
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Radoslav Stojchevski | -- | 4308 | 2025-03-20 20:15:27 | | | |
| 2 | Rita Xu | Meta information modification | 4308 | 2025-03-21 02:32:44 | | |
Video Upload Options
Cancer remains one of the most pressing challenges in modern medicine, but recent advancements are revolutionizing both therapeutic and diagnostic landscapes. This exploration of new frontiers in cancer therapy and diagnostics highlights a diverse array of innovative strategies that target the molecular mechanisms of tumorigenesis while enhancing early detection and personalized care. Cutting-edge therapies, such as small-molecule inhibitors and monoclonal antibodies, specifically target oncogene-driven pathways, offering selective toxicity over traditional chemotherapy. Immunotherapies, including immune checkpoint inhibitors, radioimmunotherapy, antibody-drug conjugates (ADCs), and chimeric antigen receptor (CAR) T-cell therapy, activate the immune system to combat malignancies, showing remarkable efficacy in oncogene-addicted cancers and hematological malignancies. Emerging approaches like cancer vaccines and oncolytic viruses further amplify immune responses, while liquid biopsy transforms diagnostics by analyzing circulating tumor markers for early detection, treatment monitoring, and resistance profiling. Artificial intelligence (AI) and machine learning amplify these advances, refining diagnosis through image analysis, predicting oncogenic mutations, and guiding personalized treatment plans. Together, these breakthroughs—including targeted therapies, immunotherapies, and technology-driven diagnostics—represent a major progress in oncology, though challenges like drug resistance, tumor heterogeneity, and accessibility persist. This summary highlights the promise and complexity of these new frontiers, paving the way for more effective, tailored cancer management.
1. Targeted Therapy and Immunotherapy: Precision Approaches to Cancer
Among the most promising novel therapeutic strategies designed to combat cancer are targeted therapy and immunotherapy, which leverage precision medicine that targets specific proteins and genetic changes driving tumor heterogeneity [1]. Unlike traditional chemotherapy, which nonselectively harms all cells, targeted therapy and immunotherapy focus on abnormal proteins or immune responses, minimizing toxicity to healthy cells [1][2]. These approaches encompass a range of techniques, including small-molecule inhibitors, monoclonal antibodies, immune checkpoint inhibitors, radioimmunotherapy, antibody-drug conjugates (ADCs), chimeric antigen receptor (CAR) T-cell therapy, cancer vaccines, and oncolytic viruses [3][4][5].
Small-molecule inhibitors are broadly used in targeted therapy designed to slow or kill tumor cells by primarily targeting protein kinases, which are highly active pro-growth signaling initiators [6] (Figure 1). Their low molecular weight allows them to diffuse through cells and target intracellular drivers that regulate proliferation and apoptosis [2]. The list of various U.S. Food and Drug Administration (FDA)-approved small-molecule inhibitors for cancer treatment is shown in (Table 1). The predominant and widely accepted class of small-molecule inhibitors includes those targeting RTKs and VEGF receptors, such as Erlotinib, Sunitinib, and others, which exert antiangiogenic and antiproliferative effects [7]. Recent breakthroughs have shown promising applications of small-molecule inhibitors in treating oncogene-driven mutations [2]. Serval inhibitors for BRAF of the MAPK pathway, such as Vemurafenib and Dabrafenib, have shown effective results against melanomas. Moreover, these inhibitors are particularly potent in treating patients with Ras and BRAF V600E mutations when used in combination with general MAPK inhibitors like Trametinib [2][8]. Despite their promise, small-molecule inhibitors have limitations, as they lead to the development of drug resistance through mechanisms that may include their influence on tumor microenvironments and the potential reactivation of both MAPK and PI3K/AKT signaling pathways [8][9]. Additionally, resistance may arise from the changes occurring in the genes coding for target proteins, deviation in signaling pathways that activate different proteins with similar functions, or mutations in the genes coding for the proteins associated with the target molecule [10][11].
| Inhibitor | Target | Mechanism of Action | FDA Approval |
|---|---|---|---|
| Erlotinib | EGFR (RTK) | Competitively inhibits ATP binding to EGFR; Blocks downstream signaling | 2004 (NSCLC), 2005 (Pancreatic cancer) |
| Gefitinib | EGFR (RTK) | Inhibits EGFR tyrosine kinase activity; reduces cell proliferation |
2003 (NSCLC) |
| Lapatinib | HER2, EGFR (RTKs) | Dual inhibitor; prevents phosphorylation and signaling | 2007 (HER+ Breast cancer) |
| Sunitinib | VEGFR, PDGFR, KIT (RTKs) | Inhibits multiple RTKs; leads to antiangiogenic and antiproliferative effects | 2006 (pNET, RCC, GIST) |
| Sorafenib | VEGFR, PDGFR, KIT (RTKs) | Inhibits RTKs and RAF kinase; blocks angiogenesis and tumorigenesis |
2005 (HCC, RCC, Thyroid cancer) |
| Vemurafenib | BRAF V600E (MAPK Pathway) |
Selectively inhibits mutant BRAF; prevents aberrant MAPK activation | 2011 (Melanoma, Erdheim–Chester disease) |
| Dabrafenib | BRAF V600E (MAPK Pathway) |
Inhibits mutant BRAF kinase; reduces MAPK-driven cell proliferation |
2013 (Melanoma, NSCLC, Anaplastic thyroid cancer) |
| Encorafenib | BRAF V600E (MAPK Pathway) |
BRAF kinase inhibitor; blocks mutant BRAF kinase; inhibits MAPK pathway signaling. | 2018 (Melanoma), 2020 (Colorectal cancer), 2023 (NSCLC) |
| Binimetinib | MEK1/2 (MAPK Pathway) |
Blocks MEK1/2 activity; inhibits downstream MAPK pathway signaling. | 2018 (Melanoma), 2023 (NSCLC) |
| Trametinib | MEK1/2 (MAPK Pathway) |
Inhibits MEK1/2; blocks MAPK activation downstream of BRAF | 2013 (Melanoma, NSCLC, Anaplastic thyroid cancer) |
| Cobimetinib | MEK1/2 (MAPK Pathway) |
Selectively inhibits MEK; suppresses MAPK signaling |
2015 (Melanoma) |
Monoclonal antibodies are immunoglobulins designed to bind specific antigens and represent the second most common form of targeted therapy [1][6]. Unlike small-molecule inhibitors, monoclonal antibodies are larger molecules that cannot enter cells. Instead, they work by targeting receptors on the surfaces of cancer cells, thereby blocking the molecules that signal proliferation or angiogenesis [2][12] (Figure 1). This approach is primarily used to target the antigens associated with oncogene signaling, thus inhibiting the pathways that promote cancer cell growth and survival [13]. The most common clinical applications of monoclonal antibodies are trastuzumab (targeting HER2), cetuximab (targeting EGFR), and pembrolizumab (targeting the PD-1/PD-L1 axis) [14][15][16]. Trastuzumab, for example, effectively disrupts oncogenic signaling by downregulating HER2, an RTK that is commonly overexpressed in HER2-positive breast cancer, thereby promoting its internalization and degradation [17][18]. Similarly, cetuximab and panitumumab bind to EGFR, preventing ligand binding and receptor dimerization, which inhibits oncogene signaling [19][20]. Moreover, the use of antibody therapy extends to tumor suppressors, as treatments with pembrolizumab or nivolumab have been shown to restore T-cell functionality against tumors, compensating for the LOF mutations in tumor suppressors[19][21].
Immunotherapy is a major treatment method and a promising therapeutic approach, especially in people with oncogene-addicted cancers—cancers that depend heavily on a single oncogene or pathway[22]. This treatment increases the ability of the immune system to recognize and eliminate cancer cells, mainly through immune checkpoint inhibitors that block pathways like PD-1, PD-L1, and CTLA-4, which tumors exploit to decrease immune responses [23]. Notably, pembrolizumab and nivolumab, previously discussed as monoclonal antibodies, overlap with this category, as they release the brakes on T cells and are very effective against oncogene-driven cancers that have mutations in genes like KRAS and EGFR [4][24]. Tumors with mutated KRAS are likely to respond better due to upregulated PD-L1 expression and immune cell infiltration, as opposed to EGFR and ALK-driven tumors, which typically have “cold” tumor microenvironments with fewer immune cells [22][25][5]. Immunotherapy may also improve outcomes for tumors with genomic instability, including mutations in tumor-suppressor genes such as TP53, STK11, and KEAP1[24][26]. Moreover, immunotherapy has also shown success when combined with chemotherapy and anti-angiogenesis agents like bevacizumab, which work synergistically to change the tumor microenvironment[25][27].
Radioimmunotherapy (RIT) is an extension of immunotherapy that combines targeted radiation with monoclonal antibodies to selectively target and destroy cancer cells[28]. This approach delivers a high dose of therapeutic or tracer radiation while minimizing exposure to normal cells[29]. Recent studies have focused on optimizing the combination of targeted radiation and immunotherapy, particularly in treatments that use alpha (radium-223 and actinium-225) and beta radionuclides (90y-ibritumomab tiuxetan), which have shown cytotoxic effects in treating leukemia, prostate cancer, and non-Hodgkin lymphoma [30][31]. Beyond its cytotoxic capabilities, RIT can influence oncogene and tumor-suppressor gene activity. For instance, a study by Guo et al. [32] identifies the correlation between p53 and RIT efficacy in tumors with wild-type TP53. The study found that, in response to RIT, the activity of p53 was upregulated, which led to increased apoptosis and better regulation of DNA damage in cancer cells. This suggests that RIT could benefit patients with functional tumor-suppressor pathways, serving as an alternative therapy in cases resistant to conventional treatments [32].
 Figure 1. Mechanisms of action of small-molecule inhibitors, monoclonal antibodies, immune checkpoint inhibitors, and radionuclides in cancer therapy. Small-molecule inhibitors target key receptors and kinases to disrupt signaling pathways and block tumor progression. Monoclonal antibodies recognize and bind specific antigens on the tumor cell surface, leading to immune-mediated destruction. Immune checkpoint inhibitors enhance T-cell activity by blocking inhibitory immune signals, restoring the immune system’s ability to recognize and eliminate tumor cells. Radioimmunotherapy combines monoclonal antibodies with radionuclides to selectively deliver cytotoxic radiation to tumor cells, increasing treatment precision while minimizing damage to normal tissues. Created in BioRender. Stojchevski, R. (2025) https://BioRender.com/k49h851.
Figure 1. Mechanisms of action of small-molecule inhibitors, monoclonal antibodies, immune checkpoint inhibitors, and radionuclides in cancer therapy. Small-molecule inhibitors target key receptors and kinases to disrupt signaling pathways and block tumor progression. Monoclonal antibodies recognize and bind specific antigens on the tumor cell surface, leading to immune-mediated destruction. Immune checkpoint inhibitors enhance T-cell activity by blocking inhibitory immune signals, restoring the immune system’s ability to recognize and eliminate tumor cells. Radioimmunotherapy combines monoclonal antibodies with radionuclides to selectively deliver cytotoxic radiation to tumor cells, increasing treatment precision while minimizing damage to normal tissues. Created in BioRender. Stojchevski, R. (2025) https://BioRender.com/k49h851.
Antibody–drug conjugates (ADCs) are a class of targeted cancer therapy that connects monoclonal antibodies with a potent cytotoxic drug (payload) through a chemical linker [33]. The monoclonal antibody offers a highly specific targeting capability, thus binding to a target antigen on the cancer cell’s surface. The presence of a chemical linker ensures that the payload, which has a highly potent cytotoxic effect, is released only inside the cancer cell, therefore minimizing the damage to healthy tissues[34]. The first FDA-approved ADC was the anti-CD33-targeted agent gemtuzumab ozogamicin in 2000, to treat patients with acute myeloid leukemia[35]. Since then, there have been eleven FDA-approved ADCs (Table 2) for targeting various tumor antigens, such as CD19, CD22, CD30, CD33, and CD79b in blood cancers (myeloma, lymphoma, and leukemia), and HER2, tissue factor, folate factor alpha, Nectin-4, and Trop-2 in solid cancers (NSCLC, breast cancer, gastric cancer, and ovarian cancer, among others[36], and many more are in advanced stages of clinical trials.
| ADC Generic Name | Target Antigen | Cytotoxic Payload | FDA Approval |
|---|---|---|---|
| Loncastuximab tesirine | CD19 | SG3199, alkylating agent (DNA targeting) |
2021 (Diffuse Large B-Cell Lymphoma—DLBCL) |
| Inotuzumab ozogamicin | CD22 | Calicheamicin (cytotoxic antibiotic) |
2017 (B-cell Acute Lymphoblastic Leukemia—ALL) |
| Brentuximab vedotin | CD30 | Monomethyl auristatin E (microtubule targeting) |
2011, 2015, 2018 (Hodgkin lymphoma—HL; 2011, 2017, 2018 (Anaplastic Large Cell Lymphoma—ALCL); 2018 (Peripheral T-Cell Lymphoma—PTCL) |
| Gemtuzumab ozogamicin | CD33 | Calicheamicin (cytotoxic antibiotic) |
2017 (Acute Myeloid Leukemia—AML) |
| Polatuzumab vedotin | CD79b | Monomethyl auristatin E (microtubule targeting) |
2019, 2023 (DLBCL) |
| Trastuzumab emtansine | HER2 | DM1 (microtubule targeting) |
2013, 2019 (HER2+ Breast Cancer) |
| Trastuzumab deruxtecan | HER2 | Topoisomerase I inhibitor (DNA targeting) |
2019, 2022 (HER2+ Breast Cancer); 2021 (Gastric Adenocarcinoma—GAC or Gastroesophageal Junction—GEJ Adenocarcinoma); 2022 (NSCLC) |
| Tisotumab vedotin | Tissue Factor | Monomethyl auristatin E (microtubule targeting) |
2021 (Cervical Cancer) |
| Mirvetuximab soravtansine–gynx | Folate Receptor Alpha |
DM4 (microtubule targeting) |
2022 (Ovarian Cancer, Fallopian Tube Cancer, and Peritoneal Cancer) |
| Enfortumab vedotin | Nectin-4 | Monomethyl auristatin E (microtubule targeting) |
2019, 2023 (Urothelial Cancer) |
| Sacituzumab govitecan | Trop-2 | SN-38 topoisomerase-1 inhibitor (DNA targeting) |
2020 (Triple-Negative Breast Cancer—TNBC); 2021 (Urothelial Cancer); 2023 (HER2- Breast Cancer, HR+ Breast Cancer) |
Chimeric antigen receptor (CAR) T-cell therapy is a novel cancer therapy that uses patients’ own T cells to fight cancer[37]. Usually, the T cells do not present receptors specific to the cancer cells’ antigens, which prevents them from attaching to the antigens and destroying the cancer cells. In CAR T-cell therapy, T cells are extracted from the patient’s blood and undergo genetic modification, which introduces a gene that encodes a cancer-specific antigen receptor on their cellular membrane, enabling them to recognize and attach to the cancer cell [38]. Then, CAR T cells are infused back into the patients, where they circulate and attack cancer cells. This therapy has shown a significantly greater promise in targeting and combating circulating blood cancers like leukemia, lymphomas, and myelomas compared to solid tumors, mostly because of the solid tumors’ inaccessibility due to their complex microenvironment [39]. So far, there are six FDA-approved CAR T-cell products for treating hematological malignancies (Table 3), and many more are in active clinical trials[40].
| CAR T-Cell Product Generic Name |
Target Antigen | FDA Approval |
|---|---|---|
| Tisagenlecleucel | CD19 | 2017 (ALL); 2018 (DLBCL); 2022 (Follicular lymphoma—FL) |
| Axicabtagene ciloleucel | CD19 | 2017, 2022 (DLBCL, PMBCL); 2021 (FL) |
| Brexucabtagene autoleucel | CD19 | 2020 (Mantle Cell Lymphoma—MCL); 2021 (ALL) |
| Lisocabtagene maraleucel | CD19 | 2021, 2022, 2024 (DLBCL, PMBCL) |
| Idecabtagene vicleucel | BCMA | 2021, 2024 (Multiple Myeloma—MM) |
| Ciltacabtagene autoleucel | BCMA | 2022, 2023 (MM) |
Despite the significant advancements and successes of targeted therapies and immune therapy, these approaches have many persistent limitations that hinder their success. Cancer cells frequently adapt, developing resistance that reduces the effectiveness of treatments like monoclonal antibodies, small-molecule inhibitors, ADCs, CAR T-cell products, and immune checkpoint inhibitors over time[41]. The resistance often stems from genetic mutations, altered signaling pathways, or changes in the tumor microenvironment, such as variable vasculature and immune suppression [42]. Additionally, these therapies can cause a spectrum of secondary effects that impact patients’ quality of life, including but not limited to skin toxicity, high blood pressure, and heart damage to severe autoimmune reactions like cytokine release syndrome (CRS), neurotoxicity, swelling, nausea, vomiting, diarrhea or constipation, allergic reactions, and hair loss [43]. Moreover, the complexity of these treatments, especially ADCs and CAR T-cell products, demands specialized manufacturing and delivery, increasing the costs and limiting their widespread availability and accessibility [44][45]. Addressing these challenges and limitations of targeted cancer therapies is crucial for improving therapeutic outcomes and treatment strategies to overcome these challenges.
Cancer vaccines can be clinically used therapeutically or preventively and are delivered in four forms: cell-based, viral/bacterial-based, peptide-based, and nucleic acid-based vaccines (Figure 2) [46][47]. These vaccines use tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) to elicit an immune response in patients that would provoke both cellular and humoral immune responses to eradicate tumors and prevent tumorigenesis [47][48]. Cell-based vaccines are prepared using whole tumor cells or cell fragments, which can be injected directly or loaded on DCs with adjuvants to enhance immunogenicity [47][49]. Viral/bacterial-based vaccines are naturally immunogenic, and their genetic material can be engineered to express tumor antigens [47]. Peptide-based vaccines contain biosynthetic peptides that represent known tumor antigens to stimulate the immune system to attack particular tumor sites [47]. Lastly, nucleic acid-based vaccines deliver genetic material that encodes tumor antigens, thus inducing MHC I-mediated CD8+ T-cell responses, making it one of the more promising approaches [47][50].
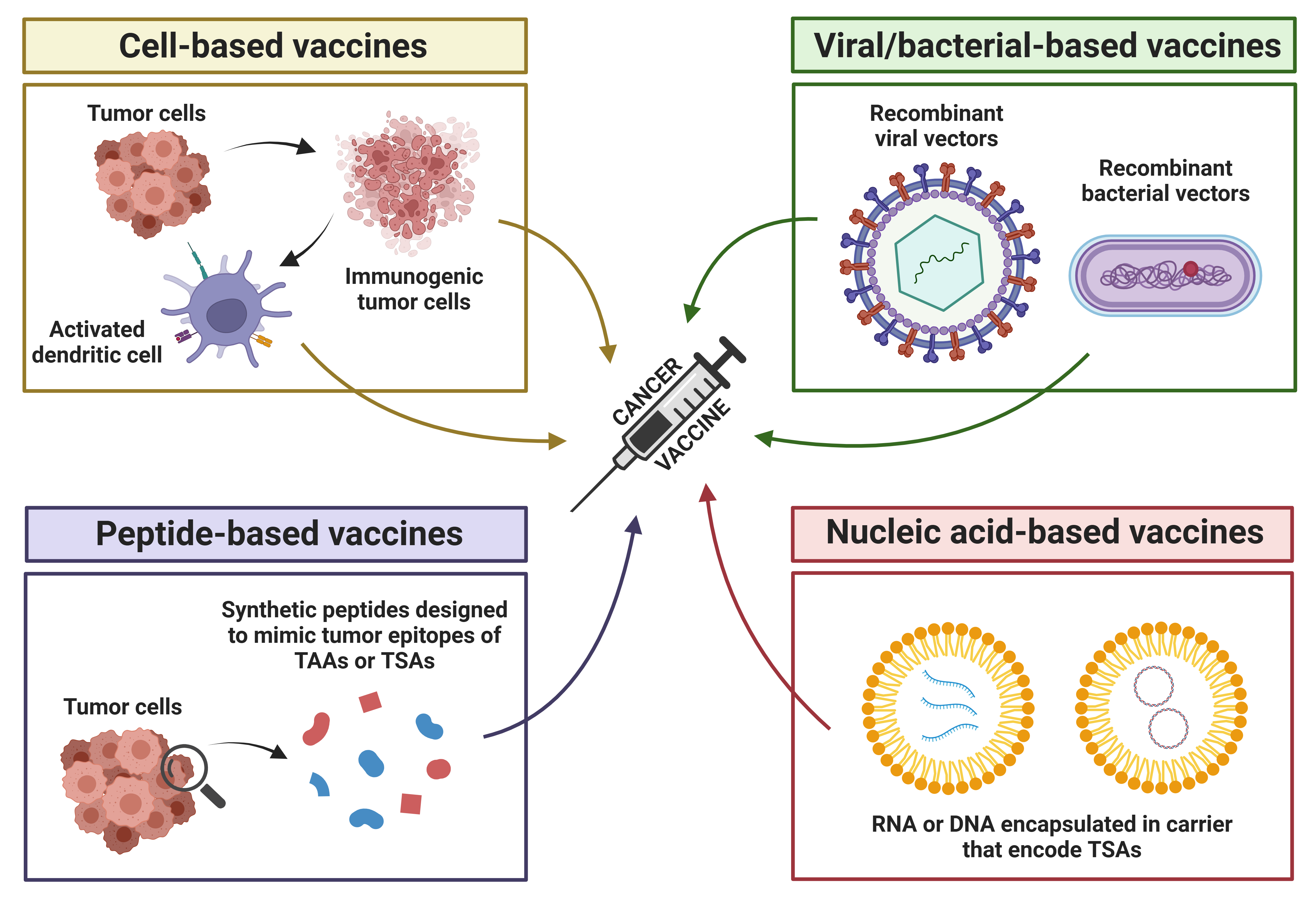
Figure 2. Types of cancer vaccines. There are four types of cancer vaccines: cell-based, viral/bacterial-based, peptide-based, and nucleic acid-based vaccines. Cell-based vaccines are prepared using whole tumor cells or tumor cell fragments. which can be injected directly or loaded onto dendritic cells along with adjuvants to enhance their immunogenicity and stimulate a stronger anti-tumor immune response. Viral/bacterial-based vaccines are designed using recombinant viral or bacterial vectors to deliver genetic material encoding cancer-specific proteins or antigens. These vectors infect host cells, enabling the expression of the target antigens and stimulating an immune response against cancer cells. Peptide-based vaccines use short biosynthetic peptides that mimic specific tumor epitopes of tumor-associated antigens (TAAs) or tumor-specific antigens (TSAs) to stimulate the immune system to recognize and attack cancer cells at specific tumor sites where the target antigens are expressed. Nucleic acid-based vaccines deliver genetic material (RNA or DNA) that encodes tumor-specific antigens. The RNA or DNA is typically encapsulated in carriers to protect it from degradation and facilitate efficient delivery into the host cells. Once inside, the genetic material is expressed, producing the target antigens, which are then presented to the immune system. This stimulates T and B cells to recognize and attack cancer cells that express these antigens. Created in BioRender. Stojchevski, R. (2025) https://BioRender.com/c04n703.
Therapeutic cancer vaccines have shown great success in clinical trials [46]. Several therapeutic vaccines that have been approved by the FDA are already in use against various cancers (Table 4) [46]. Sipuleucel-T was the first FDA-approved therapeutic cell-based vaccine for metastatic prostate cancer [51]. The prolonged disease course of advanced prostate cancer creates a window where the body can generate an immune response against the cancer cells [52]. Another example is the bacillus Calmette–Guerin (BCG) vaccine, which is a bacterial-based vaccine used to treat early-stage bladder cancer [46]. BCG uses inactivated tuberculosis bacteria, which is administered through a catheter to stimulate an immune response, causing apoptosis, necrocytosis, and oxidative stress [53][54].
While therapeutic vaccines target existing tumors, prophylactic/preventive cancer vaccines aim to reduce the initial risk of cancer development, primarily protecting against virus-induced cancers [46]. One of the two currently approved and common prophylactic cancer vaccines is the Human Papillomavirus (HPV) vaccine (Table 6), which utilizes a virus-like particle of the non-oncogenic and non-infectious papillomavirus capsid protein L1 to build an immune response that would prevent HPV from inserting itself into the host’s genome and cause nuclear aberrations [55][56]. The other prophylactic vaccine in use is the Hepatitis B vaccine, which is a common liver infection that leads to liver cirrhosis and hepatocellular carcinoma (HCC) (Table 4) [57]. A study by Cao et al. [58] showed that the Hepatitis B vaccine offers 72% protection against liver cancer post-infection.
| Vaccine Name | Type | Key Details | Prophylactic/ Therapeutics |
FDA Approval |
|---|---|---|---|---|
| Sipuleucel-T (Provenge) |
Cell-based | Autologous dendritic cells activated with PAP-GM-CSF fusion protein | Therapeutic | 2010 (Metastatic castration- resistant prostate cancer mCRPC) |
| Bacillus Calmette-Guerin (BCG) | Bacterial-based | Live attenuated bacterium; stimulates immune response against bladder tumors | Therapeutic | 1990 (Non-muscle-invasive bladder cancer NMIBC) |
| Talimogene Laherparepvec (T-VEC, Imlygic) |
Viral-based (Oncolytic) |
Modified herpes virus; lyses tumors and enhances antitumor immunity |
Therapeutic | 2015 (Melanoma) |
| Hepatitis B (HBV) Vaccine (Recombivax HB, Energix-B) |
Viral-based (Recombinant protein) |
Prevents HBV infection, indirectly reducing HCC | Prophylactic | 1986 Recombivax HB 1989 Energix-B, (Hepatitis B virus—prevents HCC) |
| HPV Vaccines (Cervarix, Gardasil 9) |
Viral-based (Virus replicon particle) |
Targets HPV strains (i.e., 16/18) directly linked to HPV-related cancer and prevents infection | Prophylactic | 2009 Cervarix, 2014 Gardasil 9 (HPV-related cancers— prevents cervical, anal, and other types of cancers) |
2. Diagnostic and Analytical Innovations: Advanced Tools for Cancer Care

ADC – Antibody–Drug Conjugate
AI – Artificial Intelligence
AKT – Protein Kinase B
ALK – Anaplastic Lymphoma Kinase
AML – Acute Myeloid Leukemia
BCG – Bacillus Calmette–Guérin
BRAF – v-Raf Murine Sarcoma Viral Oncogene Homolog B
CAD – Computer-Aided Diagnoses
CAR – Chimeric Antigen Receptor
CNN – Convolutional Neural Network
CRS – Cytokine Release Syndrome
CTCs – Circulating Tumor Cells
ctDNA – Circulating Tumor DNA
CTLA-4 – Cytotoxic T-Lymphocyte-Associated Protein 4
DAMP – Damage-Associated Molecular Patterns
DC – Dendritic Cell
EGFR – Epidermal Growth Factor Receptor
ELCAP – Early Lung Cancer Action Program
ERBB2 – Erb-B2 Receptor Tyrosine Kinase 2 (synonym for HER2)
EVs – Extracellular Vesicles
FASTK – Fas-Activated Serine/Threonine Kinase
FAT1 – FAT Atypical Cadherin 1
FDA – U.S. Food and Drug Administration
FLT3 – Fms-Like Tyrosine Kinase 3
H2AW – H2A.W Histone
HCC – Hepatocellular Carcinoma
HER2 – Human Epidermal Growth Factor Receptor 2
HPV – Human Papillomavirus
IGFALS – Insulin-Like Growth Factor Binding Protein Acid Labile Subunit
KEAP1 – Kelch-Like ECH-Associated Protein 1
KRAS – Kirsten Rat Sarcoma Viral Oncogene Homolog
LIDC – Lung Image Database Consortium
LOF – Loss of Function
MAP2K1 – Mitogen-Activated Protein Kinase Kinase 1 (MEK1)
MAPK – Mitogen-Activated Protein Kinase
MET – Mesenchymal-Epithelial Transition Factor
miRNA – MicroRNAs
ML – Machine Learning
MM – Multiple Myeloma
MRD – Minimal Residual Disease
NGS – Next-Generation Sequencing
NRAS – Neuroblastoma RAS Viral Oncogene Homolog
NSCLC – Non-Small Cell Lung Cancer
PCR – Polymerase Chain Reaction
PD-1 – Programmed Cell Death Protein 1
PD-L1 – Programmed Death-Ligand 1
PI3K – Phosphoinositide 3-Kinase
POLR3K – RNA Polymerase III Subunit K
RIT – Radioimmunotherapy
ROS – Reactive Oxygen Species
ROS1 – ROS Proto-Oncogene 1, Receptor Tyrosine Kinase
RTK – Receptor Tyrosine Kinases
SETBP1 – SET Binding Protein 1
STK11 – Serine/Threonine Kinase 11
TAA – Tumor-Associated Antigen
TME – Tumor Microenvironment
TP53 – Tumor Protein p53
TSA – Tumor-Specific Antigen
T-VEC – Talimogene Laherparepvec
VEGF – Vascular Endothelial Growth Factor
WES – Whole Exome Sequencing
References
- Padma, V.V. An Overview of Targeted Cancer Therapy. BioMedicine 2015, 5, 19.
- Shuel, S.L. Targeted Cancer Therapies: Clinical Pearls for Primary Care. Can. Fam. Physician 2022, 68, 515–518.
- Charlton, P.; Spicer, J. Targeted Therapy in Cancer. Medicine 2016, 44, 34–38.
- Shahid, K.; Khalife, M.; Dabney, R.; Phan, A.T. Immunotherapy and Targeted Therapy—The New Roadmap in Cancer Treatment. Ann. Transl. Med. 2019, 7, 595.
- Dailah, H.G.; Hommdi, A.A.; Koriri, M.D.; Algathlan, E.M.; Mohan, S. Potential Role of Immunotherapy and Targeted Therapy in the Treatment of Cancer: A Contemporary Nursing Practice. Heliyon 2024, 10, e24559.
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular Targeted Therapy: Treating Cancer with Specificity. Eur. J. Pharmacol. 2018, 834, 188–196.
- Liu, G.-H.; Chen, T.; Zhang, X.; Ma, X.-L.; Shi, H.-S. Small Molecule Inhibitors Targeting the Cancers. MedComm 2022, 3, e181.
- Hertzman Johansson, C.; Egyhazi Brage, S. BRAF Inhibitors in Cancer Therapy. Pharmacol. Ther. 2014, 142, 176–182.
- Straussman, R.; Morikawa, T.; Shee, K.; Barzily-Rokni, M.; Qian, Z.R.; Du, J.; Davis, A.; Mongare, M.M.; Gould, J.; Frederick, D.T.; et al. Tumour Micro-Environment Elicits Innate Resistance to RAF Inhibitors through HGF Secretion. Nature 2012, 487, 500–504.
- Asić, K. Dominant Mechanisms of Primary Resistance Differ from Dominant Mechanisms of Secondary Resistance to Targeted Therapies. Crit. Rev. Oncol. Hematol. 2016, 97, 178–196.
- Balik, K.; Modrakowska, P.; Maj, M.; Kaźmierski, Ł.; Bajek, A. Limitations of Molecularly Targeted Therapy. Med. Res. J. 2019, 4, 99–105.
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34.
- Singh, S.; Kumar, N.K.; Dwiwedi, P.; Charan, J.; Kaur, R.; Sidhu, P.; Chugh, V.K. Monoclonal Antibodies: A Review. Curr. Clin. Pharmacol. 2018, 13, 85–99.
- Kumar, M.; Jalota, A.; Sahu, S.K.; Haque, S. Therapeutic Antibodies for the Prevention and Treatment of Cancer. J. Biomed. Sci. 2024, 31, 6.
- Pincetic, A.; Bournazos, S.; DiLillo, D.J.; Maamary, J.; Wang, T.T.; Dahan, R.; Fiebiger, B.-M.; Ravetch, J.V. Type I and Type II Fc Receptors Regulate Innate and Adaptive Immunity. Nat. Immunol. 2014, 15, 707–716.
- Bournazos, S.; Wang, T.T.; Dahan, R.; Maamary, J.; Ravetch, J.V. Signaling by Antibodies: Recent Progress. Annu. Rev. Immunol. 2017, 35, 285–311.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Trastuzumab for Early-Stage, HER2-Positive Breast Cancer: A Meta-Analysis of 13,864 Women in Seven Randomised Trials. Lancet Oncol. 2021, 22, 1139–1150.
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-Positive Breast Cancer: Advances and Future Directions. Nat. Rev. Drug Discov. 2023, 22, 101–126.
- Zhou, J.; Ji, Q.; Li, Q. Resistance to Anti-EGFR Therapies in Metastatic Colorectal Cancer: Underlying Mechanisms and Reversal Strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328.
- Kasi, P.M.; Afable, M.G.; Herting, C.; Lukanowski, M.; Jin, Z. Anti-EGFR Antibodies in the Management of Advanced Colorectal Cancer. Oncologist 2023, 28, 1034–1048.
- Gordeev, A.; Vaal, A.; Puchkova, M.; Smirnova, I.; Doronin, A.; Znobishcheva, A.; Zhmudanova, D.; Aleksandrov, A.; Sukchev, M.; Imyanitov, E.; et al. Preclinical Comparison of Prolgolimab, Pembrolizumab and Nivolumab. Sci. Rep. 2024, 14, 23136.
- McMahon, D.J.; McLaughlin, R.; Naidoo, J. Is Immunotherapy Beneficial in Patients with Oncogene-Addicted Non-Small Cell Lung Cancers? A Narrative Review. Cancers 2024, 16, 527.
- Rotte, A. Combination of CTLA-4 and PD-1 Blockers for Treatment of Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255.
- McLean, L.; Leal, J.L.; Solomon, B.J.; John, T. Immunotherapy in Oncogene Addicted Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 2736–2751.
- Zhang, J.; Vokes, N.; Li, M.; Xu, J.; Bai, H.; Wang, J.; Wang, Z.; Zhang, J. Overcoming EGFR-TKI Resistance by Targeting the Tumor Microenvironment. Chin. Med. J. Pulm. Crit. Care Med. 2024, 2, 151–161.
- Otano, I.; Ucero, A.C.; Zugazagoitia, J.; Paz-Ares, L. At the Crossroads of Immunotherapy for Oncogene-Addicted Subsets of NSCLC. Nat. Rev. Clin. Oncol. 2023, 20, 143–159.
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017.
- Bodet-Milin, C.; Kraeber-Bodéré, F.; Eugène, T.; Guérard, F.; Gaschet, J.; Bailly, C.; Mougin, M.; Bourgeois, M.; Faivre-Chauvet, A.; Chérel, M.; et al. Radioimmunotherapy for Treatment of Acute Leukemia. Semin. Nucl. Med. 2016, 46, 135–146.
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.-K.V.; Press, O.W. Radioimmunotherapy of Human Tumours. Nat. Rev. Cancer 2015, 15, 347–360.
- Abbasi, A.; Dadashpour, M.; Alipourfard, I. Calculation of Radium-223 and Actinium-225 α-Emitter Radiopharmaceuticals Dose Rates in Treatment of Metastatic Castration-Resistant Prostate Cancer. J. Cancer Res. Ther. 2021, 17, 348–352.
- Cremonesi, M.; Ferrari, M.; Grana, C.M.; Vanazzi, A.; Stabin, M.; Bartolomei, M.; Papi, S.; Prisco, G.; Martinelli, G.; Paganelli, G.; et al. High-Dose Radioimmunotherapy with 90Y-Ibritumomab Tiuxetan: Comparative Dosimetric Study for Tailored Treatment. J. Nucl. Med. 2007, 48, 1871–1879.
- Guo, Y.; Parry, J.J.; Laforest, R.; Rogers, B.E.; Anderson, C.J. The Role of P53 in Combination Radioimmunotherapy with 64Cu-DOTA-Cetuximab and Cisplatin in a Mouse Model of Colorectal Cancer. J. Nucl. Med. 2013, 54, 1621–1629.
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93.
- Hurwitz, J.; Haggstrom, L.R.; Lim, E. Antibody–Drug Conjugates: Ushering in a New Era of Cancer Therapy. Pharmaceutics 2023, 15, 2017.
- Baron, J.; Wang, E.S. Gemtuzumab Ozogamicin for the Treatment of Acute Myeloid Leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559.
- Gogia, P.; Ashraf, H.; Bhasin, S.; Xu, Y. Antibody–Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers 2023, 15, 3886.
- Levine, B.L.; Miskin, J.; Wonnacott, K.; Keir, C. Global Manufacturing of CAR T Cell Therapy. Mol. Ther.-Methods Clin. Dev. 2017, 4, 92–101.
- De Marco, R.C.; Monzo, H.J.; Ojala, P.M. CAR T Cell Therapy: A Versatile Living Drug. Int. J. Mol. Sci. 2023, 24, 6300.
- Majzner, R.G.; Mackall, C.L. Clinical Lessons Learned from the First Leg of the CAR T Cell Journey. Nat. Med. 2019, 25, 1341–1355.
- Goyco Vera, D.; Waghela, H.; Nuh, M.; Pan, J.; Lulla, P. Approved CAR-T Therapies Have Reproducible Efficacy and Safety in Clinical Practice. Hum. Vaccines Immunother. 2024, 20, 2378543.
- Keefe, D.M.K.; Bateman, E.H. Potential Successes and Challenges of Targeted Cancer Therapies. JNCI Monogr. 2019, 2019, lgz008.
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109.
- American Cancer Society. Managing Cancer-Related Side Effects. Available online: https://www.cancer.org/cancer/managing-cancer/side-effects.html (accessed on 5 March 2025).
- Kiss, B.; Borbély, J. Business Risk Mitigation in the Development Process of New Monoclonal Antibody Drug Conjugates for Cancer Treatment. Pharmaceutics 2023, 15, 1761.
- Dropulić, B. CAR-T and Cellular Gene Therapies Are Too Expensive. Nat. Med. 2024, 30, 2714.
- Memorial Sloan Kettering Cancer Center. Cancer Vaccines: The Types, How They Work, and Which Cancers They Treat. Available online: https://www.mskcc.org/cancer-care/diagnosis-treatment/cancer-treatments/immunotherapy/cancer-vaccines (accessed on 25 January 2025).
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer Vaccines as Promising Immuno-Therapeutics: Platforms and Current Progress. J. Hematol. Oncol. 2022, 15, 28.
- Miao, L.; Zhang, Y.; Huang, L. MRNA Vaccine for Cancer Immunotherapy. Mol. Cancer 2021, 20, 41.
- Pérez-Baños, A.; Gleisner, M.A.; Flores, I.; Pereda, C.; Navarrete, M.; Araya, J.P.; Navarro, G.; Quezada-Monrás, C.; Tittarelli, A.; Salazar-Onfray, F. Whole Tumour Cell-Based Vaccines: Tuning the Instruments to Orchestrate an Optimal Antitumour Immune Response. Br. J. Cancer 2023, 129, 572–585.
- Pandya, A.; Shah, Y.; Kothari, N.; Postwala, H.; Shah, A.; Parekh, P.; Chorawala, M.R. The Future of Cancer Immunotherapy: DNA Vaccines Leading the Way. Med. Oncol. 2023, 40, 200.
- Rastogi, I.; Muralidhar, A.; McNeel, D.G. Vaccines as Treatments for Prostate Cancer. Nat. Rev. Urol. 2023, 20, 544–559.
- Madan, R.A.; Gulley, J.L. Sipuleucel-T: Harbinger of a New Age of Therapeutics for Prostate Cancer. Expert Rev. Vaccines 2011, 10, 141–150.
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the Treatment of Bladder Cancer-Current Understanding and the Prospect. Biomed. Pharmacother. 2020, 129, 110393.
- Jiang, S.; Redelman-Sidi, G. BCG in Bladder Cancer Immunotherapy. Cancers 2022, 14, 3073.
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391.
- Aggarwal, S.; Agarwal, P.; Singh, A.K. Human Papilloma Virus Vaccines: A Comprehensive Narrative Review. Cancer Treat. Res. Commun. 2023, 37, 100780.
- Flores, J.E.; Thompson, A.J.; Ryan, M.; Howell, J. The Global Impact of Hepatitis B Vaccination on Hepatocellular Carcinoma. Vaccines 2022, 10, 793.
- Cao, M.; Fan, J.; Lu, L.; Fan, C.; Wang, Y.; Chen, T.; Zhang, S.; Yu, Y.; Xia, C.; Lu, J.; et al. Long Term Outcome of Prevention of Liver Cancer by Hepatitis B Vaccine: Results from an RCT with 37 Years. Cancer Lett. 2022, 536, 215652.
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662.
- Thorne, S.H.; Liang, W.; Sampath, P.; Schmidt, T.; Sikorski, R.; Beilhack, A.; Contag, C.H. Targeting Localized Immune Suppression Within the Tumor Through Repeat Cycles of Immune Cell-Oncolytic Virus Combination Therapy. Mol. Ther. 2010, 18, 1698–1705.
- Ma, J.; Ramachandran, M.; Jin, C.; Quijano-Rubio, C.; Martikainen, M.; Yu, D.; Essand, M. Characterization of Virus-Mediated Immunogenic Cancer Cell Death and the Consequences for Oncolytic Virus-Based Immunotherapy of Cancer. Cell Death Dis. 2020, 11, 48.
- Guo, Z.S.; Liu, Z.; Bartlett, D.L. Oncolytic Immunotherapy: Dying the Right Way Is a Key to Eliciting Potent Antitumor Immunity. Front. Oncol. 2014, 4, 74.
- Yan, Z.; Zhang, Z.; Chen, Y.; Xu, J.; Wang, J.; Wang, Z. Enhancing Cancer Therapy: The Integration of Oncolytic Virus Therapy with Diverse Treatments. Cancer Cell Int. 2024, 24, 242.
- Zhu, Z.; McGray, A.J.R.; Jiang, W.; Lu, B.; Kalinski, P.; Guo, Z.S. Improving Cancer Immunotherapy by Rationally Combining Oncolytic Virus with Modulators Targeting Key Signaling Pathways. Mol. Cancer 2022, 21, 196.
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic Virus Immunotherapy: Future Prospects for Oncology. J. Immunother. Cancer 2018, 6, 140.
- Ferrucci, P.F.; Pala, L.; Conforti, F.; Cocorocchio, E. Talimogene Laherparepvec (T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers 2021, 13, 1383.
- Peter, M.; Kühnel, F. Oncolytic Adenovirus in Cancer Immunotherapy. Cancers 2020, 12, 3354.
- Hemminki, O.; dos Santos, J.M.; Hemminki, A. Oncolytic Viruses for Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 84.
- Russell, S.J.; Barber, G.N. Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines. Cancer Cell 2018, 33, 599–605.
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159.
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges and Prospects. Signal Transduct. Target. Ther. 2023, 8, 450.
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther.-Oncolytics 2019, 15, 234–247.
- Corti, C.; Cobanaj, M.; Dee, E.C.; Criscitiello, C.; Tolaney, S.M.; Celi, L.A.; Curigliano, G. Artificial Intelligence in Cancer Research and Precision Medicine: Applications, Limitations and Priorities to Drive Transformation in the Delivery of Equitable and Unbiased Care. Cancer Treat. Rev. 2023, 112, 102498.
- Armakolas, A.; Kotsari, M.; Koskinas, J. Liquid Biopsies, Novel Approaches and Future Directions. Cancers 2023, 15, 1579.
- Connal, S.; Cameron, J.M.; Sala, A.; Brennan, P.M.; Palmer, D.S.; Palmer, J.D.; Perlow, H.; Baker, M.J. Liquid Biopsies: The Future of Cancer Early Detection. J. Transl. Med. 2023, 21, 118.
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid Biopsy in Cancer: Current Status, Challenges and Future Prospects. Signal Transduct. Target. Ther. 2024, 9, 336.
- Bustin, S.A.; Siddiqi, S.; Ahmed, S.; Hands, R.; Dorudi, S. Quantification of Cytokeratin 20, Carcinoembryonic Antigen and Guanylyl Cyclase C MRNA Levels in Lymph Nodes May Not Predict Treatment Failure in Colorectal Cancer Patients. Int. J. Cancer 2004, 108, 412–417.
- Mastoraki, S.; Strati, A.; Tzanikou, E.; Chimonidou, M.; Politaki, E.; Voutsina, A.; Psyrri, A.; Georgoulias, V.; Lianidou, E. ESR1 Methylation: A Liquid Biopsy–Based Epigenetic Assay for the Follow-up of Patients with Metastatic Breast Cancer Receiving Endocrine Treatment. Clin. Cancer Res. 2018, 24, 1500–1510.
- Newman, A.M.; Bratman, S.V.; To, J.; Wynne, J.F.; Eclov, N.C.W.; Modlin, L.A.; Liu, C.L.; Neal, J.W.; Wakelee, H.A.; Merritt, R.E.; et al. An Ultrasensitive Method for Quantitating Circulating Tumor DNA with Broad Patient Coverage. Nat. Med. 2014, 20, 548–554.
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131.
- Ma, S.; Zhou, M.; Xu, Y.; Gu, X.; Zou, M.; Abudushalamu, G.; Yao, Y.; Fan, X.; Wu, G. Clinical Application and Detection Techniques of Liquid Biopsy in Gastric Cancer. Mol. Cancer 2023, 22, 7.
- Turning the Tide of Early Cancer Detection. Nat. Med. 2024, 30, 1217.
- Han, H.S.; Lee, K.-W. Liquid Biopsy: An Emerging Diagnostic, Prognostic, and Predictive Tool in Gastric Cancer. J. Gastric Cancer 2024, 24, 4.
- Fatima, S.; Ma, Y.; Safrachi, A.; Haider, S.; Spring, K.J.; Vafaee, F.; Scott, K.F.; Roberts, T.L.; Becker, T.M.; de Souza, P. Harnessing Liquid Biopsies to Guide Immune Checkpoint Inhibitor Therapy. Cancers 2022, 14, 1669.
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C.; et al. Erratum: Clonal Evolution and Resistance to EGFR Blockade in the Blood of Colorectal Cancer Patients. Nat. Med. 2015, 21, 827.
- Cossu, A.M.; Scrima, M.; Lombardi, A.; Grimaldi, A.; Russo, M.; Ottaiano, A.; Caraglia, M.; Bocchetti, M. Future Directions and Management of Liquid Biopsy in Non-Small Cell Lung Cancer. Explor. Target. Anti-Tumor Ther. 2020, 1, 239–252.
- Canale, M.; Pasini, L.; Bronte, G.; Delmonte, A.; Cravero, P.; Crinò, L.; Ulivi, P. Role of Liquid Biopsy in Oncogene-Addicted Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2019, 8, S265–S279.
- Pantel, K.; Alix-Panabières, C. Liquid Biopsy and Minimal Residual Disease—Latest Advances and Implications for Cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424.
- Johnston, A.D.; Ross, J.P.; Ma, C.; Fung, K.Y.C.; Locke, W.J. Epigenetic Liquid Biopsies for Minimal Residual Disease, What’s around the Corner? Front. Oncol. 2023, 13, 1103797.
- Pantel, K.; Alix-Panabières, C. Minimal Residual Disease as a Target for Liquid Biopsy in Patients with Solid Tumours. Nat. Rev. Clin. Oncol. 2025, 22, 65–77.
- Alba-Bernal, A.; Lavado-Valenzuela, R.; Domínguez-Recio, M.E.; Jiménez-Rodriguez, B.; Queipo-Ortuño, M.I.; Alba, E.; Comino-Méndez, I. Challenges and Achievements of Liquid Biopsy Technologies Employed in Early Breast Cancer. eBioMedicine 2020, 62, 103100.
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid Biopsy: A Step Closer to Transform Diagnosis, Prognosis and Future of Cancer Treatments. Mol. Cancer 2022, 21, 79.
- Singh, D.; Dhiman, V.K.; Pandey, M.; Dhiman, V.K.; Sharma, A.; Pandey, H.; Verma, S.K.; Pandey, R. Personalized Medicine: An Alternative for Cancer Treatment. Cancer Treat. Res. Commun. 2024, 42, 100860.
- Huang, S.; Yang, J.; Shen, N.; Xu, Q.; Zhao, Q. Artificial Intelligence in Lung Cancer Diagnosis and Prognosis: Current Application and Future Perspective. Semin. Cancer Biol. 2023, 89, 30–37.
- Chen, Y.; Han, Q.; Huang, Z.; Lyu, M.; Ai, Z.; Liang, Y.; Yan, H.; Wang, M.; Xiang, Z. Value of IVIM in Differential Diagnoses between Benign and Malignant Solitary Lung Nodules and Masses: A Meta-Analysis. Front. Surg. 2022, 9, 817443.
- Wan, Y.-L.; Wu, P.; Huang, P.-C.; Tsay, P.-K.; Pan, K.-T.; Trang, N.; Chuang, W.-Y.; Wu, C.-Y.; Lo, S. The Use of Artificial Intelligence in the Differentiation of Malignant and Benign Lung Nodules on Computed Tomograms Proven by Surgical Pathology. Cancers 2020, 12, 2211.
- Zheng, B.; Yang, D.; Zhu, Y.; Liu, Y.; Hu, J.; Bai, C. 3D Gray Density Coding Feature for Benign-malignant Pulmonary Nodule Classification on Chest CT. Med. Phys. 2021, 48, 7826–7836.
- Mehta, K.; Jain, A.; Mangalagiri, J.; Menon, S.; Nguyen, P.; Chapman, D.R. Lung Nodule Classification Using Biomarkers, Volumetric Radiomics, and 3D CNNs. J. Digit. Imaging 2021, 34, 647–666.
- Fedorov, A.; Hancock, M.; Clunie, D.; Brochhausen, M.; Bona, J.; Kirby, J.; Freymann, J.; Pieper, S.; Aerts, H.J.W.L.; Kikinis, R.; et al. DICOM Re-encoding of Volumetrically Annotated Lung Imaging Database Consortium (LIDC) Nodules. Med. Phys. 2020, 47, 5953–5965.
- Armato, S.G.; McLennan, G.; Bidaut, L.; McNitt-Gray, M.F.; Meyer, C.R.; Reeves, A.P.; Zhao, B.; Aberle, D.R.; Henschke, C.I.; Hoffman, E.A.; et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): A Completed Reference Database of Lung Nodules on CT Scans. Med. Phys. 2011, 38, 915–931.
- Amodio, V.; Yaeger, R.; Arcella, P.; Cancelliere, C.; Lamba, S.; Lorenzato, A.; Arena, S.; Montone, M.; Mussolin, B.; Bian, Y.; et al. EGFR Blockade Reverts Resistance to KRASG12C Inhibition in Colorectal Cancer. Cancer Discov. 2020, 10, 1129–1139.
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and Mutation Prediction from Non–Small Cell Lung Cancer Histopathology Images Using Deep Learning. Nat. Med. 2018, 24, 1559–1567.
- Wong, E.Y.; Chu, T.N.; Ladi-Seyedian, S.-S. Genomics and Artificial Intelligence. Urol. Clin. N. Am. 2024, 51, 27–33.
- Liu, Q.; Reed, M.; Zhu, H.; Cheng, Y.; Almeida, J.; Fruhbeck, G.; Ribeiro, R.; Hu, P. Epigenome-Wide DNA Methylation and Transcriptome Profiling of Localized and Locally Advanced Prostate Cancer: Uncovering New Molecular Markers. Genomics 2022, 114, 110474.




