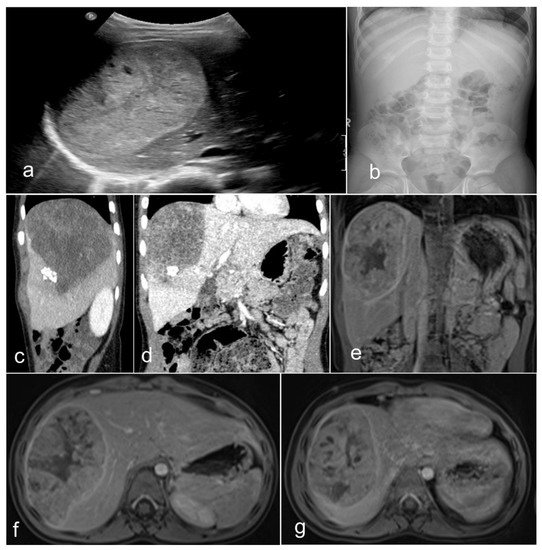
Figure 1. Imaging of a teratoid hepatoblastoma showing a heterogeneous mass encased in the liver (a), which is very close to the diaphragm (b). The magnetic resonance imaging (c–g) shows a large neoplasm in the right upper quadrant of the abdomen deriving from the right liver lobe. The neoplasm has two main portions and seems to contain different portions of tissue. The coronal view showed that the tumor is exophytic, protruding superiorly (T2 sequence). Remarkably, the transversal view typically reveals that the ventral tumor portion has a cystic nonenhancing appearance, whereas the dorsal part emerges more solid (T2 sequence). The T1 sequences—after contrast (post-gadolinium) application—(e–g) display an inhomogeneous contrast enhancement. The ventral portion of the hepatic neoplasm is intriguingly avidly enhancing, while the dorsal solid portion enhances less avidly.
3. Pathological Features
On the pathological ground, HB is separated into several histological phenotypes. They are based on patterns of differentiation. They include pure fetal epithelial, combined fetal and embryonal epithelial, macrotrabecular, mixed epithelial and mesenchymal, and mixed epithelial and mesenchymal with teratoid features [3]. The ‘small cell undifferentiated’ (SCUD) variant is no longer shown to be of significance regarding the prognosis of HB. Most of the pure SCUD neoplasms are malignant rhabdoid tumors. The resemblance of the tumor to the developing liver helps in identifying the fetal component, while more primitive areas suggest the diagnosis of an embryonal pattern. The identification of neural, or melanocytic cells, helps restrict the diagnosis of HB with teratoid features (Figure 2 and Figure 3). The ‘teratoid’ HB includes a mixture of heterologous features, such as the endoderm, neuroectoderm, or even melanin-encompassing cells with or without mesenchymal components, such as fibroblastic stroma, muscle, cartilage, or osteoid. These elements are most often notably observed in neoplasms following chemotherapy. The ‘teratoid’ HB or HB with teratoid features is a rare histologic subtype of the mixed epithelial-mesenchymal category of HB, accounting for 4% to 10% of all HBs. The presence of divergent differentiation, including neural and melanocytic, in addition to cartilaginous, osseous, skeletal muscle, and neural elements, has suggested that this tumor might originate from stem cells. The teratoid HB is an inquiring pediatric neoplasm because of the induction of stem cells in the histogenesis of this kind of pediatric liver neoplasm [24].
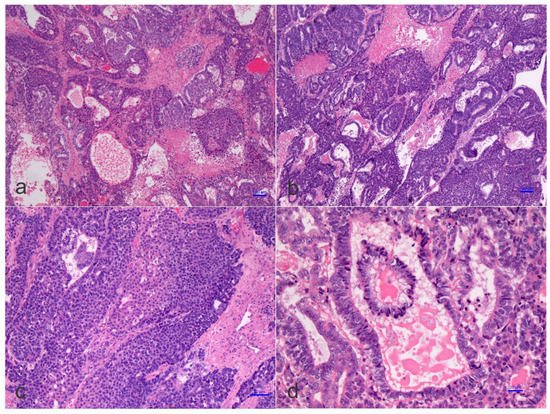
Figure 2. Histology of a mixed hepatoblastoma with teratoid features or teratoid hepatoblastoma showing fetal and embryonal portions and teratoid features with a yolk-sac-like pattern. Cords and solid portions are present and numerous mitoses are recognized with epithelial and mesenchymal components and yolk-sac-like elements (d), ((a–d) hematoxylin and eosin staining, X50 original magnification, scale bar, 100 microns).
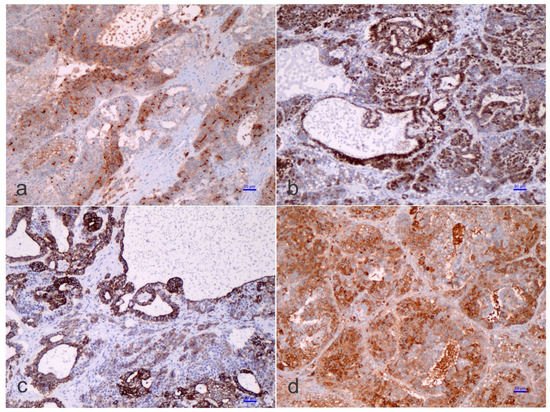
Figure 3. Immunohistochemistry of a teratoid hepatoblastoma.
Some HB may also harbor “cholangioblastic” features, which may entail uni- or bipotential neoplastic cells of the progenitor level, capable of shifting into either of two lines along with the progression of a certain tumor recalling the development of the ductal plate [25][26][27][28][29][30]. Cholangioblastic HB is a variant of epithelial HB and should not be considered part of the ‘teratoid’ concept. Increased recognition of the cholangioblastic component of an epithelial HB is crucial to address beyond using molecular studies and to anticipate the knowledge of the pathogenesis of HB.
The ‘teratoid’ elements might originate from either multipotent or, probably, less-committed cells (stem cells) [31], and chemotherapeutic approaches could provoke differentiation of the less differentiated cells, as often observed in nephroblastoma or Wilms’ tumor [32]. Immunohistostaining inconsistencies may be observed in HB, and this aspect is triggered by fluctuating epithelial, mesenchymal, and teratoid cell populations, varying intracytoplasmic constituents, such as glycogen and lipid, or cellular “anaplasia” [4][5][6]. Substantially, α-fetoprotein (AFP) is traditionally the most consistent marker found in HBs, which should also be accompanied by the expression of glypican 3, a serum marker for HB [33][34]. The Ruck et al. “small epithelial cells” with a distinguishing phenotype between the hepatic and biliary cells, carried out by immunohistochemistry and electron microscopy, are probably crucial for understanding the evolution from ontogenesis to oncogenesis [35]. These cells, which are denominated ‘oval cells’, express cytokeratin 7 (CK-7 or K7), albumin, and oval cells 6 (OV-6). The concept of oval cells has traditionally been applied to mouse models of liver cell regeneration, but the equivalent of the oval cells in humans remains poorly understood, or even unknown. These cells are predominantly found in neo-proliferative processes with anaplastic features, declining in embryonal or fetal differentiated HB. The existence of OV-6 positive cells in HB would point toward the presence of “oval cell progenitors” as the main elements of the ‘teratoid’ HB, but these concepts are heavily debated among liver pathologists and fetal liver experts. In the adult liver, oval cells (“hepatic stem cells”) with a “clonogenic” potential and a dual differentiation potential (either into hepatic or biliary cell lineages) may have been recognized since 1996 [36][37], although there is no uniformity of opinion on this. These stem cells are thought to be bone marrow-derived, though the putative liver stem cell is not yet identified with certainty. Clonogenicity refers to the ability of a determinate cell to progressively clone itself and ultimately grow into a complete colony of cloned cells. Oval cells show a distinctive co-expression of immunophenotypic stem cell markers (e.g., CD34, Thy1, and some others) concurrently with hepatic lineage cell markers (e.g., K-18 or CK-18, albumin, and others) [38][39]. The ‘immature-looking cells,’ suggested by Zimmermann [25], differ from the “true” small cell undifferentiated (SCUD)-HB cells. As indicated above, SCUD is no longer shown to be of significance regarding the prognosis of HB and most of the pure SCUD neoplasms are malignant rhabdoid tumors. These cells, with a potential toward several lines of differentiation, harbor reduced expression of hepatocyte antigen, stronger nuclear/cytoplasmic expression of β-catenin, and a decreased proliferation activity (Ki-67 labeling index) than the other cells of epithelial type. In his review, Zimmermann regarded these cells as one of the epithelial components and ‘pacemaker cells’ of the neoplasm [25]. Probably, the cells that Zimmermann was referring to correspond to the so-called blastemal cells in HB and are capable of bidirectional differentiation. The primitive glandular epithelial component is usually positive for periodic acid–Schiff (PAS) and mucicarmine, suggesting mucin production, and being positive for cytokeratin 19 (K19 or CK19)—an early bile duct lineage differentiation marker of the intermediate cytoskeleton, B-cell lymphoma 2 (BCL-2), which is a well-known anti-apoptotic protein—(cyto-)keratin 20 (K20 or CK20), and β-catenin, and focally positive for K7 (CK-7), but negative for glypican 3, delta-like protein, and claudin 1. Conversely, the embryonal and fetal components of the HB surrounding the primitive glandular epithelium are usually positive for β-catenin, glypican 3, delta-like protein, and claudin 1 but negative for K19 (CK19), BLC2, K20 (CK20), and K7 (CK7). Typically, both the embryonal components and the primitive glandular epithelium are negative for chromogranin A, neuroendocrine, and synaptophysin, as well as other neural markers. An important conundrum remains the vague differentiation of ‘teratoid’ HB from hepatic teratoma, at least for some authors. It can be considered that teratomas rare neoplasms with a rate of 7 cases per million children per year with tissue originating from all three germ layers. In the liver, four well-characterized teratomas have been reported [40][41][42][43]. Moll’s combined HB and teratoma case report should probably be considered a ‘teratoid’ HB [44]. The distinction of ‘teratoid’ HB from a teratoma needs the presence of an epithelial HB component to formulate that diagnosis.
References
- Manivel, C.; Wick, M.R.; Abenoza, P.; Dehner, L.P. Teratoid hepatoblastoma. The nosologic dilemma of solid embryonic neoplasms of childhood. Cancer 1986, 57, 2168–2174.
- Misugi, K.; Reiner, C.B. A malignant true teratoma of liver in childhood. Arch. Pathol. 1965, 80, 409–412.
- Sharma, D.; Subbarao, G.; Saxena, R. Hepatoblastoma. Semin. Diagn. Pathol. 2017, 34, 192–200.
- Czauderna, P.; Lopez-Terrada, D.; Hiyama, E.; Haberle, B.; Malogolowkin, M.H.; Meyers, R.L. Hepatoblastoma state of the art: Pathology, genetics, risk stratification, and chemotherapy. Curr. Opin. Pediatr. 2014, 26, 19–28.
- Lopez-Terrada, D.; Alaggio, R.; de Davila, M.T.; Czauderna, P.; Hiyama, E.; Katzenstein, H.; Leuschner, I.; Malogolowkin, M.; Meyers, R.; Ranganathan, S.; et al. Towards an international pediatric liver tumor consensus classification: Proceedings of the Los Angeles COG liver tumors symposium. Mod. Pathol. 2014, 27, 472–491.
- Russo, P. Liver including tumors, gallbladder, and biliary tree. In Potter’s Pathology of the Fetus, Infant and Child, 2nd ed.; Gilbert-Barness, E., Ed.; Mosby-Elsevier: Philadelphia, PA, USA, 2007; pp. 1207–1280.
- Honeyman, J.N.; Simon, E.P.; Robine, N.; Chiaroni-Clarke, R.; Darcy, D.G.; Lim, I.I.; Gleason, C.E.; Murphy, J.M.; Rosenberg, B.R.; Teegan, L.; et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014, 343, 1010–1014.
- Sergi, C.M. Hepatocellular Carcinoma, Fibrolamellar Variant: Diagnostic Pathologic Criteria and Molecular Pathology Update. A Primer. Diagnostics 2015, 6, 3.
- Shuman, C.; Beckwith, J.B.; Weksberg, R. Beckwith-Wiedemann Syndrome. In GeneReviews((R)); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; NCBI, University of Washington: Seattle, WA, USA, 1993.
- Cohen, M.M., Jr. Beckwith-Wiedemann syndrome: Historical, clinicopathological, and etiopathogenetic perspectives. Pediatr. Dev. Pathol. 2005, 8, 287–304.
- Dasouki, M.; Barr, M., Jr. Trisomy 18 and hepatic neoplasia. Am. J. Med. Genet. 1987, 27, 203–205.
- Mamlok, V.; Nichols, M.; Lockhart, L.; Mamlok, R. Trisomy 18 and hepatoblastoma. Am. J. Med. Genet. 1989, 33, 125–126.
- Maruyama, K.; Ikeda, H.; Koizumi, T. Hepatoblastoma associated with trisomy 18 syndrome: A case report and a review of the literature. Pediatr. Int. 2001, 43, 302–305.
- Schnater, J.M.; Schouten-van Meeteren, A.Y.; Heins, Y.M.; Aronson, D.C. Hepatoblastoma in a patient with a partial trisomy 9p syndrome: A case report. Cancer Genet. Cytogenet. 2005, 156, 77–79.
- Shah, R.; Tran, H.C.; Randolph, L.; Mascarenhas, L.; Venkatramani, R. Hepatoblastoma in a 15-month-old female with trisomy 13. Am. J. Med. Genet. A 2014, 164A, 472–475.
- Nagata, T.; Mugishima, H.; Shichino, H.; Suzuki, T.; Chin, M.; Koshinaga, S.; Inoue, M.; Harada, K. Karyotypic analyses of hepatoblastoma. Report of two cases and review of the literature suggesting chromosomal loci responsible for the pathogenesis of this disease. Cancer Genet. Cytogenet. 1999, 114, 42–50.
- Parada, L.A.; Limon, J.; Iliszko, M.; Czauderna, P.; Gisselsson, D.; Hoglund, M.; Kullendorff, C.M.; Wiebe, T.; Mertens, F.; Johansson, B. Cytogenetics of hepatoblastoma: Further characterization of 1q rearrangements by fluorescence in situ hybridization: An international collaborative study. Med. Pediatr. Oncol. 2000, 34, 165–170.
- Begemann, M.; Trippett, T.M.; Lis, E.; Antunes, N.L. Brain metastases in hepatoblastoma. Pediatr. Neurol. 2004, 30, 295–297.
- Endo, E.G.; Walton, D.S.; Albert, D.M. Neonatal hepatoblastoma metastatic to the choroid and iris. Arch. Ophthalmol. 1996, 114, 757–761.
- Matsunaga, T.; Sasaki, F.; Ohira, M.; Hashizume, K.; Hayashi, A.; Hayashi, Y.; Mugishima, H.; Ohnuma, N.; Japanese Study Group for Pediatric Liver, T. Analysis of treatment outcome for children with recurrent or metastatic hepatoblastoma. Pediatr. Surg. Int. 2003, 19, 142–146.
- Neu, S.M. Hepatoblastoma in an equine fetus. J. Vet. Diagn. Investig. 1993, 5, 634–637.
- Prater, P.E.; Patton, C.S.; Held, J.P. Pleural effusion resulting from malignant hepatoblastoma in a horse. J. Am. Vet. Med. Assoc. 1989, 194, 383–385.
- Loynachan, A.T.; Bolin, D.C.; Hong, C.B.; Poonacha, K.B. Three equine cases of mixed hepatoblastoma with teratoid features. Vet. Pathol. 2007, 44, 211–214.
- Kim, L.; Park, Y.N.; Kim, S.E.; Noh, T.W.; Park, C. Teratoid hepatoblastoma: Multidirectional differentiation of stem cell of the liver. Yonsei Med. J. 2001, 42, 431–435.
- Zimmermann, A. The emerging family of hepatoblastoma tumours: From ontogenesis to oncogenesis. Eur. J. Cancer 2005, 41, 1503–1514.
- Zimmermann, A. Pediatric liver tumors and hepatic ontogenesis: Common and distinctive pathways. Med. Pediatr. Oncol. 2002, 39, 492–503.
- Zimmermann, A. Hepatoblastoma with cholangioblastic features (‘cholangioblastic hepatoblastoma’) and other liver tumors with bimodal differentiation in young patients. Med. Pediatr. Oncol. 2002, 39, 487–491.
- Sergi, C.; Adam, S.; Kahl, P.; Otto, H.F. The remodeling of the primitive human biliary system. Early Hum. Dev. 2000, 58, 167–178.
- Sergi, C.; Kahl, P.; Otto, H.F. Contribution of apoptosis and apoptosis-related proteins to the malformation of the primitive intrahepatic biliary system in Meckel syndrome. Am. J. Pathol. 2000, 156, 1589–1598.
- Sergi, C.; Adam, S.; Kahl, P.; Otto, H.F. Study of the malformation of ductal plate of the liver in Meckel syndrome and review of other syndromes presenting with this anomaly. Pediatr. Dev. Pathol. 2000, 3, 568–583.
- Fiegel, H.C.; Gluer, S.; Roth, B.; Rischewski, J.; von Schweinitz, D.; Ure, B.; Lambrecht, W.; Kluth, D. Stem-like cells in human hepatoblastoma. J. Histochem. Cytochem. 2004, 52, 1495–1501.
- Variend, S.; Spicer, R.D.; Mackinnon, A.E. Teratoid Wilms’ tumor. Cancer 1984, 53, 1936–1942.
- Zhou, S.; O’Gorman, M.R.; Yang, F.; Andresen, K.; Wang, L. Glypican 3 as a Serum Marker for Hepatoblastoma. Sci. Rep. 2017, 7, 45932.
- Xiong, X.L.; Qin, H.; Yan, S.Q.; Zhou, L.S.; Chen, P.; Zhao, D. Expression of glypican-3 is highly associated with pediatric hepatoblastoma: A systemic analysis. Asian Pac. J. Cancer Prev. 2015, 16, 1029–1031.
- Ruck, P.; Xiao, J.C.; Pietsch, T.; Von Schweinitz, D.; Kaiserling, E. Hepatic stem-like cells in hepatoblastoma: Expression of cytokeratin 7, albumin and oval cell associated antigens detected by OV-1 and OV-6. Histopathology 1997, 31, 324–329.
- Haque, S.; Haruna, Y.; Saito, K.; Nalesnik, M.A.; Atillasoy, E.; Thung, S.N.; Gerber, M.A. Identification of bipotential progenitor cells in human liver regeneration. Lab. Investig. 1996, 75, 699–705.
- Haruna, Y.; Saito, K.; Spaulding, S.; Nalesnik, M.A.; Gerber, M.A. Identification of bipotential progenitor cells in human liver development. Hepatology 1996, 23, 476–481.
- Thorgeirsson, S.S. Hepatic stem cells in liver regeneration. FASEB J. 1996, 10, 1249–1256.
- Sadri, A.R.; Jeschke, M.G.; Amini-Nik, S. Advances in Liver Regeneration: Revisiting Hepatic Stem/Progenitor Cells and Their Origin. Stem Cells Int. 2016, 2016, 7920897.
- Nirmala, V.; Chopra, P.; Machado, N.O. An unusual adult hepatic teratoma. Histopathology 2003, 43, 306–308.
- Verma, M.; Agarwal, S.; Mohta, A. Primary mixed germ cell tumour of the liver--a case report. Indian J. Pathol. Microbiol. 2003, 46, 658–659.
- Winter, T.C., 3rd; Freeny, P. Hepatic teratoma in an adult. Case report with a review of the literature. J. Clin. Gastroenterol. 1993, 17, 308–310.
- Meyers, R.L. Tumors of the liver in children. Surg. Oncol. 2007, 16, 195–203.
- Moll, A.; Krenauer, A.; Bierbach, U.; Till, H.; Hirsch, W.; Leuschner, I.; Schmitz, N.; Wittekind, C.; Aigner, T. Mixed hepatoblastoma and teratoma of the liver in a 3-year-old child: A unique combination and clinical challenge. Diagn. Pathol. 2009, 4, 37.
More
Information
Subjects:
Pediatrics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
24 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




