| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christopher Lucas | + 2479 word(s) | 2479 | 2021-02-10 06:39:28 | | | |
| 2 | Karina Chen | Meta information modification | 2479 | 2021-03-04 09:30:33 | | |
Video Upload Options
Respiratory diseases are frequently characterised by epithelial injury, airway inflammation, de-fective tissue repair, and airway remodelling. This may occur in a subacute or chronic context, such as asthma and chronic obstructive pulmonary disease, or occur acutely as in pathogen challenge or acute respiratory distress syndrome (ARDS). Despite the frequent challenge of lung homeostasis, not all pulmonary insults lead to disease. Traditionally thought of as a quiescent organ, emerging evidence highlights that the lung has significant capacity to respond to injury by repairing and replacing damaged cells. This occurs with the appropriate and timely resolution of inflammation and concurrent initiation of tissue repair programmes. Airway epithelial cells are key effectors in lung homeostasis and host defence; continual exposure to pathogens, toxins, and particulate matter challenge homeostasis, requiring robust defence and repair mechanisms. As such, the epithelium is critically involved in the return to homeostasis, orchestrating the resolution of inflammation and initiating tissue repair.
1. Epithelial Roles in Tissue Repair
Lungs are continually exposed to infections, toxins, and airborne pollutants that stress homeostasis. Consequently, respiratory disorders cause a vast burden of global disease and are among the leading causes of death worldwide [1]. Although a quiescent organ at baseline the lungs have a significant reparative capacity in response to injury [2] (Figure 1A), but dysregulated inflammation and aberrant or defective repair mechanisms are increasingly linked to the pathobiology of several diseases including COPD, asthma, pulmonary fibrosis and acute respiratory distress syndrome (ARDS) [3]. Given that the pulmonary epithelium is central to host defence, homeostasis, and disease biology, this review highlights the role of airway epithelium in repair, with a particular focus on the mediators involved. While not an exhaustive assessment of the current literature, this review will focus on the interaction and interplay of epithelial regeneration and inflammatory processes.
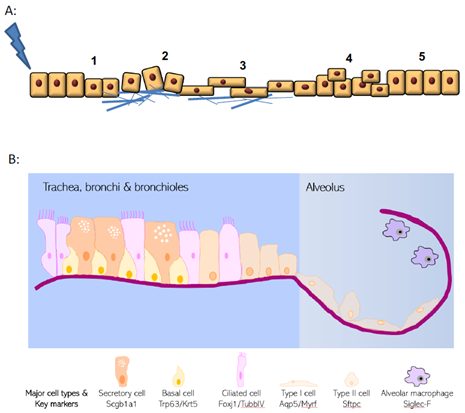
Figure 1. Hallmarks of wound healing and pulmonary epithelial organisation. (A) Upon injury, epithelial cells undergo a multistage process to repair damage. These phases include i. dedifferentiation from specialised and mature cells, ii. adhesion to extracellular matrix, iii. spreading and migration towards the wound site, iv. cellular proliferation and finally v. redifferentiation and repair. (B) Structure of the pulmonary epithelium and organisation of major epithelial cell types.
2. Epithelial Structure and Evolving Knowledge on Progenitor Populations
The lung epithelial cellular structure and composition varies significantly along its proximal–distal axis (Figure 1B). Within the trachea and conducting airways, the epithelium is arranged predominantly as a pseudostratified layer, with the most frequent cell types being ciliated cells, secretory cells, and basal cells that are adherent to the basal lamina [4]. In addition, small numbers of neuroendocrine cells and tuft cells are also present. This is in distinct contrast to the alveolar regions where thin type I cells (AT1) lie in close apposition to endothelial cells for efficient gas exchange, along with the presence of cuboidal type II cells (AT2) that produce pulmonary surfactant proteins. The three main cell types within the pulmonary epithelium that have well-documented progenitor potential are basal cells, secretory cells, and the AT2 cells [4]. However, these cells are unusual in that they display remarkable plasticity and heterogeneity in response to injury. Pulmonary progenitor cells are frequently fully differentiated epithelial cells with specialised function rather than populations of immature precursor cells. New approaches to identifying and phenotyping epithelial cells (such as single cell sequencing approaches) are continuing to reveal additional complexity and heterogeneity to respiratory epithelial cell identity [5][6][7].
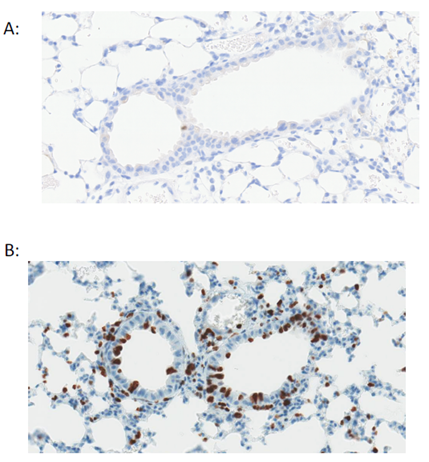
Original studies using pulsed thymidine [8] have been complemented by recent lineage tracing studies that together show the pulmonary epithelium is quiescent during homeostasis with very low rates of turnover (Figure 2). However, in response to injury, the epithelium can mount a robust response with many cells re-entering the cell cycle to divide and/or differentiate or dedifferentiate [2]. Within the trachea and proximal airways basal cells appear to be the predominant progenitor population [9]. Basal cells are characterised by expression of Trp63, cytokeratin Krt5, integrin α6, podoplanin, and nerve growth factor receptor (p75), and are present in the trachea and most of the conducting airways in human lungs (albeit in declining numbers more distally) [2]. By contrast, these cells are only present in more proximal airways in mice. They proliferate in response to epithelial injury and are capable of self-renewal and differentiation into secretory and ciliated cells [9]. They can also restore denuded tracheal xenografts implanted into immunodeficient mice [10].
Figure 2. Pulmonary epithelial cells are quiescent during homeostasis. Staining for Ki67+ve cells (brown) shows very low proliferation in the mouse airway under basal conditions (A) with an increase in proliferation after airway epithelial injury with naphthalene (B).
In distal airway epithelium, a more prominent role for secretory cells as the major progenitor population has been described, although it should be noted that rodent lungs lack basal cells in this region, in contrast to the small numbers of basal cells that also populate this region in humans. Secretory cells are characterised by expression of the secretoglobins Scgb1a1 and Scgb3a2. Under homeostatic conditions, distal airway secretory cells self-renew and differentiate into ciliated cells [11] (which are characterised by the expression of the transcription factor Foxj1 and the cytoskeletal component Tubb4a). Secretory cell injury is most frequently modelled by systemic administration of naphthalene which targets cytochrome p450 (Cyp2f2)-expressing secretory cells. In response to naphthalene-induced injury, those secretory cells that survive (Cyp2f2 negative variant club cells) proliferate and reconstitute both secretory and ciliated cells [11][12]. Furthermore, secretory cells are capable of mediating tracheal epithelial repair, albeit making a minor contribution in the context of sulfur dioxide (SO2)-induced injury [11]. In addition, after marked basal cell injury (induced by a Krt5–diphtheria toxin system) secretory cells are capable of dedifferentiating and repopulating the basal cell population with similar self-renewal rates as ‘normal’ basal cells, with these secretory cell-derived basal cells also able to promote epithelial repair and give rise to all three main epithelial cell types after influenza infection [13].
Within the alveolar region, the best described progenitor cell is the AT2 cell, which proliferates after injury and can repopulate AT1 cells [14][15]. However, other candidate alveolar progenitor cell populations exist. These include a lineage negative progenitor that subsequently expresses Trp63 and Krt5 (traditional basal cell markers) and is able to migrate into the alveoli to repair areas of severe damage after influenza injury [16][17], an α6β4 integrin-expressing cell type that proliferates in response to bleomycin-induced alveolar injury and proliferates and expands clonally in ex vivo culture [18], and a H2-K1 high distal airway epithelial population that can differentiate into alveolar structures [19]. In addition, a population of cells at the bronchoalveolar duct junction (BADJ) that co-expresses both AT2 and secretory markers (surfactant protein C and Scgb1a1, respectively) has been reported to have both alveolar and airway progenitor potential [20]. Given that some AT2s co-express these markers, confirmation of this cell type as a bona fide and independent progenitor population is awaited.
In summary, these studies demonstrate that while the respiratory epithelium has specific region-defined cellular modes of repair, significant plasticity exists in the cells that are able to respond to injury, and that injury-specific and magnitude of injury-specific regenerative process may be invoked. While the cellular players in epithelial reconstitution are increasingly delineated, much work is needed to define the local microenvironmental cues that accelerate healing.
3. Epithelial Cell–Immune Cell Crosstalk
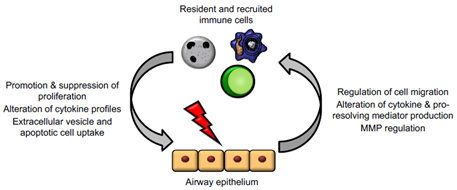
The crosstalk between epithelial cells and immune cells (particularly granulocytes and macrophages) is critical for the appropriate progression of inflammation, resolution, and repair in the lung (Figure 3). As well as being important barrier cells segregating organs from potential hostile environments, epithelial cells are important for regulating immune cell trafficking, triggering changes in mediator and cytokine production, altering phagocytosis during inflammation resolution, and changing production of proteins/MMP to allow for epithelial repair. Epithelium and immune cells can also work together for the generation of certain signals, like specialised pro-resolving mediators (SPMs) which require transcellular synthesis [21]. The role of immune cells in resolution and repair (and some of the ways in which epithelial cells promote these functions) have been extensively reviewed elsewhere.
Figure 3. Epithelial cells and immune cells engage bidirectional communication to regulate tissue repair. Activated immune cells, including macrophages, neutrophils, and T lymphocytes, regulate epithelial cell responses to promote proliferation, alteration of mediator and cytokine production, and downstream signalling cascades. In turn, injured epithelium can themselves regulate immune cell trafficking and promote a shift from inflammation to resolution and repair functions.
Immune cells, in turn, are responsible for helping to lay down scaffolding proteins, promoting epithelial migration and cellular survival and preventing a shift from beneficial repair to fibrotic processes. Neutrophils act as the first responder in inflammation and help neutralise the wound area of infection, helping to promote early stages of inflammation. Regarding the later steps of tissue repair, neutrophils serve as major producers of reactive oxygen species (ROS), nitric oxide (NO), TGF-β, and other mediators that promote epithelial cell migration and proliferation. There is also evidence that infiltration of neutrophils into mucosal epithelium (within the intestine) triggers increased epithelial permeability [22]. It is important to recognise that a balance of neutrophil numbers and activation is key as prolonged neutrophil influx can potentially impair tissue repair, perhaps by maintaining a pro-inflammatory mediator milieu or impairing a shift to repair phenotypes in macrophages and epithelial cells.
Macrophages have a more established role in tissue repair, though the precise molecular mechanisms of action for these cells in tissue repair are still largely unknown. A role for macrophages in tissue repair was first suggested nearly 50 years ago, and multiple mouse models with depleted macrophages (or impaired cellular migration) have impaired wound healing [23][24][25]. Often, macrophages have a critical, temporally restricted role in tissue healing. This is elegantly highlighted in salamander limb repair after amputation, whereby macrophage presence around the initial time of injury is essential for regeneration of the amputated limb. Furthermore, the defective limb repair observed in macrophage-depleted states can be rescued by the combination of allowing macrophage replenishment and then causing additional amputational injury [23]. Some of the roles of macrophages are the same as neutrophils—production of mediators, cytokines, and growth factors that promote epithelial cell proliferation; regulation of oxidative stress; and modulation of epithelial barrier properties—but distinct macrophage roles also exist. For one, macrophages remain a major phagocytic cell, and clearance of debris, wounded epithelial cell fragments, and apoptotic neutrophils is critical for promoting a space for repair and for triggering production of many downstream cellular signals. Indeed, macrophages (along with cough and the mucociliary escalator) is an important part of airway clearance within the healthy lung. Generally, tissue-resident macrophages and recruited monocytes (often differentiated by cytokines and growth factors in the local tissue microenvironment) significantly contribute to tissue repair, regeneration, and the mechanisms of fibrosis, highlighting their substantial plasticity [26]. IFN-γ- or LPS-stimulated macrophages are instrumental in the initial stages, where phagocytosis aids in pathogen killing and clearing debris, whereas IL-4-treated macrophages support angiogenesis and matrix production in the later stages of wound healing [26][27]. The unique roles at the different repair stages have been reviewed elsewhere [28].
Within lung, alveolar macrophages are the most widely studied, and are major regulators of matrix metalloproteinases (MMPs), which are critical for epithelial cell migration [29][30]. Lung epithelial cells, as well as macrophages, are also capable of producing IL-10, with alveolar macrophages having high expression of the IL-10 receptor, which acts to limit inflammatory responses at least partly via JAK1–STAT3 pathways [31][32]. Finally, there is evidence that macrophages may promote matrix deposition and provide scaffolding, which would allow for better epithelial cell migration and re-epithelialisation. In a mouse model investigating the effect of recruited macrophages to the site of skin repair after mechanical injury, peritoneal and tissue-resident macrophages in the skin, spleen, and liver in LysMCre/iDTR mice were depleted at the various stages of healing. Depletion in the inflammatory phase (2 and 1 days prior to wounding as well as at day 2 and 4 post-wounding) resulted in delayed re-epithelialisation and reduced collagen formation. By contrast, depletion of macrophages in the tissue formation phase (mid-stage of the repair response 3, 4, 6, and 8 dpi) was shown to delay wound closure and lead to haemorrhage in the wound tissue [33][34][35]. Within the lung, a second population of tissue resident macrophages also exists, namely interstitial macrophages, with both similarities and differences when compared to alveolar macrophages; their function remains relatively poorly described[36].
It is important to note that dysregulated macrophage function, such as incomplete efferocytosis, can contribute to fibrosis and improper wound healing as well as autoimmune diseases such as systemic lupus erythematous and type I diabetes [37]. For example, in the case of type I diabetes, which occurs as a result of the destruction of insulin producing B cells in the pancreas, aberrant efferocytosis of apoptotic pancreatic cells leading to necrosis is thought to contribute to the release of autoantigens. In addition, impaired efferocytosis is seen in a multitude of diseases, including diabetes and asthma, with evidence that efficient apoptotic cell sensing and clearance is critical for efficient tissue repair [38]. For example, slow wound healing in diabetes is associated with accumulated apoptotic cells at the wound site [37]. Therefore, critical to a healthy repair process is the control of macrophage function and signals that disrupt normal healing and lead to fibrotic scar generation by epithelium and macrophages.
Like the innate immune system, epithelial cells can trigger and respond to adaptive immune cells. Damaged cells relay signals to natural killer (NK) cells, T cells, and innate lymphoid cells (ILCs), among others. For example, the cytokines IL-25 and IL-33 produced by epithelial cells induce Th2-type adaptive responses where increased expression of both cytokines has been found in patients with idiopathic pulmonary fibrosis (IPF) [39][40][41], and IL-33 has an inhibitory effect on mast cell functions [42]. Lymphocytes can directly respond to these signals, trafficking into the injured space, or can relay these signals on to other cell types [43]. Furthermore, certain T cell populations (γδ T cells) can reside in the intraepithelial spaces; these can provide epithelial growth factors and help regulate epithelial cell apoptosis [44]. Depletion of adaptive immune cells leads to more severe lung injury, for example, regulatory T cells promote tissue repair by promoting Th1 and Th17 cell responses [45]. The direct correlation of depleted T cell populations (and NK cells) to tissue repair seen in other organ systems (like the skin) has not yet been established [44][46]. Lymphocytes are also major producers of cytokines, including IL-22 (a major driver of epithelial cell proliferation and repair), IL-4/IL-13 (which may regulate the balance between epithelial cell healing and fibrosis), and amphiregulin (an EGF family member which has been linked to tissue repair and remodelling) [47][48][49]. While cytokine production and the contribution of adaptive immune cells to dysregulated healing is well characterised, the contributions of these cells in regular pulmonary epithelial healing are less clear. Generally, both innate and adaptive immune cells appear to have highly regulated and wide-ranging roles in regulating and responding to epithelial injury; further investigation into the signals that mediate these responses may reveal significant novel targets to promote repair.
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800.
- Kotton, D.N.; Morrisey, E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014, 20, 822–832, doi:10.1038/nm.3642.
- Spella, M.; Lilis, I.; Stathopoulos, G.T. Shared epithelial pathways to lung repair and disease. Eur. Respir. Rev. 2017, 26, 170048, doi:10.1183/16000617.0048-2017.
- Hermanns, M.I.; Unger, R.E.; Kehe, K.; Peters, K.; Kirkpatrick, C.J. Lung epithelial cell lines in coculture with human pulmo-nary microvascular endothelial cells: Development of an alveolo-capillary barrier in vitro. Lab. Investig. 2004, 84, 736–752, doi:10.1038/labinvest.3700081.
- Angelidis, I.; Simon, L.M.; Fernandez, I.E.; Strunz, M.; Mayr, C.H.; Greiffo, F.R.; Tsitsiridis, G.; Ansari, M.; Graf, E.; Strom, T.-M.; et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 2019, 10, 1–17, doi:10.1038/s41467-019-08831-9.
- Schiller, H.B.; Montoro, D.T.; Simon, L.M.; Rawlins, E.L.; Meyer, K.B.; Strunz, M.; Braga, F.A.V.; Timens, W.; Koppelman, G.H.; Budinger, G.R.S.; et al. The Human Lung Cell Atlas: A High-Resolution Reference Map of the Human Lung in Health and Disease. Am. J. Respir. Cell Mol. Biol. 2019, 61, 31–41, doi:10.1165/rcmb.2018-0416tr.
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nat. Cell Biol. 2020, 587, 619–625, doi:10.1038/s41586-020-2922-4.
- Evans, M.J.; Bils, R.F. Identification of Cells Labeled with Tritiated Thymidine in the Pulmonary Alveolar Walls of the Mouse1,2,3. Am. Rev. Respir. Dis. 1969, 100, 372–378, doi:10.1164/arrd.1969.100.3.372.
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L.M.; et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771.
- Liu, J.Y.; Nettesheim, P.; Randell, S.H. Growth and differentiation of tracheal epithelial progenitor cells. Am. J. Physiol. Cell. Mol. Physiol. 1994, 266, L296–L307, doi:10.1152/ajplung.1994.266.3.l296.
- Rawlins, E.L.; Okubo, T.; Xue, Y.; Brass, D.M.; Auten, R.L.; Hasegawa, H.; Wang, F.; Hogan, B.L. The Role of Scgb1a1+ Clara Cells in the Long-Term Maintenance and Repair of Lung Airway, but Not Alveolar, Epithelium. Cell Stem Cell 2009, 4, 525–534, doi:10.1016/j.stem.2009.04.002.
- Hong, K.U.; Reynolds, S.D.; Giangreco, A.; Hurley, C.M.; Stripp, B.R. Clara Cell Secretory Protein–Expressing Cells of the Airway Neuroepithelial Body Microenvironment Include a Label-Retaining Subset and Are Critical for Epithelial Renewal after Progenitor Cell Depletion. Am. J. Respir. Cell Mol. Biol. 2001, 24, 671–681, doi:10.1165/ajrcmb.24.6.4498.
- Tata, P.R.; Mou, H.; Pardo-Saganta, A.; Zhao, R.; Prabhu, M.; Law, B.M.; Vinarsky, V.; Cho, J.L.; Breton, S.; Sahay. A.; et al. Dedifferentiation of com-mitted epithelial cells into stem cells in vivo. Nature 2013, 503, 218–223.
- Adamson, I.Y.; Bowden, D.H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab. Investig. 1974, 30, 35–42.
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036, doi:10.1172/jci68782.
- Vaughan, A.E.; Brumwell, A.N.; Xi, Y.; Gotts, J.E.; Brownfield, D.G.; Treutlein, B.; Tan, K.; Tan, V.; Liu, F.C.; Looney, M.R.; et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nat. Cell Biol. 2015, 517, 621–625, doi:10.1038/nature14112.
- Zuo, W.; Zhang, T.; Wu, D.Z.; Guan, S.P.; Liew, A.-A.; Yamamoto, Y.; Wang, X.; Lim, S.J.; Vincent, M.; Lessard, M.; et al. p63+Krt5+ distal airway stem cells are essential for lung regeneration. Nat. Cell Biol. 2015, 517, 616–620, doi:10.1038/nature13903.
- Chapman, H.A.; Li, X.; Alexander, J.P.; Brumwell, A.; Lorizio, W.; Tan, K.; Sonnenberg, A.; Wei, Y.; Vu, T.H.; et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 2011, 121, 2855–2862.
- Kathiriya, J.J.; Brumwell, A.N.; Jackson, J.R.; Tang, X.; Chapman, H.A. Distinct Airway Epithelial Stem Cells Hide among Club Cells but Mobilize to Promote Alveolar Regeneration. Cell Stem Cell 2020, 26, 346–358.
- Lee, J.-H.; Bhang Dong, H.; Beede, A.; Huang Tian, L.; Stripp, B.R.; Bloch, K.D.; Wagers, A.J.; Tseng, Y.-H.; Ryeom, S.; Kim, C.F.; et al. Lung Stem Cell Differentiation in Mice Directed by Endothelial Cells via a BMP4-NFATc1-Thrombospondin-1 Axis. Cell 2014, 156, 440–455.
- Levy, B.D.; Vachier, I.; Serhan, C.N. Resolution of Inflammation in Asthma. Clin. Chest Med. 2012, 33, 559–570, doi:10.1016/j.ccm.2012.06.006.
- Sumagin, R.; Robin, A.Z.; Nusrat, A.; Parkos, C.A. Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to reg-ulate the epithelial barrier and neutrophil recruitment. Mucosal Immunol. 2014, 7, 905–915, doi:10.1038/mi.2013.106.
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb re-generation. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420.
- Petrie, T.A.; Strand, N.S.; Yang, C.T.; Rabinowitz, J.S.; Moon, R.T. Macrophages modulate adult zebrafish tail fin regenera-tion. Development 2014, 141, 2581–2591.
- Nagaoka, T.; Kaburagi, Y.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Steeber, D.A.; Tedder, T.F.; Sato, S. Delayed Wound Healing in the Absence of Intercellular Adhesion Molecule-1 or L-Selectin Expression. Am. J. Pathol. 2000, 157, 237–247, doi:10.1016/s0002-9440(10)64534-8.
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462, doi:10.1016/j.immuni.2016.02.015.
- Murray, P.J.; Allen, J.E.; Biswas Subhra, K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20.
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606, doi:10.1016/j.ajpath.2015.06.001.
- Shapiro, S.D.; Senior, R.M. Matrix metalloproteinases. Matrix degradation and more. Am. J. Respir. Cell Mol. Biol. 1999, 20, 1100–1102.
- Legrand, C.; Gilles, C.; Zahm, J.M.; Polette, M.; Buisson, A.C.; Kaplan, H.; Birembaut, P.; Tournier, J.M.; et al. Airway epithe-lial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J. Cell Biol. 1999, 146, 517–529.
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93, doi:10.1038/nri3600.
- Murray, P.J. The primary mechanism of the IL-10-regulated antiinflammatory response is to se-lectively inhibit transcription. Proc. Natl. Acad. Sci. USA 2005, 102, 8686–8691.
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11, doi:10.1016/j.semcdb.2016.08.006.
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419.
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential Roles of Macrophages in Diverse Phases of Skin Repair. J. Immunol. 2010, 184, 3964–3977, doi:10.4049/jimmunol.0903356.
- Gibbings, S.L.; Thomas, S.M.; Atif, S.M.; McCubbrey, A.L.; Desch, A.N.; Danhorn, T.; Leach, S.M.; Bratton, D.L.; Henson, P.M.; Janssen, W.J.; et al. Three Unique Interstitial Macrophages in the Murine Lung at Steady State. Am. J. Respir. Cell Mol. Biol. 2017, 57, 66–76.
- Abdolmaleki, F.; Farahani, N.; Hayat, S.M.G.; Pirro, M.; Bianconi, V.; Barreto, G.E.; Sahebkar, A. The Role of Efferocytosis in Autoimmune Diseases. Front. Immunol. 2018, 9, 1645, doi:10.3389/fimmu.2018.01645.
- Bosurgi, L.; Cao, Y.G.; Cabeza-Cabrerizo, M.; Tucci, A.; Hughes, L.D.; Kong, Y.; Weinstein, J.S.; Licona-Limon, P.; Schmid, E.T.; Pelorosso, F.; et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Sci-ence 2017, 356, 1072–1076, doi:10.1126/science.aai8132.
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103, doi:10.1097/aci.0000000000000133.
- Hams, E.; Armstrong, M.E.; Barlow, J.L.; Saunders, S.P.; Schwartz, C.; Cooke, G.; Fahy, R.J.; Crotty, T.B.; Hirani, N.; Flynn, R.J.; et al. IL-25 and type 2 in-nate lymphoid cells induce pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2014, 111, 367–372.
- Luzina, I.G.; Kopach, P.; Lockatell, V.; Kang, P.H.; Nagarsekar A.; Burke A.P.; Hasday, J.D.; Todd, N.W.; Atamas, S.P.; et al. Interleukin-33 poten-tiates bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2013, 49, 999–1008.
- Jung, M.Y.; Smrž, D.; Desai, A.; Bandara, G.; Ito, T.; Iwaki, S.; Kang, J.H.; Andrade, M.V.; Hilderbrand, S.C.; Brown, J.M.; et al. IL-33 induces a hyporesponsive phe-notype in human and mouse mast cells. J. Immunol. 2013, 190, 531–538.
- Jovanovic, K.; Siebeck, M.; Gropp, R. The route to pathologies in chronic inflammatory diseases characterized by T helper type 2 immune cells. Clin. Exp. Immunol. 2014, 178, 201–211, doi:10.1111/cei.12409.
- Havran, W.L.; Jameson, J.M.; Witherden, D.A. Epithelial Cells and Their Neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G627–G630, doi:10.1152/ajpgi.00224.2005.
- Tan, W.; Zhang, C.; Liu, J.; Miao, Q. Regulatory T‐cells promote pulmonary repair by modulating T helper cell immune re-sponses in lipopolysaccharide‐induced acute respiratory distress syndrome. Immunology 2019, 157, 151–162, doi:10.1111/imm.13060.
- Liu, Q.; Smith, C.W.; Zhang, W.; Burns, A.R.; Li, Z. NK Cells Modulate the Inflammatory Response to Corneal Epithelial Abrasion and Thereby Support Wound Healing. Am. J. Pathol. 2012, 181, 452–462, doi:10.1016/j.ajpath.2012.04.010.
- McAleer, J.P.; Kolls, J.K. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol. Rev. 2014, 260, 129–144, doi:10.1111/imr.12183.
- Wirsdörfer, F.; Jendrossek, V. The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung. Front. Immunol. 2016, 7, doi:10.3389/fimmu.2016.00591.
- Monticelli, L.A.; Sonnenberg, G.F.; Artis, D. Innate lymphoid cells: Critical regulators of allergic in-flammation and tissue repair in the lung. Curr. Opin. Immunol. 2012, 24, 284–289.