
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pawel Madejski | + 4713 word(s) | 4713 | 2022-02-08 05:09:50 | | | |
| 2 | Conner Chen | -29 word(s) | 4684 | 2022-02-15 01:46:40 | | | | |
| 3 | Conner Chen | -29 word(s) | 4684 | 2022-02-15 01:55:27 | | | | |
| 4 | Conner Chen | Meta information modification | 4684 | 2022-02-15 01:56:23 | | |
Video Upload Options
With the increase in electricity consumption around the world, electricity demands are increasing every day. During electricity generation using energy technologies based on fossil fuels, the emission of harmful pollutants into the environment (gaseous, liquid, and solid) occurs as the emission of NOx, SOx, dust, CO2, and wastewater (e.g., from flue-gas treatment installations). A great deal of effort in modern low-emission energy technologies was directed at activities leading to decreased gaseous pollutant emissions. The emission of carbon dioxide (CO2), treated as one of the main reasons for global warming when fossil fuel is burned, cannot be avoided. The carbon capture utilization and storage (CCUS) methods and technologies are among the many ways to reduce CO2 emissions.
1. Introduction
-
Reducing the use of fossil fuels by:
- ○ improving the efficiency of energy conversion processes;
- ○ reducing the demand for energy;
- ○ using renewable (non-fossil fuel) energy sources, such as hydropower, wind, biomass, solar cells, and nuclear power;
- ○ increasing the use of green hydrogen, which is produced by splitting water using electricity from renewable energy.
-
Replace technologies using fossil fuels with a low carbon to hydrogen C/H2 ratio by replacing coal and oil with gaseous fuels.
-
Capturing CO2 from fuel combustion in power plants and other industrial processes and storing it in appropriate geological structures, in exhausted or exploited gas or crude oil deposits (intensification of crude oil extraction, enhanced oil recovery (EOR)), or at the bottom of oceans.
-
Limiting deforestation processes and thus storing more CO2 in biomass.
-
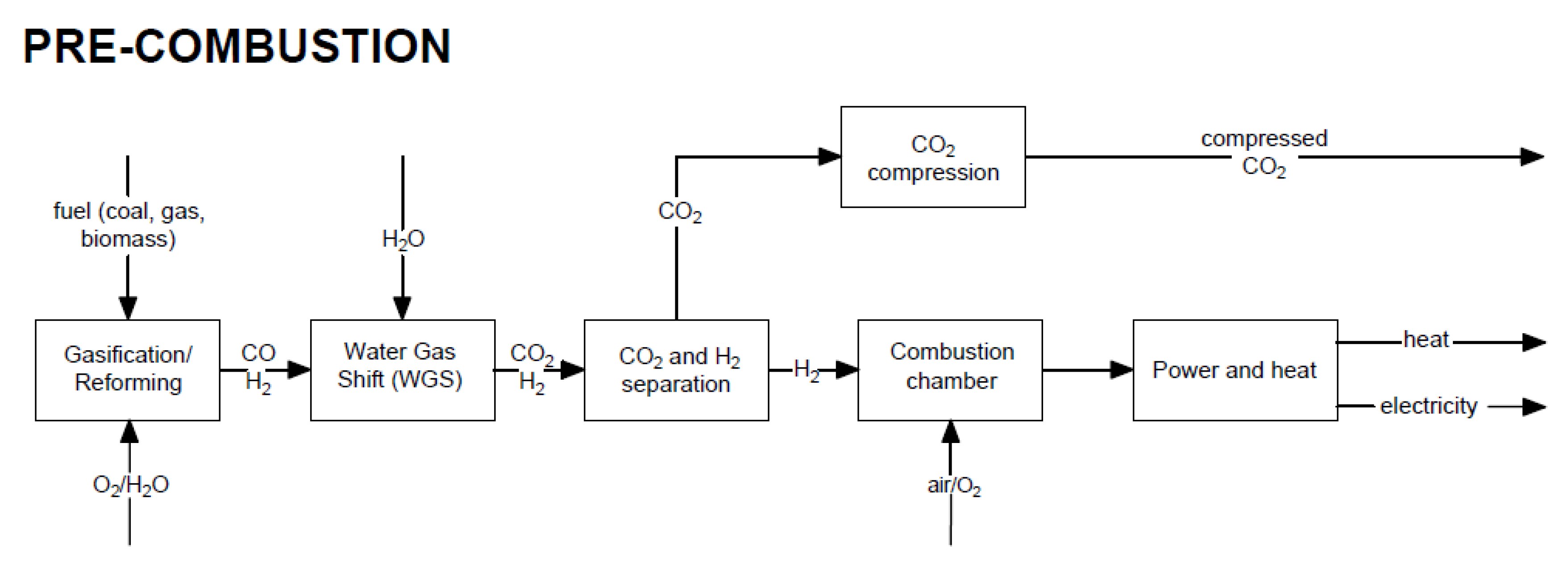
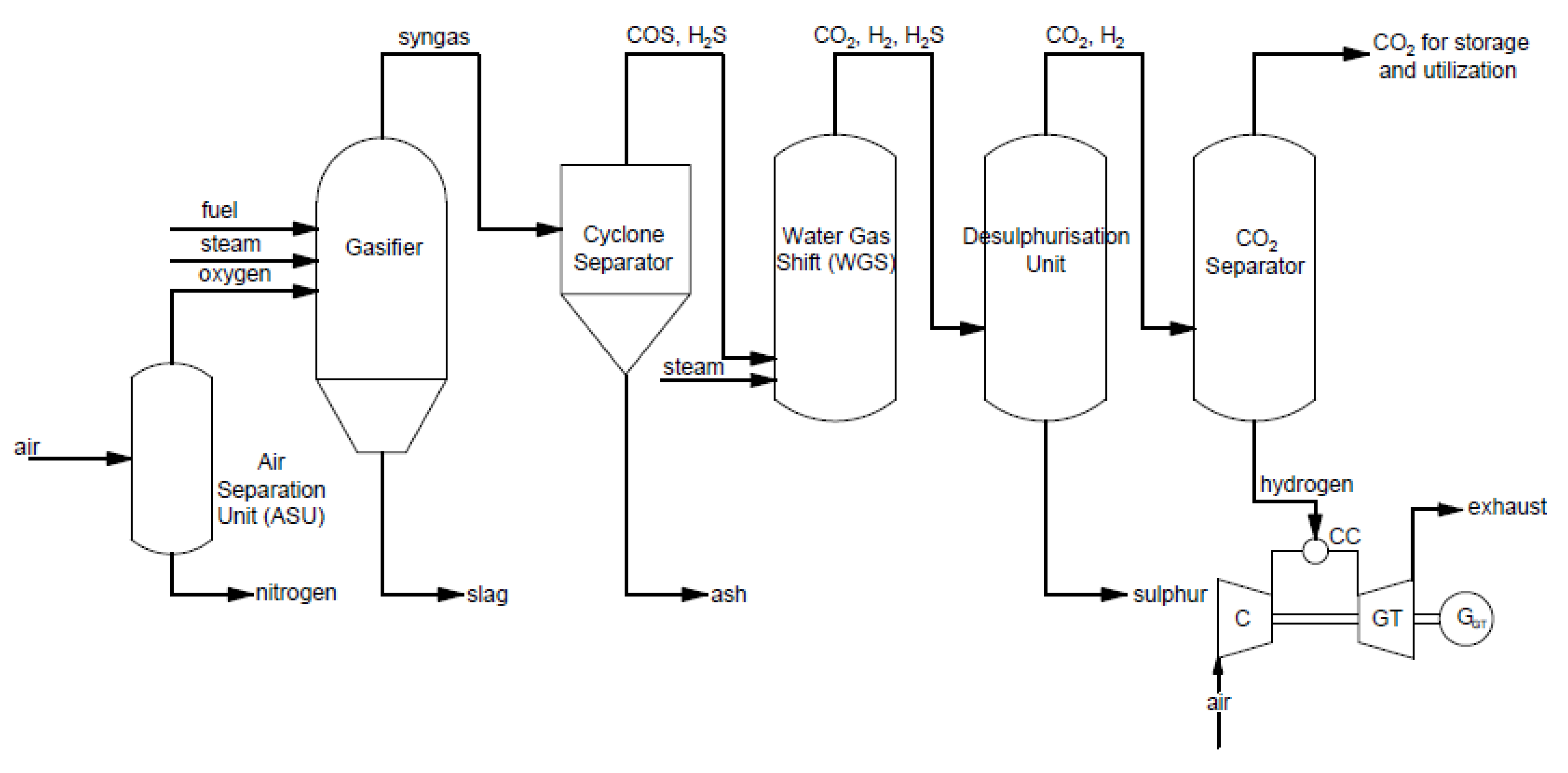
Pre-combustion carbon capture occurs before the combustion process (through fuel gasification with oxygen, e.g., integrated IGCC coal gasification technology).
-
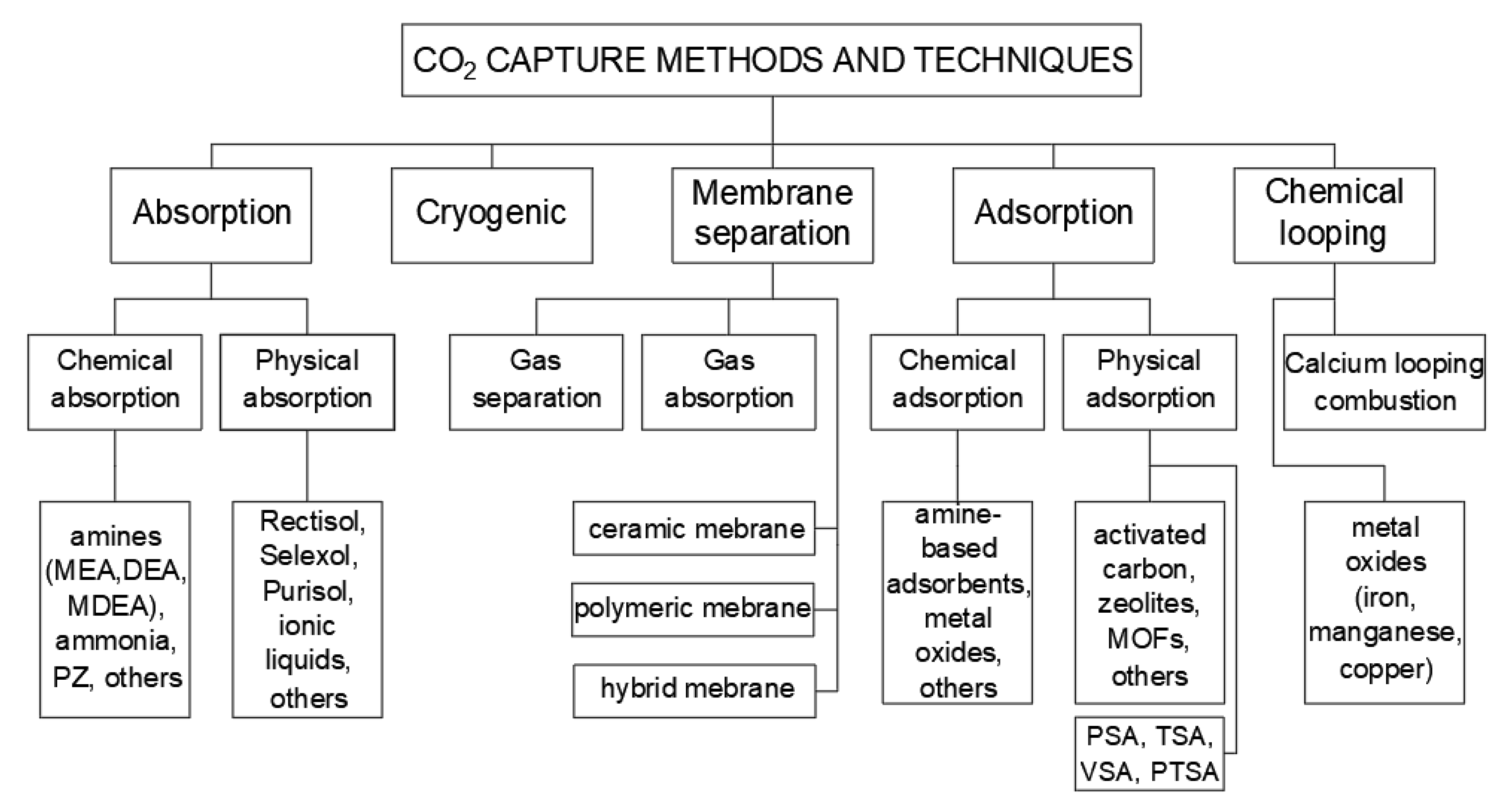
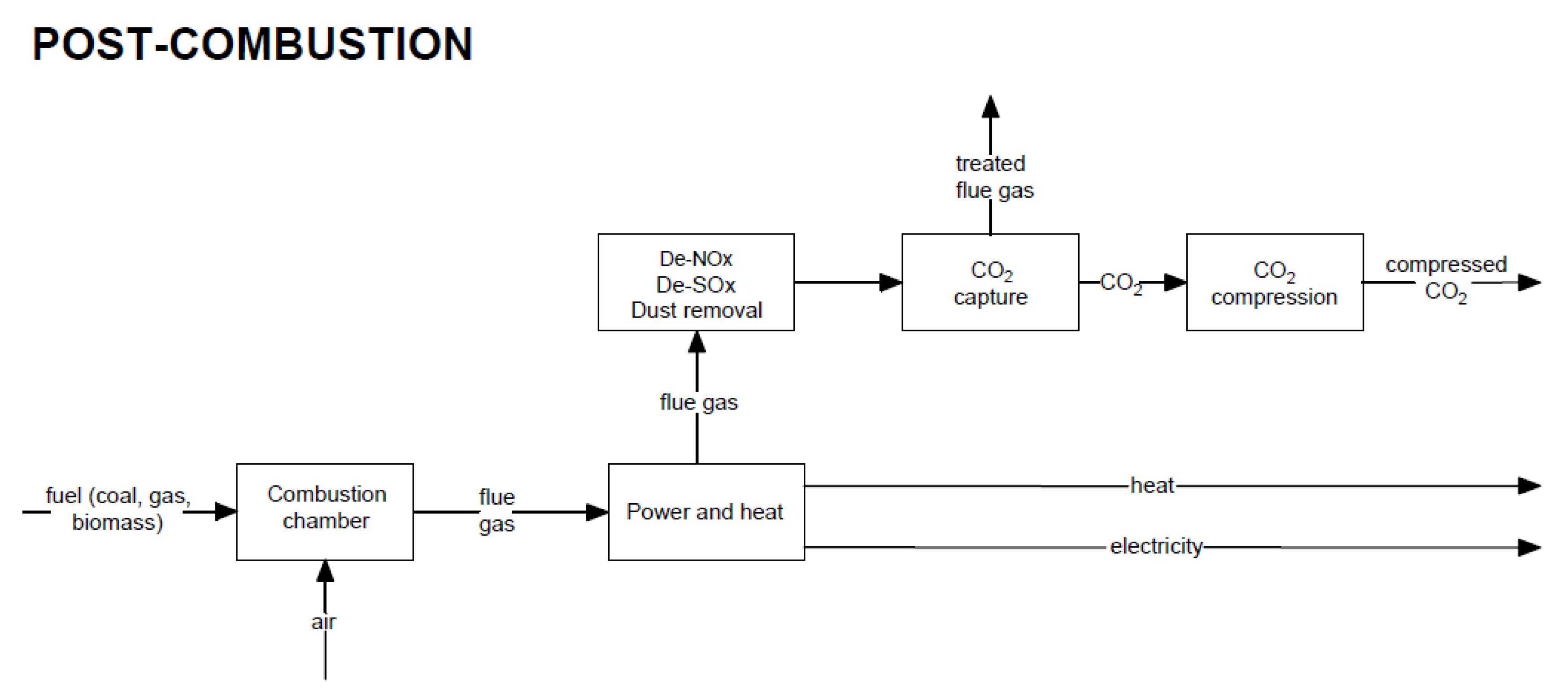
Post-combustion carbon capture occurs after the combustion process (capturing CO2 from flue gas, e.g., using chemical absorption, physical adsorption, membrane separation, or the use of a chemical loop).
-
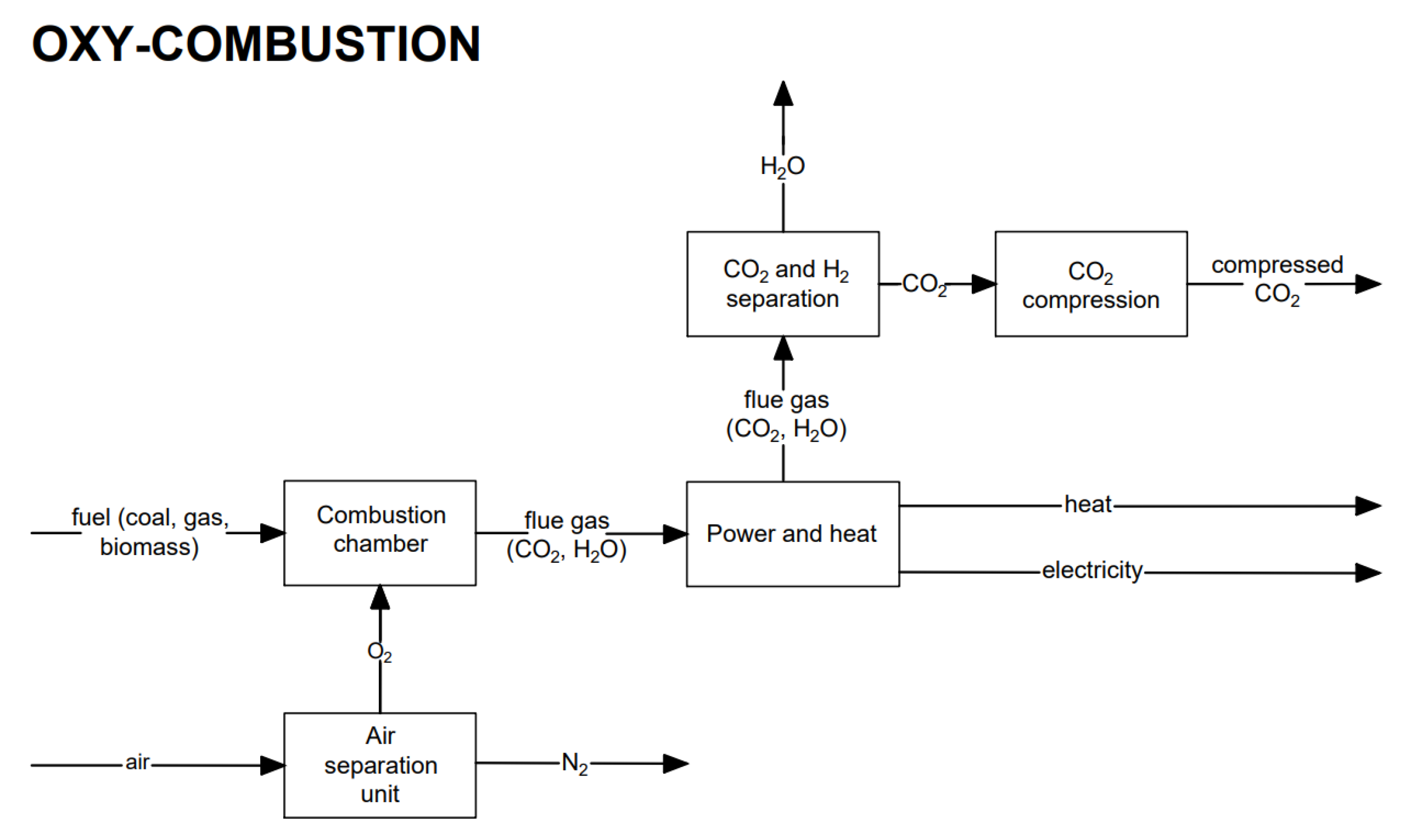
Oxy-combustion carbon capture occurs after the combustion process in an oxygen atmosphere by separating CO2 generated during the oxy-combustion process, e.g., using an oxygen gas turbine. Oxygen atmosphere can be obtained by removing nitrogen from the air before the combustion process.
2. Pre-Combustion CO2 Capture
3. Post-Combustion CO2 Capture
- (a) Absorption solvent-based methods
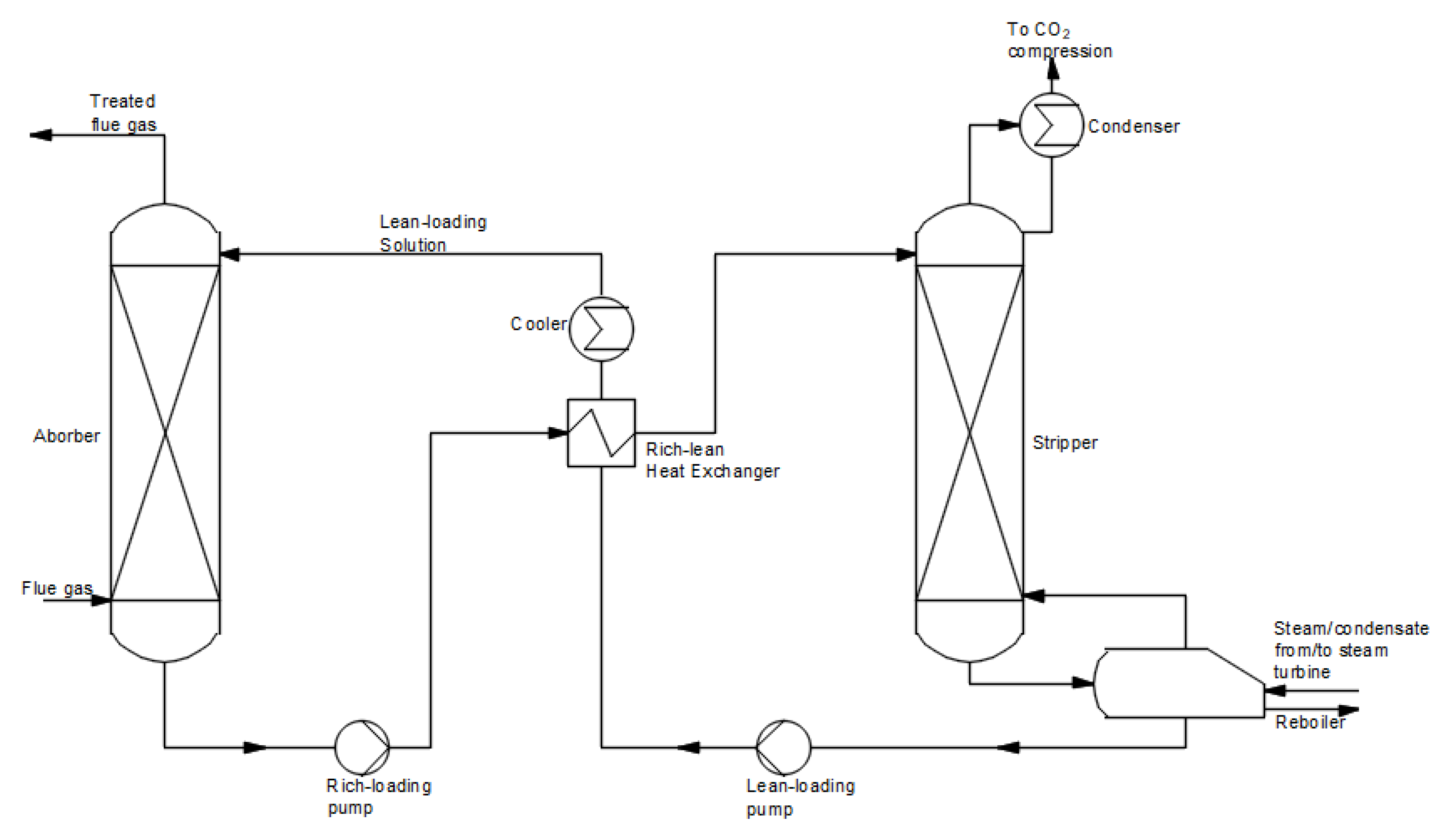
-
(b) Adsorption–physical separation
-
(c) Membrane separation
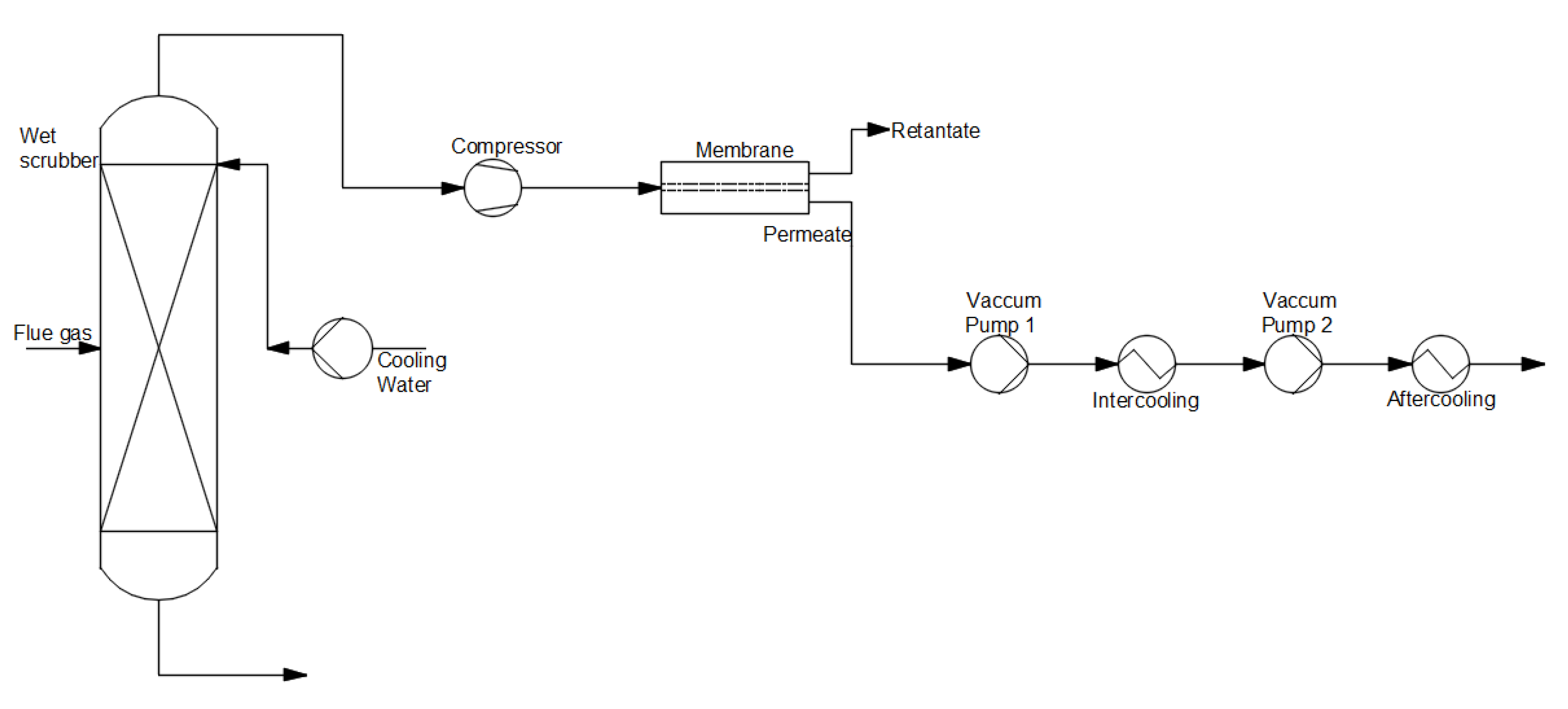
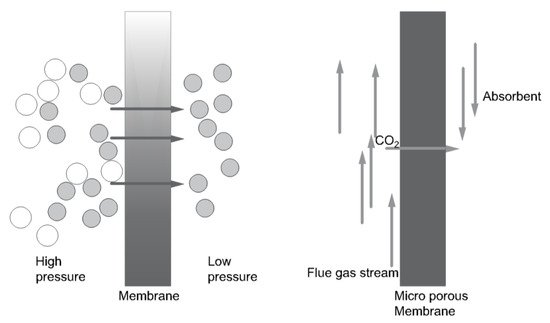
-
(d) Chemical looping combustion (CLC) and calcium looping process (CLP)
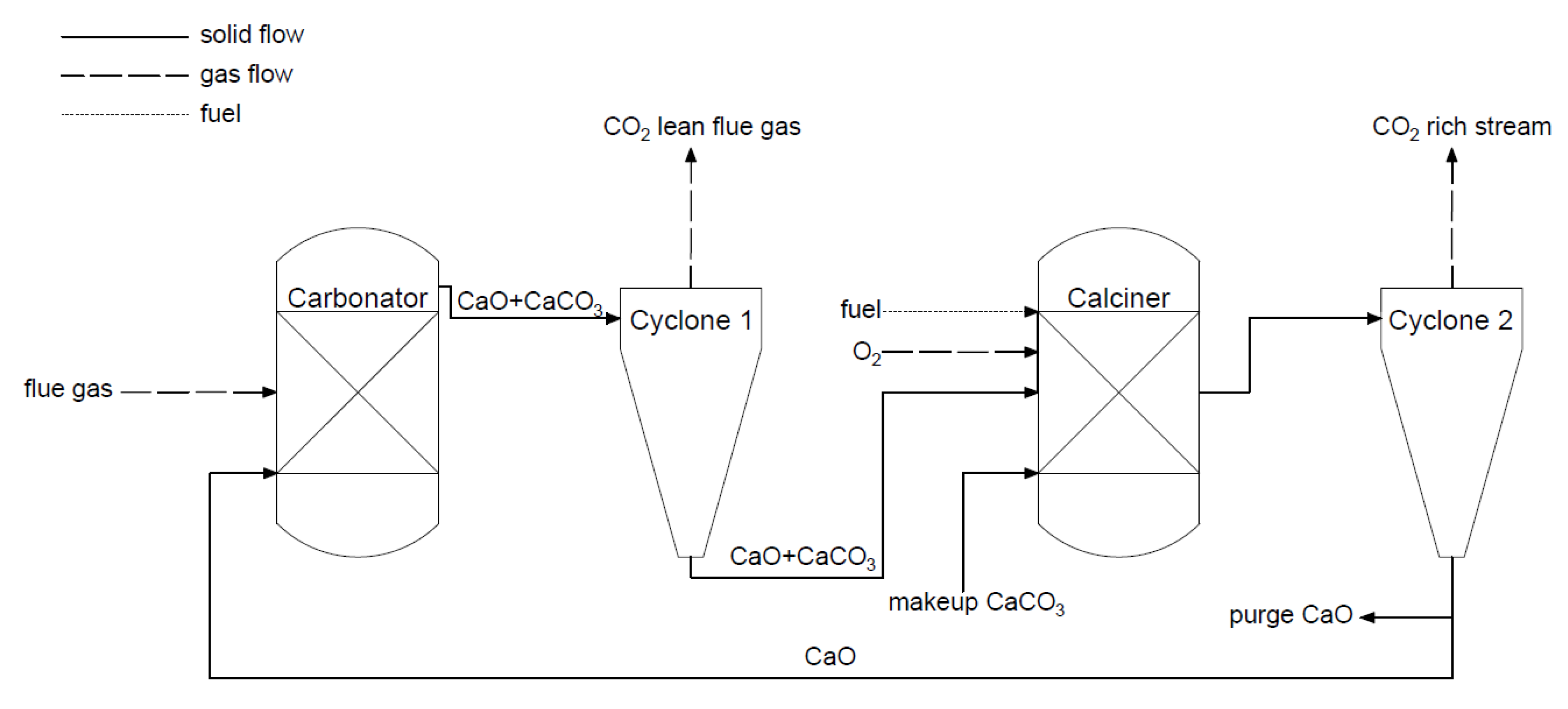
-
(e) Cryogenic method
-
(f) Application of absorption-based post-combustion capture method
-
(g) Converting CO2 into value-added chemicals
4. Oxy-Combustion CO2 Capture
References
- Spliethoff, H. Power Generation from Solid Fuels; Springer: Berlin/Heidelberg, Germany, 2010.
- Chmielniak, T. Technologie Energetyczn, 2nd ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2021.
- Sarkar, S. Fuels and Combustion; University Press: New Delhi, India, 2009.
- Madejski, P. (Ed.) Thermal Power Plants, New Trends and Recent Developments; Intech Open Limited: London, UK, 2018; ISBN 978-1-78923-079-6.
- Madejski, P.; Janda, T.; Modliński, N.; Nabagło, D. A Combustion Process Optimization and Numerical Analysis for the Low Emission Operation of Pulverized Coal-Fired Boiler. In Developments in Combustion Technology; Kyprianidis, K., Skvaril, J., Eds.; In Intech Open Limited: London, UK, 2016.
- Energy Technology Perspectives 2020, Special Report on Carbon Capture Utilisation and Storage CCUS in Clean Energy Transitions; IEA: Paris, France, 2020; Available online: https://iea.blob.core.windows.net/assets/7f8aed40-89af-4348-be19-c8a67df0b9ea/Energy_Technology_Perspectives_2020_PDF.pdf (accessed on 2 December 2021).
- Centrum Informacji o Rynku Energii, Cena Emisji CO2 Może Wzrosnąć o Ponad 50% do 2030—Wynika z Projektu UE. Available online: https://www.cire.pl/artykuly/materialy-problemowe/186670-cena-emisji-co2-moze-wzrosnac-o-ponad-50-do-2030-wynika-z-projektu-ue (accessed on 20 October 2021).
- Nord, L.; Bolland, O. Carbon Dioxide Emission Management in Power Generation; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2020.
- Peridas, G.; Mordick Schmidt, B. The role of carbon capture and storage in the race to carbon neutrality. Electr. J. 2021, 34, 106996.
- Wienchol, P.; Szlȩk, A.; Ditaranto, M. Waste-to-energy technology integrated with carbon capture—Challenges and opportunities. Energy 2020, 198, 117352.
- Holz, F.; Scherwath, T.; del Granado, P.C.; Skar, C.; Olmos, L.; Ploussard, Q.; Ramos, A.; Herbst, A. A 2050 perspective on the role for carbon capture and storage in the European power system and industry sector. Energy Econ. 2021, 104, 105631.
- Gładysz, P.; Stanek, W.; Czarnowska, L.; Sładek, S.; Szlęk, A. Thermo-ecological evaluation of an integrated MILD oxy-fuel combustion power plant with CO2 capture, utilisation, and storage—A case study in Poland. Energy 2018, 144, 379–392.
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663.
- Olabi, A.G.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 1117102022.
- Wanga, Y.; Zhaoa, L.; Ottoa, A.; Robiniusa, M.; Stoltena, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665.
- Kuropka, J. Możliwości Ograniczania Emisji Ditlenku Węgla ze Spalin Energetycznych. Available online: http://www.pzits.not.pl/docs/ksiazki/Pol_%202012/Kuropka%20179-188.pdf (accessed on 18 October 2021).
- Vega, F.; Cano, M.; Camino, S.; Fernandez, L.M.G.; Portillo, E.; Navarrete, B. Solvents for Carbon Dioxide Capture. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Intechopen: London, UK, 2018; ISBN 978-1-78923-575-3.
- Artanto, Y.; Jansen, J.; Pearson, P.; Puxty, G.; Cottrell, A.; Meuleman, E.; Feron, P. Pilot-scale evaluation of AMP/PZ to capture CO2 from flue gas of an Australian brown coal–fired power station. Int. J. Greenh. Gas Control 2014, 20, 189–195.
- Boot-Handford, M.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189.
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176.
- Barzgali, F.; Lai, S.; Mani, F. Novel non-aqueous amine solvents for reversible CO2 capture. Energy Procedia 2014, 63, 1795–1804.
- Mahi, M.; Mokbel, I.; Negadi, L.; Dergal, F.; Jose, J. Experimental solubility of carbon dioxide in monoethanolamine, or diethanolamine or N-methyldiethanolamine (30 wt%) dissolved in deep eutectic solvent (choline chloride and ethylene glycol solution). J. Mol. Liq. 2019, 289, 111062.
- Sifat, N.S.; Haseli, Y. A critical review of CO2 Capture Technologies and Prospects for Clean Power Generetion. Energies 2019, 12, 4143.
- Osman, A.I.; Hefny, M.; Maksoud, M.I.A.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2020, 19, 797–849.
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823.
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460.
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. Sep. Purif. Technol. 2020, 238, 116448.
- Rakowski, J.; Bocian, P.; Celińska, A.; Świątkowski, B.; Golec, T. Zastosowanie Pętli Chemicznej w Energetyce. Available online: http://elektroenergetyka.pl/upload/file/2016/4/Rakowski_04_2016.pdf (accessed on 15 October 2021).
- Bhavsar, S.; Najera, M.; More, A.; Veser, G. Chemical-looping processes for fuel-flexible combustion and fuel production. In Reactor and Process Design in Sustainable Energy Technology, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; pp. 233–280.
- Tilak, P.; El-Halwagi, M.M. Process integration of Calcium Looping with industrials plants for monetizing CO2 into value-added products. Carbon Resour. Convers. 2018, 1, 191–199.
- Knapik, E.; Kosowski, P.; Stopa, J. Cryogenic liquefaction and separation of CO2 using nitrogen removal unit cold energy. Chem. Eng. Res. Des. 2018, 131, 66–79.
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278.
- Mostafavi, E.; Ashrafi, O.; Navarri, P. Assessment of process modifications for amine-based post-combustion carbon capture processes. Clean. Eng. Technol. 2021, 4, 100249.
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490.
- Lungkadee, T.; Onsree, T.; Tangparitkul, S.; Janwiruch, N.; Nuntaphan, A.; Tippayawong, N. Technical and economic analysis of retrofitting a post-combustion carbon capture system in a Thai coal-fired power plant. Energy Rep. 2021, 7, 308–313.
- Otitoju, O.; Oko, E.; Wang, M. Technical and economic performance assessment of post-combustion carbon capture using piperazine for large scale natural gas combined cycle power plants through process simulation. Appl. Energy 2021, 292, 116893.
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.V.; Abu Zahra, M.R.M. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2017, 185, 1433–1449.
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799.
- Agarwal, A.S.; Rode, E.; Sridhar, N.; Hill, D. Conversion of CO2 to Value-Added Chemicals: Opportunities and Challenges. In Handbook Climate Change Mitigigation and Adaptation; Chen, W.Y., Suzuki, T., Lackner, M., Eds.; Springer: New York, NY, USA, 2015; pp. 1–40.
- Pal, T.K.; De, D.; Bharadwaj, P.K. Metal–organic frameworks for the chemical fixation of CO2 into cyclic carbonates. Coord. Chem. Rev. 2020, 408, 213173.
- Kumaravel, V.; Barlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value Added Products. ACS Energy Lett. 2020, 5, 486–529.
- McGrath, O.J. Biological Conversion of Carbon Dioxide to Value-Added Chemicals. Grad. Thesis Diss. Probl. Rep. 2021, 8293.
- Nocito, F.; Dibenedetto, A. Atmospheric CO2 mitigation technologies: Carbon capture utilization and storage. Curr. Opin. Green Sustain. Chem. 2020, 21, 34–43.
- CS Energy, Callide Oxyfuel Project. Available online: https://www.csenergy.com.au/what-we-do/generating-energy/callide-power-station/callide-oxyfuel-project (accessed on 20 October 2021).
- Global CCS Institue. The Compostilla Project OXYCFB300. Available online: https://www.globalccsinstitute.com/archive/hub/publications/137158/Compostilla-project-OXYCFB300-carbon-capture-storage-demonstration-project-knowledge-sharing-FEED-report.pdf (accessed on 20 October 2021).
- Project Negative CO2 Emission Gas Power Plant. Available online: https://nco2pp.mech.pg.gda.pl (accessed on 20 October 2021).
- Ziółkowski, P.; Madejski, P.; Amiri, M.; Kuś, T.; Stasiak, K.; Subramanian, N.; Pawlak-Kruczek, H.; Badur, J.; Niedźwiecki, Ł.; Mikielewicz, D. Thermodynamic Analysis of Negative CO2 Emission Power Plant Using Aspen Plus, Aspen Hysys, and Ebsilon Software. Energies 2021, 14, 6304.
- Ziółkowski, P.; Badur, J.; Pawlak-Kruczek, H.; Niedzwiecki, L.; Kowal, M.; Krochmalny, K. A novel concept of negative CO2 emission power plant for utilization of sewage sludge. In Proceedings of the 6th International Conference on Contemporary Problems of Thermal Engineering CPOTE 2020, Gliwice, Poland, 21–24 September 2020; pp. 531–542.
- Cormos, C.C. Oxy-combustion of coal, lignite and biomass: A techno-economic analysis for a large scale Carbon Capture and Storage (CCS) project in Romania. Fuel 2016, 169, 50–57.
- Kindra, V.; Rogalev, A.; Lisin, E.; Osipov, S.; Zlyvko, O. Techno-economic analysis of the oxy-fuel combustion power cycles with near-zero emissions. Energies 2021, 14, 5358.





