Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jehan El-Jawhari | + 3191 word(s) | 3191 | 2021-05-26 05:58:23 | | | |
| 2 | Dean Liu | -10 word(s) | 3181 | 2021-08-04 10:39:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
El-Jawhari, J. Bone Marrow. Encyclopedia. Available online: https://encyclopedia.pub/entry/12751 (accessed on 08 February 2026).
El-Jawhari J. Bone Marrow. Encyclopedia. Available at: https://encyclopedia.pub/entry/12751. Accessed February 08, 2026.
El-Jawhari, Jehan. "Bone Marrow" Encyclopedia, https://encyclopedia.pub/entry/12751 (accessed February 08, 2026).
El-Jawhari, J. (2021, August 04). Bone Marrow. In Encyclopedia. https://encyclopedia.pub/entry/12751
El-Jawhari, Jehan. "Bone Marrow." Encyclopedia. Web. 04 August, 2021.
Copy Citation
Bone marrow (BM) is a reliable source of multipotent mesenchymal stromal cells (MSCs), which have been successfully used for treating osteonecrosis.
multipotent mesenchymal stromal cells
osteonecrosis
autologous bone marrow
regenerative therapy
1. Introduction
Bone marrow-multipotent mesenchymal stromal cells (BM-MSCs), the key bone regenerating cells, were first identified as attached to plastic culture surfaces and characterized by a self-renewal capacity to form a colony and the ability to differentiate into bone, cartilage and fat cells [1]. With accumulating scientific knowledge on the MSC roles in tissue regeneration, their other functions, including immunoregulation, proliferation, angiogenesis support and pro-survival abilities, became important to consider for application in regenerative therapies [2][3][4]. The best-characterized and most frequently used source of MSCs is BM, even with the very low frequency of these progenitor cells, i.e., 0.01–0.001% of nucleated BM cells [1][5].
Osteonecrosis (ON), also named avascular necrosis, is a multifactorial and painful bone disease characterized by vascular insufficiency, progressive collapse of subchondral bone and cartilage damage. This disease often develops in the femoral head, but it could also affect other joints, mostly in young or middle-aged individuals. One main mechanism of ON pathogenesis is vascular damage due to clotting, embolism, increased intraosseous pressure and direct blood vessel injury [6]. ON can also be caused by direct trauma or infections. The most common non-traumatic conditions that can trigger ON are corticosteroids, alcohol abuse, Gaucher’s disease, sickle cell disease, Systemic Lupus Erythematosus, diabetes, chronic renal diseases and chemotherapy (Figure 1) [6]. Over the last three decades, autologous MSCs have attracted considerable interest as cellular regenerative therapies for ON [7]. For enhancing therapeutic outcomes, it is also important to consider the effects of patients’ age as ON is common among young and old populations but can occur in elderly individuals according to the underlying conditions [7][8][9]. Here, we review how these predisposing causes of ON and age can affect the biological characteristics of MSCs.
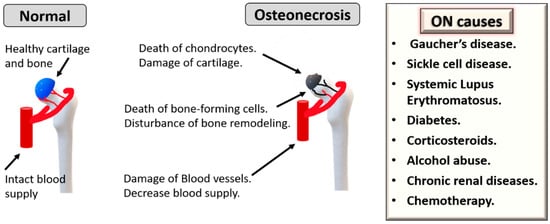
Figure 1. Underlying causes and pathogenesis of osteonecrosis (ON). Chronic diseases can cause ON, such as sickle cell disease, Gaucher’s disease, Systemic Lupus Erythematosus, diabetes, chronic renal disease and chemotherapy. Additionally, corticosteroids and alcohol abuse are other triggers.
2. MSC Therapy for Osteonecrosis
2.1. Pre-Clinical Studies
Extensive experimental research (in vitro and in vivo studies) has confirmed the unique and multifaceted regenerative capacities of MSCs. Because of their unique biological properties, MSCs represent a promising therapeutic tool for ON (Figure 2). Importantly, MSCs provide angiogenic support function mostly due to the production of Vascular endothelial growth factor (VEGF) and control osteoclastogenesis via the release of soluble factors such as RANKL and OPG [10][11]. These cells also have essential differentiation capacities needed to repair damaged bone and cartilage tissues in ON [12]. More recently, their high proliferation and ability of suppression of immune response associated with tissue damage via the production of immunosuppressive factors, indoleamine 2,3 dioxygenase (IDO), Transforming growth factor-beta (TGF-β), prostaglandin E2 (PGE-2) and IL-10 have been considered equally important [13][14][15]. A controlled inflammatory reaction is required for the normal bone healing process and is deemed necessary for timely fracture repair and tissue regeneration in ON [14].
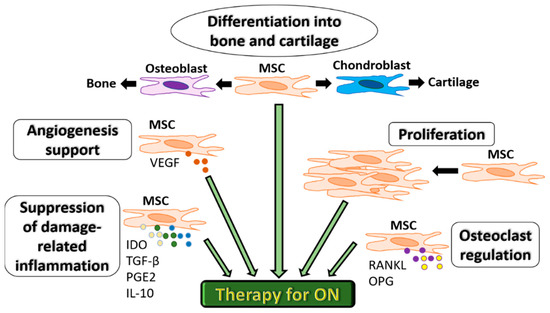
Figure 2. Functional characteristics of MSCs for therapeutic use in ON. MSCs can differentiate into bone or cartilage, support angiogenesis via VEGF secretion, proliferate to compensate for bone cell death, regulate osteoclastogenesis via RANKL and OPG and suppress inflammation associated with tissue damage via the production of immunosuppressive factors IDO, TGF-β, PGE-2 and IL-10.MSC: mesenchymal stromal cells. IDO: indoleamine 2,3 dioxygenase.
An important factor in the therapy of ON is the use of 3D scaffolds that are used as bone fillers and additionally support the repair functions of MSCs [16]. These scaffolds also overcome the disadvantages of autografts and allografts, such as limited accessibility and side effects of immune rejection and infection [16]. The needed criteria for scaffolds used in regenerative therapies include biocompatibility, biodegradability, porosity, and mechanical support to avoid bone collapse during the repair process. Several natural and synthetic materials are used to fabricate scaffolds [16][17]. Polymers are commonly used in ON treatment include as poly lactide-co-glycolide (PLGA), poly ε-caprolactone (PCL), Cervi Cornus Colla (CCC), polyethylene glycol maleate citrate (PEGMC), polylactide (PLA), polymethyl methacrylate (PMMA), and peptide-based hydrogels, but they lack mechanical stability [16]. Other materials include natural polymers, such as hyaluronic acid and collagen reinforced with demineralized bone matrix (DBM), and inorganic components of bone, e.g., calcium phosphate (CP), β-tricalcium calcium phosphate (TCP) and hydroxyapatite (HA) [16]. Using porous HA or TCP scaffolds together with BM MSCs enhanced bone regeneration in osteonecrosis, as seen in experimental models [18]. Popular materials for synthetic scaffolds also include ceramics, bio-glass and porous titanium (Ti) [16].
In a composite approach, bioactive cells and growth factors are included to ‘functionalize’ the scaffolds and enhance the osteoinductivity. A combination of scaffolds and MSCs is a popular method to repair necrotic areas, as the scaffolds help direct MSCs to the necrotic areas and deliver mechanical support for these cells. Ceramic scaffolds loaded with BM-MSCs promoted the healing of bone defect in a canine model of femoral head ON [19]. Porous tantalum scaffolds were used to load MSCs following surface cover with Bio-Gide® collagen membrane [20]. This hybrid therapy has helped induce bone and cartilage repair of femoral head necrosis in a rabbit model [20]. The seeding collagen scaffold with hypoxia-pretreated BM MSCs induced bone regeneration and angiogenesis in rabbit femoral head ON as hypoxia increase viability and osteogenic capacity of MSCs [21]. In addition to MSCs, more complex constructs can be potentially used as scaffolds loaded with both adipose-derived MSCs and endothelial cells, shown as a potential therapy for ON in a rat model [22].
Another successful approach for ON therapy is the use of scaffolds functionalized by growth factors to ensure the sustained release of these factors, which would enhance MSC regenerative functions and blood vessel formation. This combination also solves the problems of local application of growth factors, such as their short half-life in vivo or heterogenous ossification related to the applications of BMPs [23]. Encapsulation of growth factors, such as BMP-2 or VEGF [24][25] in the PLGA microsphere, showed great results in experimental models of bone necrosis. Combined platelet-rich plasma (PRP) with scaffold has been shown to have the capacity to heal defects in a rabbit model of ON [26]. Another potential tool includes platelet-derived growth factors that can be used in different formats. Allogeneic platelet lysate or PRP prepared from young, healthy donors has a great potential enhancing chondrogenic and osteogenic potential of MSCs. This combination therapy is popularly considered for traumatic bone damage and various degenerative joint diseases [27][28]. In a case study, a combined local injection of adipose tissue-MSCs with platelet-rich plasma showed satisfactory outcomes in an experimental model of femoral head ON [29]. Another approach for enhancing MSC-based therapy is the use of genetically modified MSCs. The bFGF- gene overexpressed lentivirus-transfected BM MSCs combined with cancellous bone is one example that was tested in a rabbit model of ON with bone healing success [30]. Furthermore, synthesized adenovirus-mediated BMP-2 and bFGF modified BMSCs combined with DBM were effective in the repair of a canine femoral head defect [31].
2.2. Clinical Studies
Initial studies on using autologous MSCs for the treatment of ON were based on cultured MSCs from bone marrow (BM) aspirates and have demonstrated excellent patient safety and efficacy profiles as proved by pain relief and radiological signs of healing [32][33]. A phase I/II study in sickle cell disease patients who had ON showed a high success rate when using autologous MSCs up to 60 months of follow-up [34]. Similarly, Gangii et al. showed that autologous BM-MSCs were effective to treat the early stage of ON for the 60-month follow up [35]. In common, the outcome of MSC-based therapy shows better outcomes in the early stages of ON and traumatic rather than non-traumatic patients [36][37].
Using uncultured autologous MSCs are particularly preferred for ease of extraction, safety and minimal need for handling, processing, and ethics [7]. Pioneering work by Hernigou et al. of using BM autologous MSCs to treat patients with femoral head ON in combination with core decompression has indicated the potential therapeutic value of these cells, particularly in a dose-dependent fashion [38]. The same group showed that the numbers of BM MSCs are reduced in ON and suggested better techniques to increase the yield of harvested progenitor cells, such as optimizing the BM aspiration method and using BM concentrates [39]. While the benefits of autologous BM MSCs in ON seem evident, more focus on assessing the numbers and, particularly, the biological fitness of therapeutic autologous MSCs is needed to develop these therapies further.
Core decompression is a classical surgical treatment for ON but usually effective for small necrotic lesions. The combination of core decompression with autologous BM was associated with improved clinical scores and radiological signs [40]. Some clinical studies have included other supporting factors for MSCs. Injection of concentrated BM and platelet-rich plasma was used for 77 ON patients and showed clinical improvement in 86% of the tested group [41]. In another study, a combination of core decompression, autologous BM-MSCs and calcium sulphate/calcium ceramic scaffold showed satisfactory outcomes. However, that study lacked controls of single therapeutic tools [42]. Other functional scaffolds were tested, and Kuroda et al. showed that using slow-release scaffolds to deliver human FGF was an advantage to promote regeneration of bone necrosis in femoral ON patients [43].
The BM is a common source for MSCs used for ON, and the clinical delivery of BM MSCs as mononuclear cells was found to be better than using whole BM, likely because of concentrated cells [44]. Adipose stromal cells were also applied into hip joints and resulted in positive clinical outcomes [45]. In another study, a combination of umbilical cord MSCs and BM mononuclear cells improved ON patient symptoms [46]. Furthermore, the delivery of MSCs is commonly directed into the area of ON, but the systemic infusion could be another route of delivery. Co-infusion of BM cells and umbilical cord MSCs through femoral artery resulted in clinical improvement as conducted in Phase I/II clinical study on 30 femoral head patients [46]. Additionally, Mao et al. found that intra-arterial MSCs was effective in treating early-stage ON [47]. However, additional research is still required to determine whether the local delivery or the systemic infusion of MSCs is more effective in ON therapy.
In summary, the great potential of autologous MSCs alone or in combination with scaffolds and growth factors for tissue repair in ON is evident [6][48]. However, different ON-underlying factors could hamper the regenerative potential of autologous MSCs. As there are multiple causes for the development of ON, it is essential to assess the patient’s suitability for autologous MSC use carefully and consider how the underlying cause of ON might affect the biological fitness of these regenerative cells. This knowledge will help to optimize autologous MSC-based regenerative therapies for ON. Furthermore, the effect of a patient’s age should be taken into account based on the recent advances in this area of research.
3. Age-Related Changes in MSCs
ON is common in the young/middle age group [7][49]. While chronological aging is related to the numbers of years, the process of biological aging can be promoted by environment, diet, disease, heredity and lifestyle [50]. At the cellular level, aging is a complex process caused by an accumulation of various types of damages in cells over time [51][52]. The free radical theory refers to the damage caused by intermediate oxygen molecules resulting from cellular metabolism. In older age, oxidative stress outgrows the level of anti-oxidant enzymes resulting in increasing levels of reactive oxygen species (ROS) [53]. Another theory suggests that replication errors and extrinsic factors (such as radiation and ROS) progressively cause damage to the DNA, ultimately leading to cell senescence or death [50][54][55]. These general theories apply to MSC ageing characterized by a decline in their number [56], increased ROS leading to a shift towards adipogenic differentiation [57], a decline in telomere length [58], as well as increased DNA damage [59].
3.1. Pre-Clinical Studies
Previous in vitro studies have used the long-term passaged MSCs in vitro as an indication of ageing. These studies showed that several changes are associated with in vitro ageing of MSCs [60]. These changes include reduced survival and proliferation, increased senescence and ROS expression levels, decreased differentiation potential, and increased genetic instability [60]. However, recent studies on MSC healthy aging in vivo (as discussed below) could change our understanding of aged MSCs and their use in therapy for ON.
3.2. Clinical/Human Studies
The CFU-F assay has been the oldest method for enumerating BM-MSCs by counting the numbers of single cell-derived colonies based on first the MSC ability of plastic-adherence and second their ability to proliferate forming a colony. Over the years, several groups have performed CFU-F experiments to examine the age-related changes in the number of aspirated iliac crest BM-MSCs (Table 1). The variable results obtained can be due to different factors. For example, different volumes of BM aspirates have been used (Table 1), but larger-volume aspirations could lead to the dilution of BM aspirates with blood [61] and subsequently produce artificially low MSC frequencies, as MSCs are not present in the peripheral blood [62][63]. Further processing of the BM aspirate for MSC isolation as well as using different media and different scoring criteria, could also impact the CFU-F frequency [64][65][66] (Table 1).
Table 1. List of previous studies investigating age-related changes in the numbers of BM MSCs counted by the CFU-F assay.
| Age (Years) | Volume (mL) | Isolation | Media | Colony Definition | CFU-F | Ref |
|---|---|---|---|---|---|---|
| Y: 22–44, O: 66–74 | 10 mL | DC | α-MEM + 10%FCS | >16 cells | No change | [67] |
| 13–79, no groups | 4 × 2 mL pooled | DC | α-MEM + 10%FCS + ASC + Dex | >8 cells | No change | [68] |
| Y: 0–18, O: 59–75 | NR | DC | DMEM + 10%FCS | NR | Decline | [58] |
| Y: 19–40, O: > 40 | NR | DC | DMEM + 10%FCS | >50 cells | Decline | [69] |
| Y: 6–16, O: 29–76 | NR | PA | DMEM + 20%FCS | NR | NS decline | [70] |
| 1–52, no groups | NR | DC | DMEM + 20%FCS | >50 cells | No change | [71] |
| 22–80, no groups | 8 mL | PA | StemMacs medium | >50 cells | Decline in women | [72] |
| Y: 20–40, I: 41–60, O: >60 | 10 mL | PA | StemMacs medium | >50 cells | Decline | [73] |
| 14–59, no groups | 30 mL, 3 × 10 mL | PA | DMEM/Ham’s F12 + 10% FCS + bFGF + heparin | >50 cells | Decline | [74] |
| Y: <45, I: 45–65, O: >65 | NR | DC | α-MEM + 10% human serum | >50 cells | Decline | [56] |
BM: Bone marrow, I: intermediate, O: Old, Y: Young, PA: Plastic adhesion, DC: Density centrifugation α-MEM: Alpha–Minimum essential medium, DMEM: Dulbecco’s minimum essential medium, FCS: Fetal calf serum, ASC: sodium ascorbate, Dex: dexamethasone, bFGF: basic fibroblast growth factor, NR: Not reported, NS: Non-significant.
Irrespective of these compounding factors, a general trend for a decline in BM MSC numbers has been confirmed in several recent studies. Most recent data from our laboratory (n = 67 donors, 33 females and 34 males) indicated a significant age-related decline in the number of colonies in relation to donor age in both males and females [73]. Interestingly, while the overall median CFU-F frequency was the lowest in the old donor group (61–89 years old), the decline was the steepest between young donors (19–40 years old) and the donors of the intermediate age group (41−60 years old) [73]. Similarly, another study has found a decline with age, but no groups were analyzed [74]. This was also noted in the study by Siegel et al., which implied that the decline in MSC numbers begins in the fifth decade of life [56].
Enumeration of uncultured BM-MSCs has also been attempted by flow cytometry using several surface markers [75][76][77]. CD271 (low-affinity nerve growth factor receptor) in combination with CD45 (pan-hematopoietic lineage cell marker) has been recently reported to provide the best gating strategy for BM-MSCs [77][78][79]. In two of our recent studies, BM-MSCs were quantified volumetrically using the CD45lowCD271+ phenotype [72][73], and in both studies, we observed trends for age-related decline in the numbers of MSCs [72][73]. However, a steeper decline was observed in the intermediate age group (41–60 years old) as compared to the young donor group (19–40 years old), rather than in the donors of the old age group (61–89 years old) compared to the intermediate age group, in agreement with CFU-F findings [73]. Overall, these studies indicate that autologous MSCs in relatively young ON patients are likely to be higher in number compared to those from the middle-age group.
Using uncultured MSCs, our group has recently shown no significant age-related increase in adipogenic or osteogenic differentiation transcripts or ROS levels in uncultured MSCs from old donors compared to young donors [73][80]. This contrasts with the higher ROS levels found in minimally cultured MSCs from older donors [81]. Very recently, another group evaluated the number and functionalities of MSCs from young and old donors and found no age-related differences in growth kinetics, tri-lineage potential, gene expression profiles and immunosuppressive properties [82]. When the same biological characteristics were compared between early and late passage MSCs, they displayed significant differences. Although this indicted little implication of donor age on MSC immunomodulatory functions, more research would be needed to assess this function in other ON-underlying diseases. This research will be particularly important for therapeutic MSCs as defective immunosuppression has been recently reported with a link to complicated bone healing [83].
The age-related changes of the extra cellular matrix (ECM) could affect the functions of MSCs and their use for ON therapy [84]. The ECM is formed of proteoglycans, fibrous proteins (mainly collagens), minerals and water. In joints, proteoglycans absorb water and act primarily to resist load compression and create flexibility. In contrast, collagens enable cartilage to resist sheer stress. With aging, changes in these ECM components can lead to osteochondral tissue damages. As the age increases, the molecular weight of proteoglycans is decreased, and the proteolytic enzyme activity is increased [85][86], leading to reduced proteoglycan aggregates and higher levels of serum aggrecan fragments [87]. Similarly, thickness and cross-linking of collagen type II fibers are detected with aging [85]. Furthermore, an age-mediated increase in the expression and activity of collagenase activity by chondrocytes could lead to articular surface fibrillation and erosion [88]. Such disturbance in ECM homeostasis could affect the reparatory performance of intrinsic or implanted therapeutic MSCs as ECM is essential for cell survival, proliferation and communication. Collagen type II enhances chondrogenesis and osteogenesis of MSCs by facilitating osteogenic marker RUNX2 stimulation via the integrin α2β1-FAK-JNK signaling pathway [89]. Another component of ECM proteoglycans, Syndecan-1, is involved in osteo-adipogenic balance during the early induction of MSC lineage differentiation [90]. In addition to ECM, an inflammatory microenvironment such as that seen in osteoarthritis can also negatively affect MSC function by suppressing chondrogenic and osteogenic differentiation [91]. Consequently, it is important to consider the joint microenvironment to optimize MSC-based therapy for ON, particularly in aged patients. Suggestions of using young ECM to rejuvenate therapeutic MSCs also necessitates further investigations [92][93].
Summarizing the current literature in relation to age-related changes in BM-MSCs, it becomes apparent that young individuals are likely to have several-fold more MSCs in aspirated BM compared to donors over 40 years old. This can have implications on the dose of MSCs delivered into the damaged femoral head in ON patients. However, middle-aged and older donors show significant variability in relation to their MSC numbers, possibly because their skeletal biological ages are not the same as their chronological ages and may have been affected by environmental causes listed at the beginning of this section. With regards to MSC functions, such as differentiation, anti-oxidative and bone-remodeling capacities, age-related declines reported using cultured MSCs may represent an artefact of culture-expanded in vitro, and different types of assays need to be developed for the assessment of these functions in uncultured MSCs. In relation to ON treatment with autologous MSCs, these findings indicate that MSC dose-determination based on rapid laboratory tests [72], rather than ‘predicted’ from patients age, is needed for standardization of these therapies.
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147.
- Li, H.; Shen, S.; Fu, H.; Wang, Z.; Li, X.; Sui, X.; Yuan, M.; Liu, S.; Wang, G.; Guo, Q. Immunomodulatory Functions of Mesenchymal Stem Cells in Tissue Engineering. Stem Cells Int. 2019, 20199671206.
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. Am. 2005, 87, 1430–1437.
- Katagiri, W.; Kawai, T.; Osugi, M.; Sugimura-Wakayama, Y.; Sakaguchi, K.; Kojima, T.; Kobayashi, T. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 8.
- Jones, E.A.; Kinsey, S.E.; English, A.; Jones, R.A.; Straszynski, L.; Meredith, D.M.; Markham, A.F.; Jack, A.; Emery, P.; McGonagle, D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002, 46, 3349–3360.
- Elgaz, S.; Bonig, H.; Bader, P. Mesenchymal stromal cells for osteonecrosis. J. Transl. Med. 2020, 18, 399.
- Hernigou, P.; Trousselier, M.; Roubineau, F.; Bouthors, C.; Chevallier, N.; Rouard, H.; Flouzat-Lachaniette, C.H. Stem Cell Therapy for the Treatment of Hip Osteonecrosis: A 30-Year Review of Progress. Clin. Orthop. Surg. 2016, 8, 1–8.
- Shigemura, T.; Nakamura, J.; Kishida, S.; Harada, Y.; Ohtori, S.; Kamikawa, K.; Ochiai, N.; Takahashi, K. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: Prospective MRI study. Rheumatology 2011, 50, 2023–2028.
- Dima, A.; Pedersen, A.B.; Pedersen, L.; Baicus, C.; Thomsen, R.W. Association of common comorbidities with osteonecrosis: A nationwide population-based case-control study in Denmark. BMJ Open 2018, 8, e020680.
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 9130–9138.
- Abe, T.; Sumi, K.; Kunimatsu, R.; Oki, N.; Tsuka, Y.; Nakajima, K.; Ando, K.; Tanimoto, K. The effect of mesenchymal stem cells on osteoclast precursor cell differentiation. J. Oral. Sci. 2019, 61, 30–35.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- El-Jawhari, J.J.; Jones, E.; Giannoudis, P.V. The roles of immune cells in bone healing; what we know, do not know and future perspectives. Injury 2016, 47, 2399–2406.
- Goodman, S.B.; Maruyama, M. Inflammation, Bone Healing and Osteonecrosis: From Bedside to Bench. J. Inflamm. Res. 2020, 13913–13923.
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 101191.
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact. Mater. 2020, 5, 584–601.
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 8416–8433.
- Maruyama, M.; Nabeshima, A.; Pan, C.C.; Behn, A.W.; Thio, T.; Lin, T.; Pajarinen, J.; Kawai, T.; Takagi, M.; Goodman, S.B.; et al. The effects of a functionally-graded scaffold and bone marrow-derived mononuclear cells on steroid-induced femoral head osteonecrosis. Biomaterials 2018, 18739–18746.
- Peng, J.; Wen, C.; Wang, A.; Wang, Y.; Xu, W.; Zhao, B.; Zhang, L.; Lu, S.; Qin, L.; Guo, Q.; et al. Micro-CT-based bone ceramic scaffolding and its performance after seeding with mesenchymal stem cells for repair of load-bearing bone defect in canine femoral head. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 316–325.
- Liu, B.; Yang, F.; Wei, X.; Zhang, X.; Zhang, Y.; Wang, B.; Liu, G.; Xie, H.; Yang, J.; Wang, W.; et al. An exploratory study of articular cartilage and subchondral bone reconstruction with bone marrow mesenchymal stem cells combined with porous tantalum/Bio-Gide collagen membrane in osteonecrosis of the femoral head. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1123–1132.
- Fan, L.; Zhang, C.; Yu, Z.; Shi, Z.; Dang, X.; Wang, K. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and osteogenesis in rabbit femoral head osteonecrosis. Bone 2015, 81544–81553.
- Ismail, T.; Osinga, R.; Todorov, A.; Haumer, A., Jr.; Tchang, L.A.; Epple, C.; Allafi, N.; Menzi, N.; Largo, R.D.; Kaempfen, A.; et al. Engineered, axially-vascularized osteogenic grafts from human adipose-derived cells to treat avascular necrosis of bone in a rat model. Acta Biomater. 2017, 63236–63245.
- Phipps, M.C.; Monte, F.; Mehta, M.; Kim, H.K. Intraosseous Delivery of Bone Morphogenic Protein-2 Using a Self-Assembling Peptide Hydrogel. Biomacromolecules 2016, 17, 2329–2336.
- Wang, C.K.; Ho, M.L.; Wang, G.J.; Chang, J.K.; Chen, C.H.; Fu, Y.C.; Fu, H.H. Controlled-release of rhBMP-2 carriers in the regeneration of osteonecrotic bone. Biomaterials 2009, 30, 4178–4186.
- Zhang, H.X.; Zhang, X.P.; Xiao, G.Y.; Hou, Y.; Cheng, L.; Si, M.; Wang, S.S.; Li, Y.H.; Nie, L. In vitro and in vivo evaluation of calcium phosphate composite scaffolds containing BMP-VEGF loaded PLGA microspheres for the treatment of avascular necrosis of the femoral head. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 60298–60307.
- Zhang, X.L.; Wang, Y.M.; Chu, K.; Wang, Z.H.; Liu, Y.H.; Jiang, L.H.; Chen, X.; Zhou, Z.Y.; Yin, G. The application of PRP combined with TCP in repairing avascular necrosis of the femoral head after femoral neck fracture in rabbit. Eur. Rev. Med. Pharm. Sci. 2018, 22, 903–909.
- Altaie, A.; Baboolal, T.G.; Wall, O.; Jones, E.; McGonagle, D. Platelet lysate enhances synovial fluid multipotential stromal cells functions: Implications for therapeutic use. Cytotherapy 2018, 20, 375–384.
- Vun, J.; Panteli, M.; Jones, E.; Giannoudis, P.V. The in vitro effects of platelet products on the biophysiological functions of human bone marrow mesenchymal stromal cells: A systematic review. Eur. Cell. Mater. 2021, 41269–41315.
- Pak, J.; Lee, J.H.; Jeon, J.H.; Lee, S.H. Complete resolution of avascular necrosis of the human femoral head treated with adipose tissue-derived stem cells and platelet-rich plasma. J. Int. Med. Res. 2014, 42, 1353–1362.
- Zhang, F.; Peng, W.X.; Wang, L.; Zhang, J.; Dong, W.T.; Wu, J.H.; Zhang, H.; Wang, J.B.; Zhao, Y. Role of FGF-2 Transfected Bone Marrow Mesenchymal Stem Cells in Engineered Bone Tissue for Repair of Avascular Necrosis of Femoral Head in Rabbits. Cell. Physiol. Biochem. 2018, 48, 773–784.
- Peng, W.X.; Wang, L. Adenovirus-Mediated Expression of BMP-2 and BFGF in Bone Marrow Mesenchymal Stem Cells Combined with Demineralized Bone Matrix For Repair of Femoral Head Osteonecrosis in Beagle Dogs. Cell. Physiol. Biochem. 2017, 43, 1648–1662.
- Muller, I.; Vaegler, M.; Holzwarth, C.; Tzaribatchev, N.; Pfister, S.M.; Schutt, B.; Reize, P.; Greil, J.; Handgretinger, R.; Rudert, M. Secretion of angiogenic proteins by human multipotent mesenchymal stromal cells and their clinical potential in the treatment of avascular osteonecrosis. Leukemia 2008, 22, 2054–2061.
- Zhao, D.; Cui, D.; Wang, B.; Tian, F.; Guo, L.; Yang, L.; Liu, B.; Yu, X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012, 50, 325–330.
- Daltro, G.C.; Fortuna, V.; de Souza, E.S.; Salles, M.M.; Carreira, A.C.; Meyer, R.; Freire, S.M.; Borojevic, R. Efficacy of autologous stem cell-based therapy for osteonecrosis of the femoral head in sickle cell disease: A five-year follow-up study. Stem Cell Res. Ther. 2015, 6110.
- Gangji, V.; De Maertelaer, V.; Hauzeur, J.P. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone 2011, 49, 1005–1009.
- Hauzeur, J.P.; De Maertelaer, V.; Baudoux, E.; Malaise, M.; Beguin, Y.; Gangji, V. Inefficacy of autologous bone marrow concentrate in stage three osteonecrosis: A randomized controlled double-blind trial. Int. Orthop. 2018, 42, 1429–1435.
- Sen, R.K.; Tripathy, S.K.; Aggarwal, S.; Marwaha, N.; Sharma, R.R.; Khandelwal, N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: A randomized control study. J. Arthroplast. 2012, 27, 679–686.
- Hernigou, P.; Beaujean, F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin. Orthop. Relat. Res. 2002, 405, 14–23.
- Hernigou, P.; Poignard, A.; Manicom, O.; Mathieu, G.; Rouard, H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J. Bone Jt. Surg. Br. 2005, 87, 896–902.
- Tabatabaee, R.M.; Saberi, S.; Parvizi, J.; Mortazavi, S.M.; Farzan, M. Combining Concentrated Autologous Bone Marrow Stem Cells Injection With Core Decompression Improves Outcome for Patients with Early-Stage Osteonecrosis of the Femoral Head: A Comparative Study. J. Arthroplast. 2015, 30 (Suppl. 9), 11–15.
- Martin, J.R.; Houdek, M.T.; Sierra, R.J. Use of concentrated bone marrow aspirate and platelet rich plasma during minimally invasive decompression of the femoral head in the treatment of osteonecrosis. Croat. Med. J. 2013, 54, 219–224.
- Civinini, R.; De Biase, P.; Carulli, C.; Matassi, F.; Nistri, L.; Capanna, R.; Innocenti, M. The use of an injectable calcium sulphate/calcium phosphate bioceramic in the treatment of osteonecrosis of the femoral head. Int. Orthop. 2012, 36, 1583–1588.
- Kuroda, Y.; Asada, R.; So, K.; Yonezawa, A.; Nankaku, M.; Mukai, K.; Ito-Ihara, T.; Tada, H.; Yamamoto, M.; Murayama, T.; et al. A pilot study of regenerative therapy using controlled release of recombinant human fibroblast growth factor for patients with pre-collapse osteonecrosis of the femoral head. Int. Orthop. 2016, 40, 1747–1754.
- Rastogi, S.; Sankineani, S.R.; Nag, H.L.; Mohanty, S.; Shivanand, G.; Marimuthu, K.; Kumar, R.; Rijal, L. Intralesional autologous mesenchymal stem cells in management of osteonecrosis of femur: A preliminary study. Musculoskelet. Surg. 2013, 97, 223–228.
- Pak, J. Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain Physician 2012, 15, 75–85.
- Cai, J.; Wu, Z.; Huang, L.; Chen, J.; Wu, C.; Wang, S.; Deng, Z.; Wu, W.; Luo, F.; Tan, J. Cotransplantation of bone marrow mononuclear cells and umbilical cord mesenchymal stem cells in avascular necrosis of the femoral head. Transpl. Proc. 2014, 46, 151–155.
- Mao, Q.; Wang, W.; Xu, T.; Zhang, S.; Xiao, L.; Chen, D.; Jin, H.; Tong, P. Combination treatment of biomechanical support and targeted intra-arterial infusion of peripheral blood stem cells mobilized by granulocyte-colony stimulating factor for the osteonecrosis of the femoral head: A randomized controlled clinical trial. J. Bone Min. Res. 2015, 30, 647–656.
- Gomez-Barrena, E.; Padilla-Eguiluz, N.G.; Rosset, P.; Hernigou, P.; Baldini, N.; Ciapetti, G.; Gonzalo-Daganzo, R.M.; Avendano-Sola, C.; Rouard, H.; Giordano, R.; et al. Osteonecrosis of the Femoral Head Safely Healed with Autologous, Expanded, Bone Marrow-Derived Mesenchymal Stromal Cells in a Multicentric Trial with Minimum 5 Years Follow-Up. J. Clin. Med. 2021, 10, 508.
- Hernigou, P.; Poignard, A.; Zilber, S.; Rouard, H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J. Orthop. 2009, 43, 40–45.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Ganguly, P.; El-Jawhari, J.J.; Giannoudis, P.V.; Burska, A.N.; Ponchel, F.; Jones, E.A. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transpl. 2017, 26, 1520–1529.
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880.
- Li, T.S.; Marban, E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 2010, 28, 1178–1185.
- Jin, K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74, PMC2995895.
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485.
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schafer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11146.
- Kanda, Y.; Hinata, T.; Kang, S.W.; Watanabe, Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011, 89, 250–258.
- Baxter, M.A.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004, 22, 675–682.
- Wagner, W.; Ho, A.D.; Zenke, M. Different facets of aging in human mesenchymal stem cells. Tissue Eng. Part. B Rev. 2010, 16, 445–453.
- Yang, Y.K. Aging of mesenchymal stem cells: Implication in regenerative medicine. Regen. Ther. 2018, 9120–9122.
- Cuthbert, R.; Boxall, S.A.; Tan, H.B.; Giannoudis, P.V.; McGonagle, D.; Jones, E. Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy 2012, 14, 431–440.
- Kuznetsov, S.A.; Mankani, M.H.; Gronthos, S.; Satomura, K.; Bianco, P.; Robey, P.G. Circulating skeletal stem cells. J. Cell Biol. 2001, 153, 1133–1140.
- Kuznetsov, S.A.; Mankani, M.H.; Leet, A.I.; Ziran, N.; Gronthos, S.; Robey, P.G. Circulating connective tissue precursors: Extreme rarity in humans and chondrogenic potential in guinea pigs. Stem Cells 2007, 25, 1830–1839.
- Fragkakis, E.M.; El-Jawhari, J.J.; Dunsmuir, R.A.; Millner, P.A.; Rao, A.S.; Henshaw, K.T.; Pountos, I.; Jones, E.; Giannoudis, P.V. Vertebral body versus iliac crest bone marrow as a source of multipotential stromal cells: Comparison of processing techniques, tri-lineage differentiation and application on a scaffold for spine fusion. PLoS ONE 2018, 13, e0197969.
- Dal Pozzo, S.; Urbani, S.; Mazzanti, B.; Luciani, P.; Deledda, C.; Lombardini, L.; Benvenuti, S.; Peri, A.; Bosi, A.; Saccardi, R. High recovery of mesenchymal progenitor cells with non-density gradient separation of human bone marrow. Cytotherapy 2010, 12, 579–586.
- Horn, P.; Bork, S.; Wagner, W. Standardized isolation of human mesenchymal stromal cells with red blood cell lysis. Methods Mol. Biol. 2011, 69823–69835.
- Stenderup, K.; Justesen, J.; Eriksen, E.F.; Rattan, S.I.; Kassem, M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J. Bone Min. Res. 2001, 16, 1120–1129.
- Muschler, G.F.; Nitto, H.; Boehm, C.A.; Easley, K.A. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J. Orthop. Res. 2001, 19, 117–125.
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173.
- Kuznetsov, S.A.; Mankani, M.H.; Bianco, P.; Robey, P.G. Enumeration of the colony-forming units-fibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Res. 2009, 2, 83–94.
- Li, J.; Wong, W.H.; Chan, S.; Chim, J.C.; Cheung, K.M.; Lee, T.L.; Au, W.Y.; Ha, S.Y.; Lie, A.K.; Lau, Y.L.; et al. Factors affecting mesenchymal stromal cells yield from bone marrow aspiration. Chin. J. Cancer Res. 2011, 23, 43–48.
- El-Jawhari, J.J.; Cuthbert, R.; McGonagle, D.; Jones, E.; Giannoudis, P.V. The CD45lowCD271high Cell Prevalence in Bone Marrow Samples May Provide a Useful Measurement of the Bone Marrow Quality for Cartilage and Bone Regenerative Therapy. J. Bone Jt. Surg. Am. 2017, 99, 1305–1313.
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45(low)CD271(+) Phenotype. Stem Cells Int. 2019, 20195197983.
- Rebolj, K.; Veber, M.; Drobnic, M.; Malicev, E. Hematopoietic stem cell and mesenchymal stem cell population size in bone marrow samples depends on patient’s age and harvesting technique. Cytotechnology 2018, 70, 1575–1583.
- Harichandan, A.; Buhring, H.J. Prospective isolation of human MSC. Best Pract. Res. Clin. Haematol. 2011, 24, 25–36.
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118.
- Kouroupis, D.; Sanjurjo-Rodriguez, C.; Jones, E.; Correa, D. Mesenchymal Stem Cell Functionalization for Enhanced Therapeutic Applications. Tissue Eng. Part. B Rev. 2019, 25, 55–77.
- Josephson, A.M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K.S.; Muinos Lopez, E.; Litwa, H.P.; Neibart, S.S.; Kadiyala, M.; Wong, M.Z.; et al. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 6995–7004.
- Zha, K.; Yang, Y.; Tian, G.; Sun, Z.; Yang, Z.; Li, X.; Sui, X.; Liu, S.; Zhao, J.; Guo, Q. Nerve growth factor (NGF) and NGF receptors in mesenchymal stem/stromal cells: Impact on potential therapies. Stem Cells Transl. Med. 2021.
- Ganguly, P.; Burska, A.N.; Davis, C.L.M.; El-Jawhari, J.J.; Giannoudis, P.V.; Jones, E.A. Intrinsic Type 1 Interferon (IFN1) Profile of Uncultured Human Bone Marrow CD45(low)CD271(+) Multipotential Stromal Cells (BM-MSCs): The Impact of Donor Age, Culture Expansion and IFNalpha and IFNbeta Stimulation. Biomedicines 2020, 8, 214.
- Block, T.J.; Marinkovic, M.; Tran, O.N.; Gonzalez, A.O.; Marshall, A.; Dean, D.D.; Chen, X.D. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res. Ther. 2017, 8, 239.
- Andrzejewska, A.; Catar, R.; Schoon, J.; Qazi, T.H.; Sass, F.A.; Jacobi, D.; Blankenstein, A.; Reinke, S.; Krüger, D.; Streitz, M.; et al. Multi-Parameter Analysis of Biobanked Human Bone Marrow Stromal Cells Shows Little Influence for Donor Age and Mild Comorbidities on Phenotypic and Functional Properties. Front. Immunol. 2019, 10.
- El-Jawhari, J.J.; Kleftouris, G.; El-Sherbiny, Y.; Saleeb, H.; West, R.M.; Jones, E.; Giannoudis, P.V. Defective Proliferation and Osteogenic Potential with Altered Immunoregulatory Phenotype of Native Bone Marrow-Multipotential Stromal Cells in Atrophic Fracture Non-Union. Sci. Rep. 2019, 9, 17340.
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017, 4020–4030.
- Buckwalter, J.A.; Woo, S.L.; Goldberg, V.M.; Hadley, E.C.; Booth, F.; Oegema, T.R.; Eyre, D.R. Soft-tissue aging and musculoskeletal function. J. Bone Jt. Surg. Am. 1993, 75, 1533–1548.
- Mitani, H.; Takahashi, I.; Onodera, K.; Bae, J.W.; Sato, T.; Takahashi, N.; Sasano, Y.; Igarashi, K.; Mitani, H. Comparison of age-dependent expression of aggrecan and ADAMTSs in mandibular condylar cartilage, tibial growth plate, and articular cartilage in rats. Histochem. Cell Biol. 2006, 126, 371–380.
- Germaschewski, F.M.; Matheny, C.J.; Larkin, J.; Liu, F.; Thomas, L.R.; Saunders, J.S.; Sully, K.; Whittall, C.; Boyle, Y.; Peters, G.; et al. Quantitation OF ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthr. Cartil. 2014, 22, 690–697.
- Forsyth, C.B.; Cole, A.; Murphy, G.; Bienias, J.L.; Im, H.J.; Loeser, R.F.J. Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1118–1124.
- Chiu, L.H.; Lai, W.F.; Chang, S.F.; Wong, C.C.; Fan, C.Y.; Fang, C.L.; Tsai, Y.H. The effect of type II collagen on MSC osteogenic differentiation and bone defect repair. Biomaterials 2014, 35, 2680–2691.
- Yu, C.; Peall, I.W.; Pham, S.H.; Okolicsanyi, R.K.; Griffiths, L.R.; Haupt, L.M. Syndecan-1 Facilitates the Human Mesenchymal Stem Cell Osteo-Adipogenic Balance. Int. J. Mol. Sci. 2020, 21, 3884.
- Atesok, K.; Fu, F.H.; Sekiya, I.; Stolzing, A.; Ochi, M.; Rodeo, S.A. Stem cells in degenerative orthopaedic pathologies: Effects of aging on therapeutic potential. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 626–636.
- Lynch, K.; Pei, M. Age associated communication between cells and matrix: A potential impact on stem cell-based tissue regeneration strategies. Organogenesis 2014, 10, 289–298.
- Sun, Y.; Li, W.; Lu, Z.; Chen, R.; Ling, J.; Ran, Q.; Jilka, R.L.; Chen, X.D. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011, 25, 1474–1485.
More
Information
Subjects:
Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
31 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




