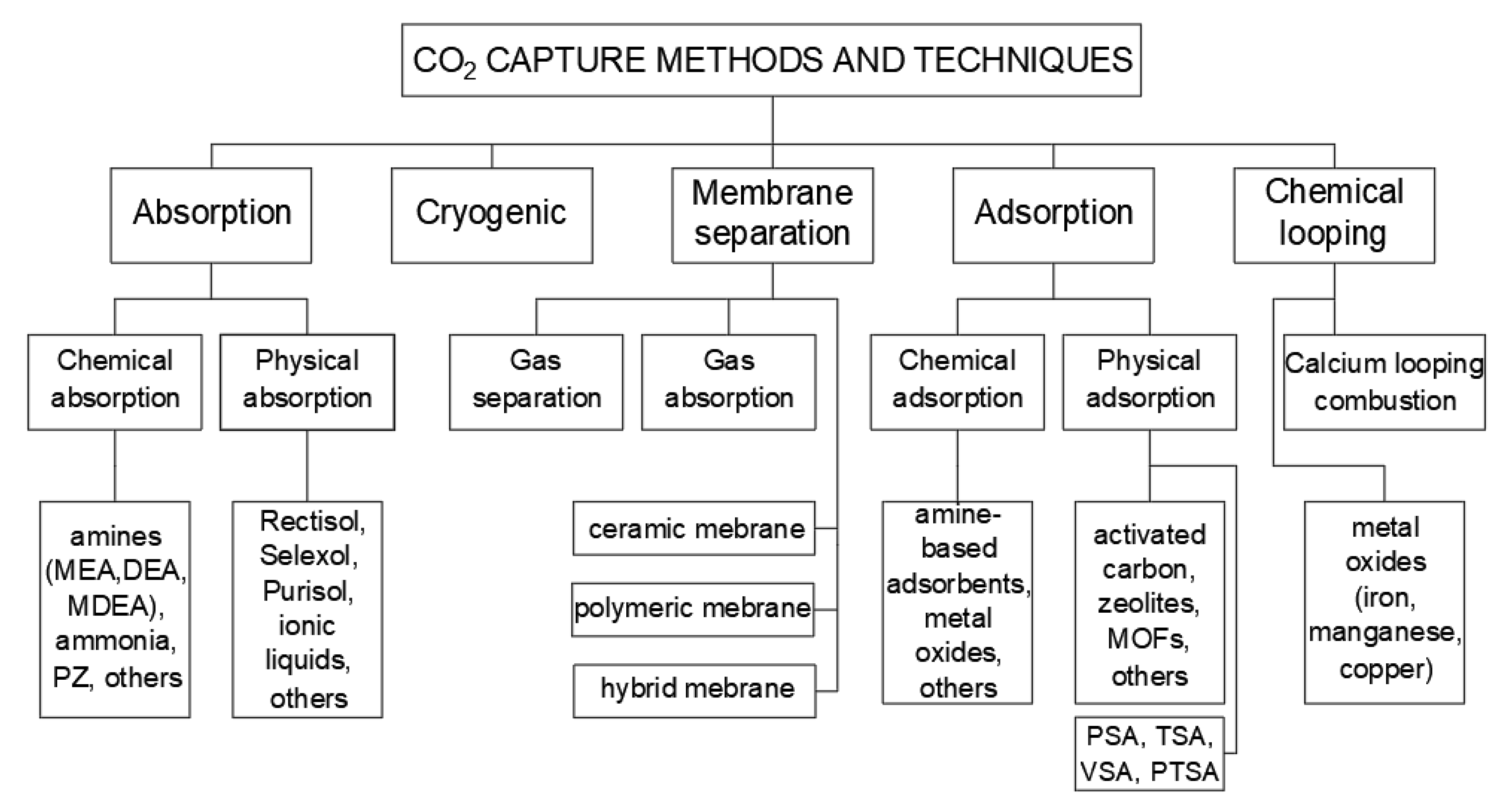
2. Pre-Combustion CO2 Capture
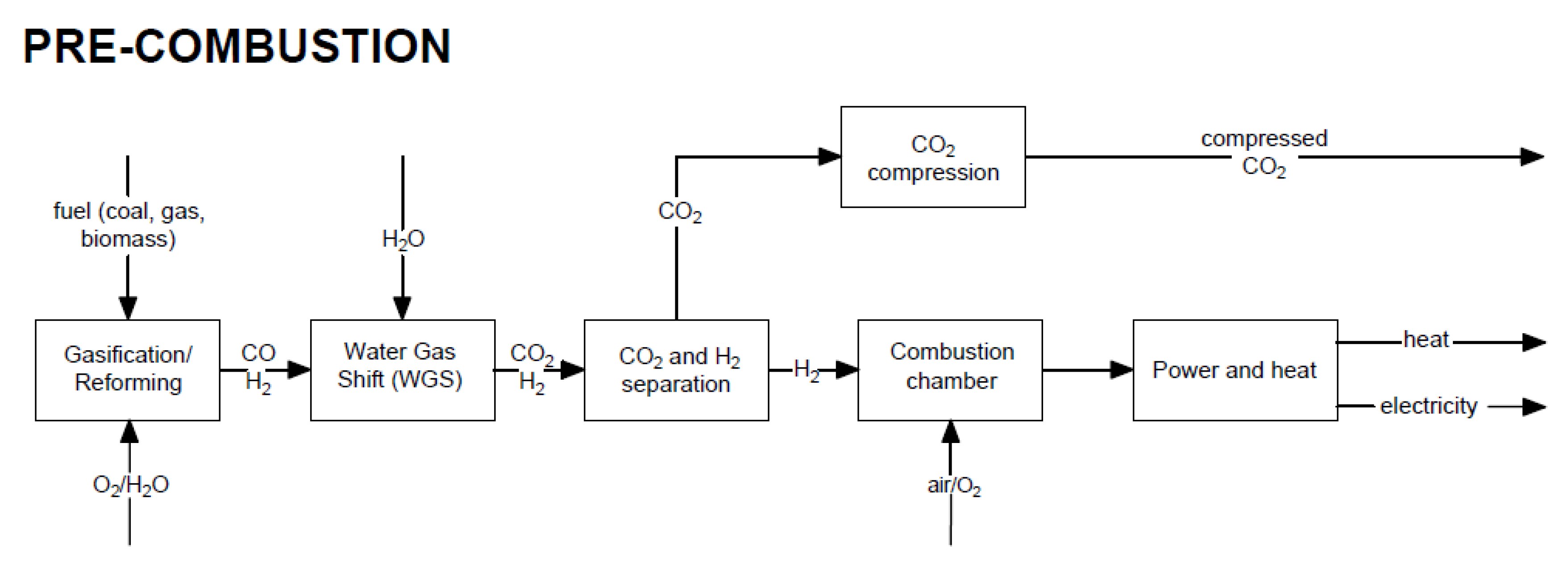
In this method, the fuel (coal, gas, biomass) is not completely combusted in the reactor, but is converted into a mixture of CO and H2 in the reforming or gasification process. Subsequently, using the water–gas shift, CO2 and H2 are produced. Figure 2 shows a block diagram of the pre-combustion CO2 capture method in a power plant.
Figure 2. Block diagram of electricity generation and heat production with the use of the pre-combustion CO2 capture method.
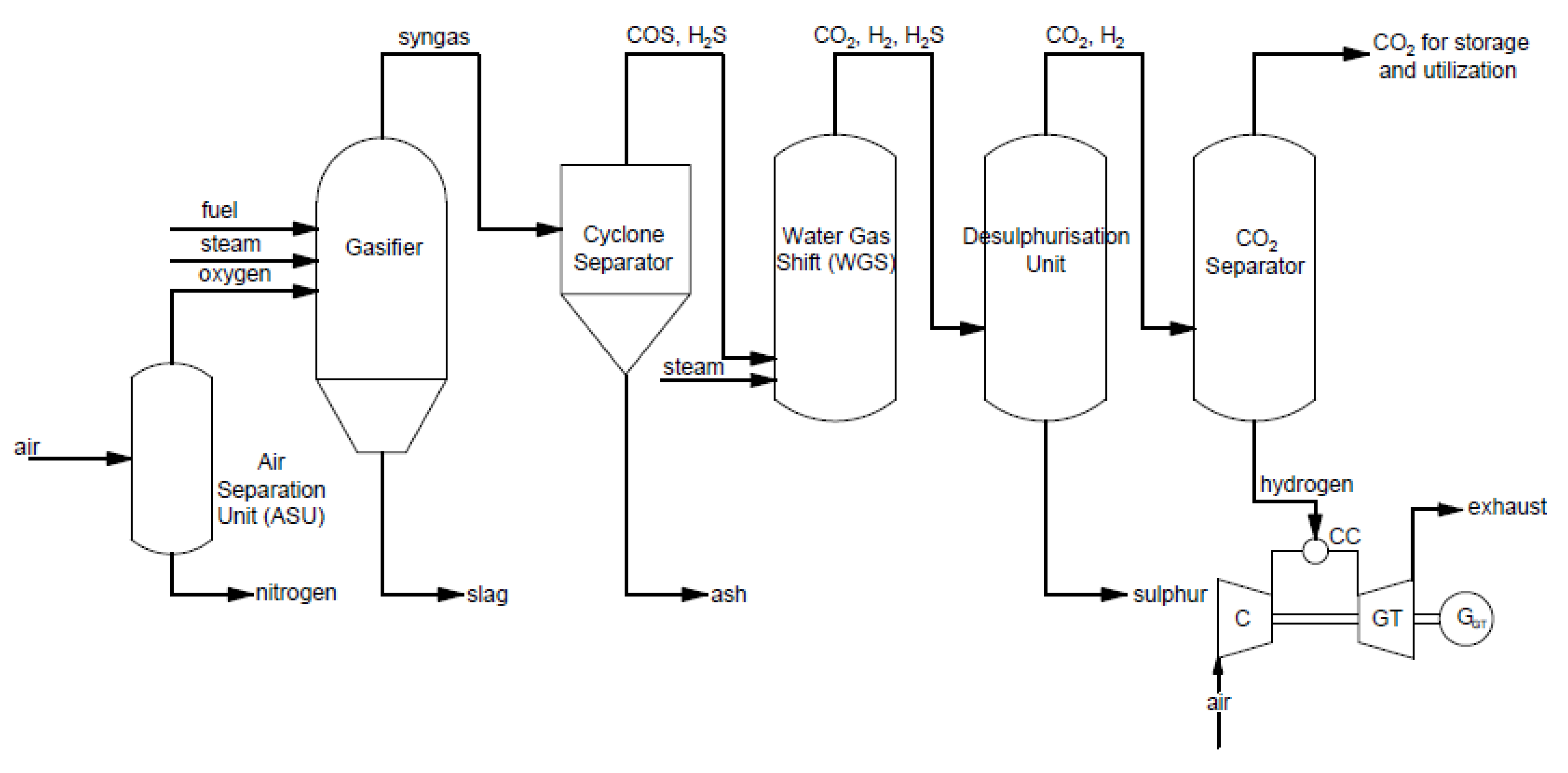
Pre-combustion capture is used, e.g., in an integrated gasification combined cycle (IGCC). Carbon dioxide is removed after the gasification process. An example of a typical process for power and heat generation in a gas turbine with pre-combustion capture is shown in Figure 3.
Figure 3. Scheme of integrated gasification combined cycle (IGCC) for electricity generation using a gas turbine using the pre-combustion CO2 capture method.
In this process, steam and oxygen are provided to the gasifier to produce syngas enriched in hydrogen and carbon monoxide. Then, syngas is sent to a cyclone separator, where it is filtered to remove ash. After, the conversion of syngas and steam to CO
2 and H
2 occurs in the water–gas shift reactor. The received gas needs to be purified of sulfur in the desulfurization unit. Subsequently, CO
2 is captured in the CO
2 separator and is sent for storage or utilization. Received hydrogen is provided to the gas turbine as fuel [
18]. Pre-combustion methods are very effective in CO
2 separation on the grounds of the high concentration of CO
2 in fuel before combustion. On the other hand, these processes are expensive due to the need for a gasification unit.
Pre-combustion carbon capture uses physical and chemical methods to capture CO
2 from processed syngas. Chemical absorbents, such as carbonates and physical solvents, such as polypropylene glycol and methanol, are commercially used in industries to capture CO
2. The cost expenditure and energy consumption of carbon capture depend on the utilities and capture process. An effective solvent or absorbent pre-combustion carbon capture technology can achieve more than 90% CO
2 capture, but, at the same time, reduces plant efficiency [
19]. The calcium looping process is another method of pre-combustion CO
2 capture, where CO
2 capture is achieved effectively at a low cost. This method involves the sorption of CaO with CO
2 and the desorption of CaCO
3 to release CO
2 at an optimal temperature. This cycling process repeats multiple times, and waste heat from the gasifier is used to reduce the heat consumption of the CO
2 capture process. The CaL pre-combustion carbon capture method is highly effective. Low-cost and CO
2 capture is achieved by decreasing energy consumption [
20]. The pre-combustion carbon capture demo plant in Port Arthur, United States, has successfully captured 1 million tons of CO
2 since operating without problems. This plant proved that using the dual pressure swing adsorption (PSA) technology method, hydrogen >99.9% purification and a high-efficiency CO
2 capture can be achieved. When the streaming gas has low pressure, hydrogen purification is performed, and the tail gas is sent to undergo vacuum pressure swing adsorption (VPSA) to separate purified CO
2. If the streaming gas has high pressure, CO
2 capture is achieved without VPSA first, and hydrogen purification is achieved from the exiting gas [
21].
3. Post-Combustion CO2 Capture
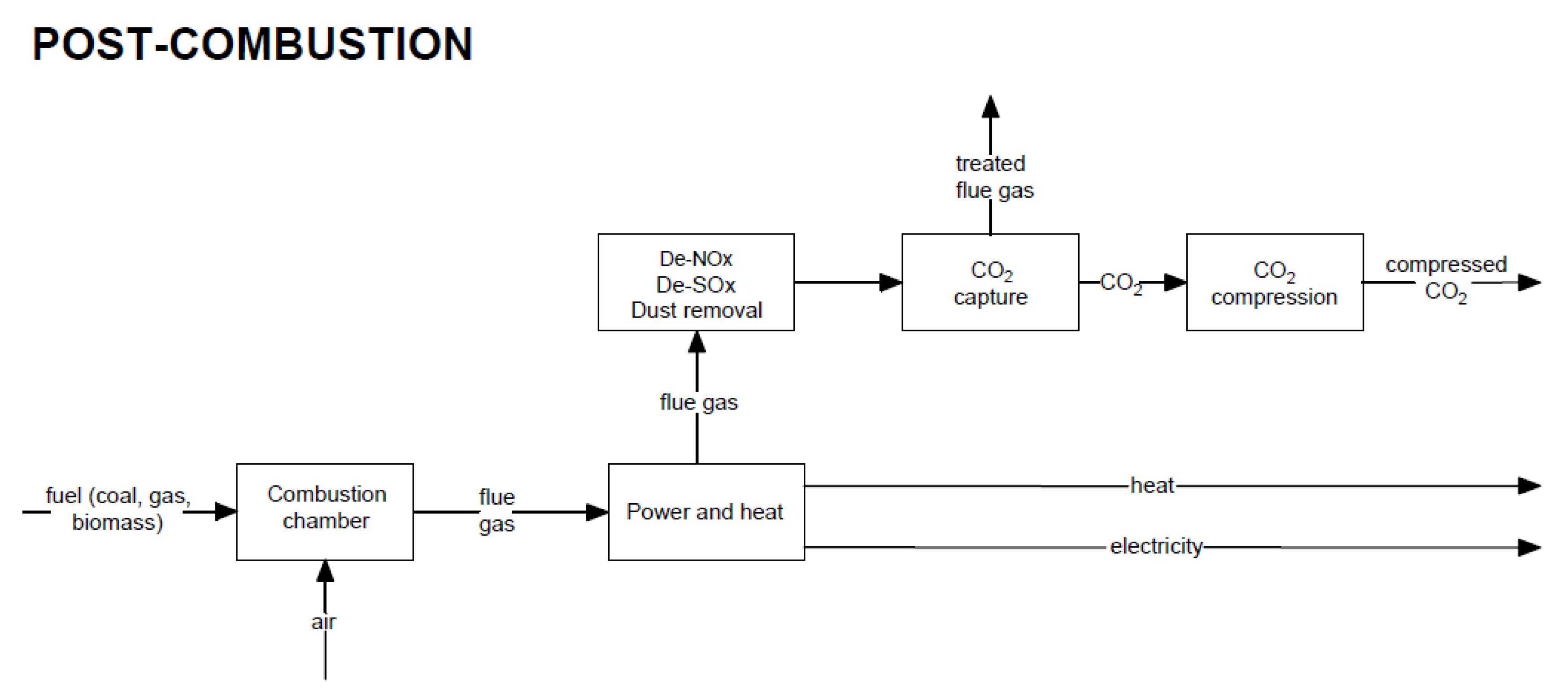
Post-combustion CO2 capture methods are based on removing carbon dioxide from flue gas. The capture unit is placed after the purification systems, such as desulphurization, denitrogenation, and dedusting installations. Figure 4 shows a general block diagram of the post-combustion capture technique.
Figure 4. Block diagram of electricity generation and heat production through the use of the post-combustion CO2 capture method.
In existing conventional power units, post-combustion technologies are the most frequently considered [
2,
8]; nevertheless, there is one main barrier to using these methods. Since the partial pressure of CO
2 in the flue gas is low (flue gas is under atmospheric pressure and the concentration of carbon dioxide is within 13–15%), the driving force for CO
2 is also low [
22]. Post-combustion technologies can be divided up according to the type of process used for capturing carbon dioxide, as follows:
- (a) Absorption solvent-based methods
Chemical absorption is the most recognizable method of CO
2 capture. It relies on a reaction between carbon dioxide and a chemical solvent. Solvents that are usually used are alkanolamines, such as monoethanolamine (MEA), diethanolamine (DEA), or methyl diethanolamine (MDEA) in aqueous solution [
23]. A schematic diagram of chemical absorption is shown in
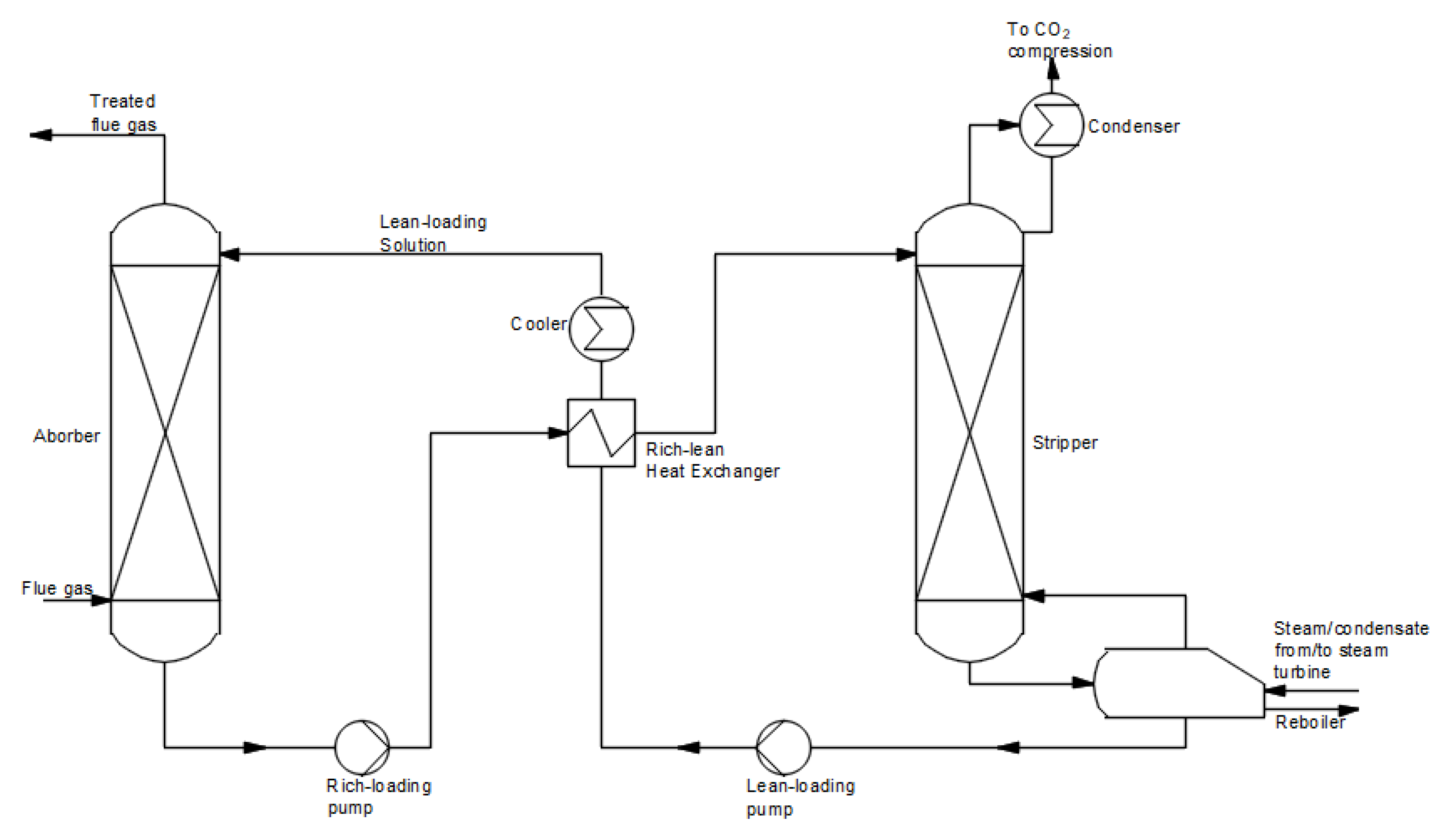
Figure 5. The process takes place in two stages. In the first stage, the flue gas reacts with the solvent in the absorber to capture CO
2. Subsequently, the rich loading solution is carried to the stripper to regenerate CO
2 at elevated temperatures. The solution without CO
2 (lean-loading solution) is sent back to the absorber column. A high purity carbon dioxide stream from the desorber is transferred for compression and storage or utilization. The chemical absorption process has been used for a long time in the chemical industry. The typically used 30% MEA and MDEA solutions achieve a high process efficiency and a high degree of carbon dioxide purity [
23]. The chemical absorption method is a very energy-consuming process due to the need to supply a large amount of heat to the desorber. It is assumed that approx. 30% (37%) of the heat supplied to the steam in the boiler should be directed to the CCS installation in the case of a steam unit fired with hard coal (lignite), depending on the absorber used (for ammonia, the amount of heat needed for regeneration is 22% for hard coal and 27% for lignite). Chemical absorption technologies are used in power plants fired with solid fuel, and they are the only ones that are commercially available. It is assessed that the amine method can capture approx. 85–95% carbon dioxide included in flue gas with a purity above 99.95% [
2].
Figure 5. Scheme of the post-combustion CO
2 capture method using a chemical absorption process (based on [
22]).
Nowadays, apart from conventional solvents (amine-based-MEA, DEA, ammonia, piperazine), there are other solvents developed for the CO
2 capture process. Solvent blends offer the ability to improve absorption properties by combining types properly. Primary and secondary amines have high absorption rates, and tertiary amines are characterized by a high capacity [
8]. For example, blending MEA with a little PZ can improve the absorption rate (PZ is 50 times faster than MEA) [
24]. Another possibility is to use a solution of 2-amino-2-methyl1-propanol (AMP) promoted with PZ. Artanto et al. showed that a mixture of 25 wt% AMP and 5 wt% PZ can be a good substitution for MEA [
25]. Ionic liquids (ILs) are novel alternatives for amines. These low melting salts are comprised of a large organic cation and an arbitrary anion, which can be combined freely, obtaining a great variety of compound properties. ILs can physically or chemically absorb CO
2, depending on pressure [
8]. A review [
26] and articles [
24,
27] have presented deep insight into this technology. In the case of reducing energy consumption, there are new generation solvents—water-free solvents and biphasic solvents—that have been proposed. The presence of water in a solvent enhances the energy demand for the regeneration process. Novel water-free solvents, such as non-aqueous organic amine blends (methanol, ethylene glycol), aminosilicones, or amines with a superbase have been observed [
24]. In [
28], researchers showed that solvent mixtures based on ethylene glycol, used in the chemisorption process, can achieve CO
2 capture efficiencies of up to 95%. Deep eutectic solvents (DESs), such as choline chloride and ethylene glycol at a 1:2 mole ratio are getting more attention. They are fluids consisting of organic halide salts and metal salts or a hydrogen bond donor. DESs have similar properties to ILs, but they are cheaper and environmentally friendlier. According to [
29], using DESs can decrease the vapor pressure of a solvent, achieve a lower effect of corrosion, and needs less energy in the regeneration process.
The physical absorption method is based on using a chemically inert solvent, which absorbs CO
2 physically. Absorption occurs in water or organic absorbers (methanol, N-methyl-2-pyrrolidone, dimethyl ether). This method achieves the best results for low temperatures and high pressures of the separated gas. Therefore, it is used to capture CO
2 from the coal gasification process. In this method, there are distinguished processes, with the use of solvent such as Selexol
TM, Rectisol
TM, Ifpexol
TM, Fluor
TM, Purisol
TM, Sulfinol
TM, and Morphysorb
TM [
23,
24,
30].
Adsorption is a process that uses a solid surface to remove carbon dioxide from a mixture. Physical separation relies on adsorption, absorption, and cryogenic separation methods. It can be physical (Van der Waals forces for adhesion CO
2—physisorption) or chemical (covalent bonding between compounds—chemisorption) [
31]. Physical adsorption uses various porous materials (such as activated carbon, alumina, metallic oxides, or zeolites [
6]) to absorb carbon dioxide. Activated carbons contain amorphous carbon, and it is low-cost material with the advantage of having a large surface area and the possibility of modifying its pore structure. However, the weak binding energy with carbon dioxide causes this material to need to be highly microporous to be useful for carbon capture [
8]. Zeolites (crystalline aluminosilicates) have good adsorption properties for CO
2 capture, but they are hydrophilic. The presence of water weakens these properties by reducing the strength of interactions between coupled compounds [
26]. A new approach is to use metal–organic frameworks (MOFs) in adsorption processes. MOFs consist of metal ions or ion clusters linked by organic ligands and bridges that create strong coordination bonds. On account of this, MOFs are characterized as having benefits such as ease of design and synthesis, a high porosity, and tailored pore properties [
32]. One of the other adsorption materials is silica. Silicas are non-carbonaceous substances with a large surface area and pore size, and they are highly mechanically stable. Mesoporous silica materials use amine-based substances for CO
2 capture [
8,
31]. The methods of adsorption are as follows: pressure swing adsorption (PSA), temperature swing adsorption (TSA), vacuum swing adsorption (VSA), and pressure–temperature swing adsorption (PTSA).
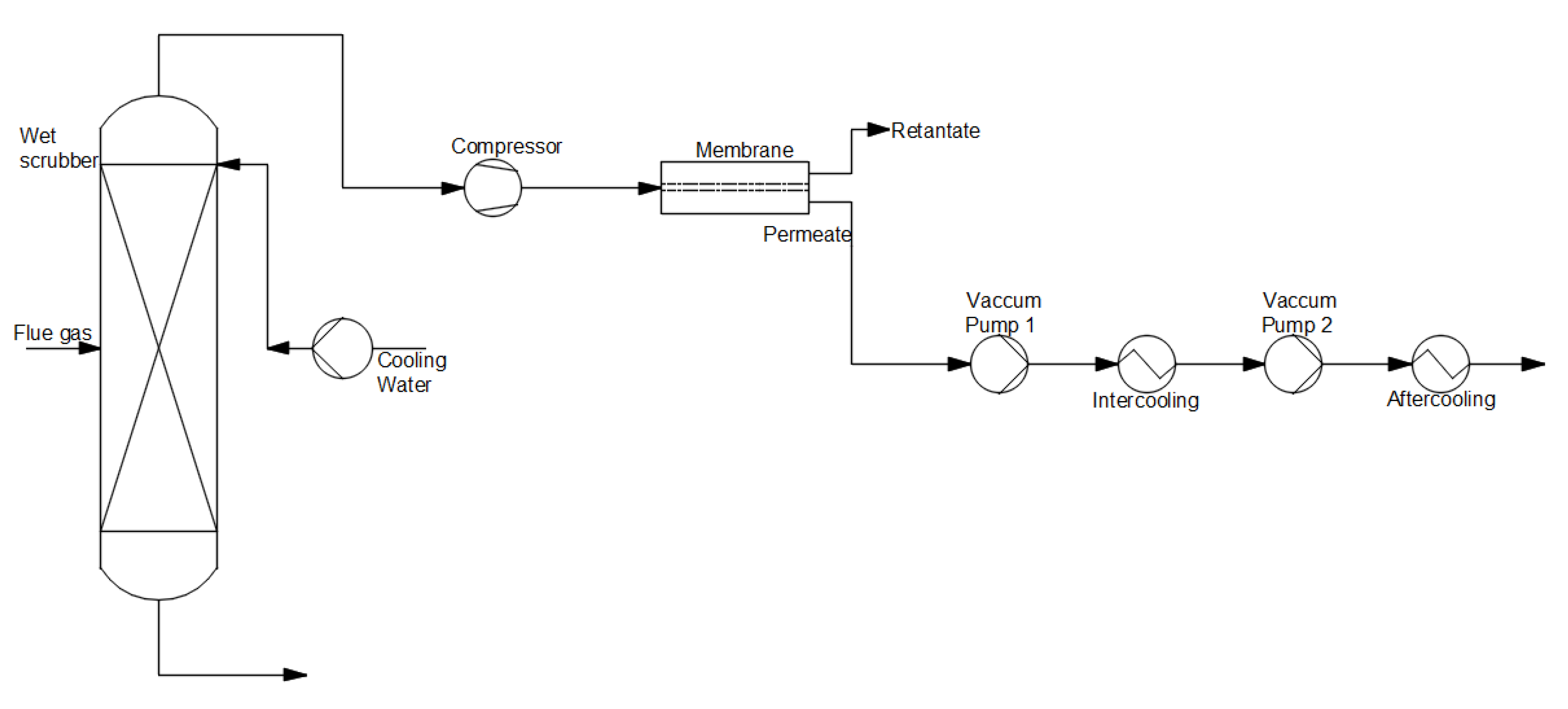
Figure 6 depicts the membrane separation process. In the first place, flue gas is directed to an absorber to cool to the operating temperature of the membrane. Subsequently, flue gas is transported to the membrane. This method uses a spiral wound, flat sheet, and hollow fiber modules [
30].
Figure 6. Scheme of the post-combustion CO
2 capture method using a membrane separation process (based on [
22]).
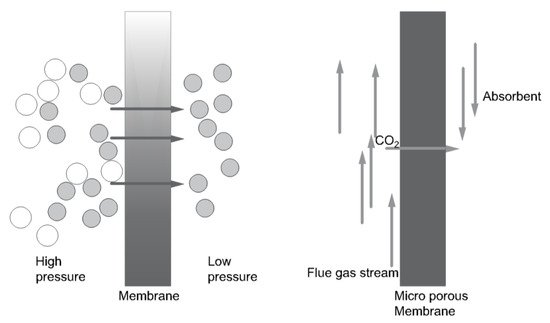
There are two types of membrane capture technology: gas separation membranes and gas absorption membranes. With a gas separation membrane, gas with CO
2 is introduced at the high-pressure side of the membrane. Carbon dioxide is recovered at the low-pressure side. A solid microporous membrane is used to enable gas flow and absorption in the gas absorption system. This system has a high removal rate of CO
2, on the grounds of minimization of flooding, foaming, channeling, and entrainment. The principles of both membrane systems are shown in
Figure 7 [
22,
30].
Figure 7. Principle of (
left) a gas separation membrane, (
right) a gas absorption membrane (based on [
30]).
Membranes should characterize relevant properties for gas separation—proper permeability and selectivity. There are three types of membrane materials: polymeric membranes (organic), ceramic membranes (inorganic), and hybrid membranes [
22]. A polymeric membrane has a lower cost of production than the others with a relatively high gas flux and it is mechanically stable [
33]. Nevertheless, it has generally low selectivity CO
2/N
2—less than 100, and it is supposed to be 200 [
32]. Ceramic membranes, especially zeolites and their derivatives, obtain high selectivities. However, the production of ceramic membranes is more difficult [
22]. Hybrid membranes (modified on the surface of inorganic membranes) provide advantages of both membranes, polymeric and ceramic. They have the flexibility and low cost of production of a polymer and the high selectivity of an inorganic material [
8,
22]. For post-combustion capture, commercially available polymeric membranes, such as PRISM, Polaris, PolyActive, PermSelect, and Medal, are introduced in [
34]. A new approach is to use metal–organic frameworks (MOFs) in the experimental stage. These offer many properties that are useful for membranes, such as large surface areas, adjustable pore sizes, and controllable pore-surface properties [
32].
Chemical looping technology uses two reactors, an air reactor and a fuel reactor. These reactors typically circulate fluidized beds that are coupled for carrier transport. In the air reactor, oxidation of an “oxygen carrier”, usually metal particles, such as iron, manganese, or copper, occurs with the oxygen from air. As a result of the reaction, metal oxides are formed. These compounds are carried to a second reactor, where they react with the fuel. Metal oxides are reduced during combustion, producing energy and flue gas as a stream of CO
2 and H
2O. The flue gas can be condensed to receive pure CO
2 [
35,
36].
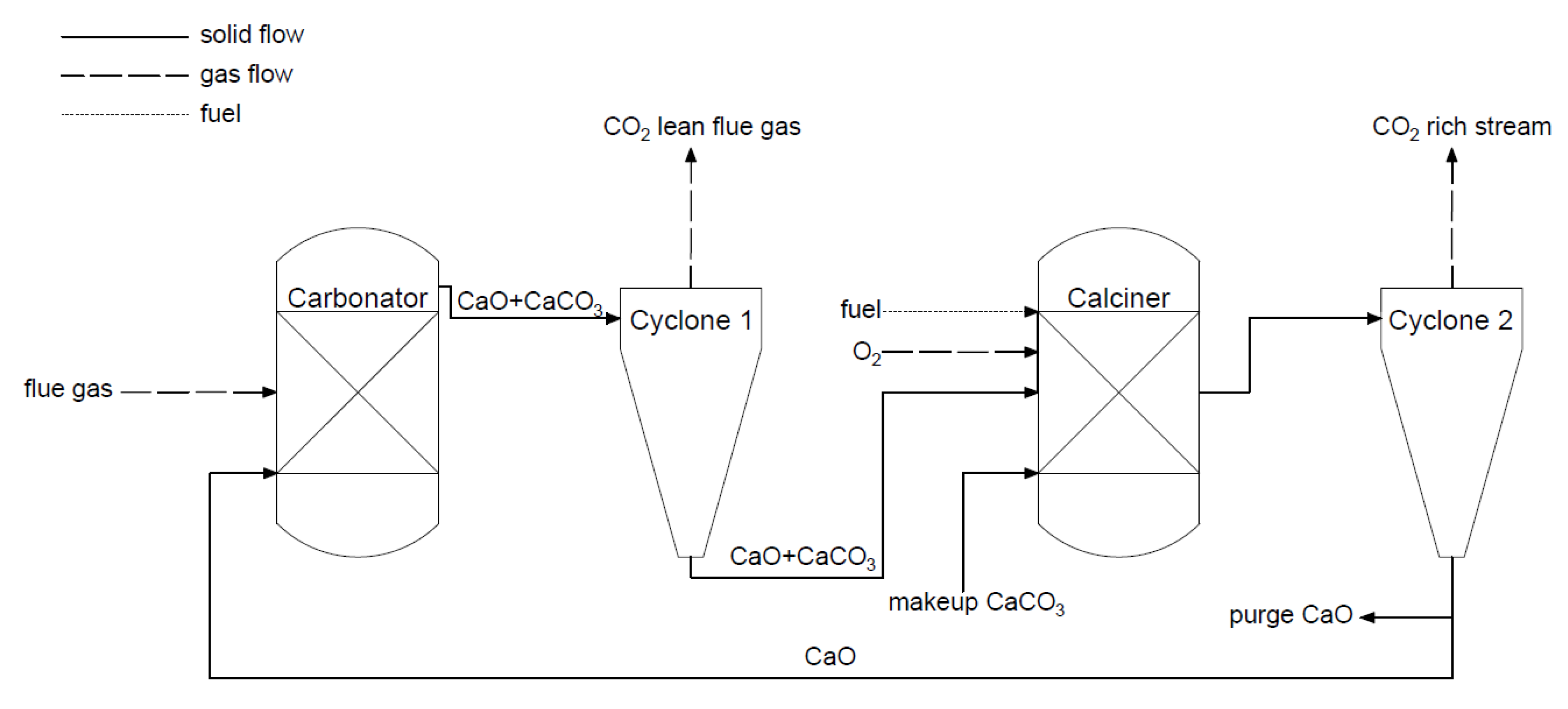
The calcium looping process is a type of chemical looping. The process (
Figure 8) is based on a reversible reaction between calcium oxide and carbon dioxide. The reaction of bounding CaO and CO
2 is called carbonation, and takes place in the first reactor. Subsequently, the formed calcium carbonate in the carbonator is transported to the second reactor, called a calciner, where the reversible reaction occurs and high purity CO
2 stream is produced (>95%). In the calciner, the heat needed for the reaction is generated by burning fuel in the oxygen atmosphere, and sometimes the CLC capture method is considered a kind of oxy-combustion method. The reactors (circulating fluidized bed (CFB)) are coupled to transport solid and cyclones separate solid and gaseous mass streams. Calcium looping technology has a few advantages. It uses a cheap sorbent (lime) and the flue gas is partially desulfurized. Moreover, the process uses fluidized beds, and this mature high-temperature technology can generate power [
6,
37].
Figure 8. Scheme of the calcium looping process (adapted from [
37]).
The cryogenic method of carbon capture technology uses liquefied natural gas (LNG) to provide cold energy to capture CO
2. Cryogenic CO
2 capture is used in oxyfuel combustion technology as well as in post-combustion carbon capture technology to separate CO
2 from flue gas. With cryogenic CO
2 capture, it is possible to produce high purity CO
2 of up to 99.17%. This method includes a few processes, such as compression, expansion, separation, and cooling. The cryogenic method is less preferred because of its high operational cost [
38]. The cryogenic method used in post-combustion carbon capture is carried out using various methods [
38,
39].
The absorption-based post-combustion capture is the most widely used method due to its efficiency and lower energy consumption. Monoethanolamine (MEA), methyl di-ethanol amine (MDEA) and piperazine (PZ) are the most extensively used amine solvents in large-scale industries [
40]. There are many classified technologies used in adsorption and absorption, as well as in membrane separation. Chao et al. analyzed the challenges and compared the commercial use of PCC technology using solvents for absorbents, bed configurations for adsorption, and membrane processes. Among the adsorbent processes, temperature swing adsorption is very effective for both adsorptions, using solid and solvents, compared to pressure swing adsorption (PSA) and vacuum swing adsorption (VSA). TSA is more efficient than PSA and VSA, but it consumes a large amount of energy during regeneration. In the case of chemical absorption, MEA is the best and most used solvent for CO
2 capture. MEA shows a good absorption and desorption rate when mixed with other solvents as well. However, the solvent absorption process requires a high energy consumption for the regeneration of solvents, and solvent losses may occur due to evaporation and chemical degradation, leading to reduced absorption capacity [
41]. Lungkadee et al. showed simulation analyses of retrofitting a post-carbon capture unit with a 300 MW power plant. The amine-based PCC technology used MEA amine for the carbon capture process, and was estimated to cost less than 55 $/ton of CO
2 capture. The absorber and desorber used in this process were designed to have 90% CO
2 capture capacity and 30 wt% of MEA. About 63.075 kg/s of CO
2 was captured from flue gas at a flow rate of 458 kg/s with 15% CO
2 content [
42]. Another simulation analysis of natural gas combined cycle (NGCC) power plants with PZ solvent showed better performance when compared to that of MEA solvent. The use of 40 wt% PZ solvent showed significant improvements in capture efficiency, energy consumption, and capture cost compared to that of using 30 wt% MEA solvent. The lowest CO
2 capture cost was obtained at 34.65 $/ton of CO
2 using 40 wt% PZ solvent [
43]. Hadri et al. showed a comparison of 30 different amine solutions (30 wt%) used for post-combustion carbon capture. The amine solutions were analyzed using a solvent screening setup, where amine was passed at 1 bar pressure with a gas containing 15% CO
2, and CO
2 loading was measured. Compared to that of other amines, hexamethylenediamine had the highest CO
2 loading of 1.35 and triethanolamine had the lowest CO
2 loading of 0.39 [
44].
CO
2 can be utilized to satisfy the needs of various industries as fuels and chemicals or beverages and food [
45]. Technologies that allow to convert CO
2 into value-added chemicals are still being developed because of their economic and environmental benefits. In contrast to physical processes, the valence state of CO
2 changes [
46]. This process can be used to produce chemical feedstock (polymers, plastics, carbonates [
47]), as well as energy carriers (methane, ethane, methanol, syngas). Among the chemical conversions, it is possible to distinguish thermochemical, electrochemical (photoelectrochemical [
48]), and biological processes, where enzymes are used [
49]. Because of the high stability of CO
2, there is a thermodynamic barrier in the CO
2 conversion process [
50]. A crucial component in most processes connected with converting CO
2 into value-added chemicals is hydrogen. It should be produced using renewable energy sources to maintain an environmentally friendly effect.
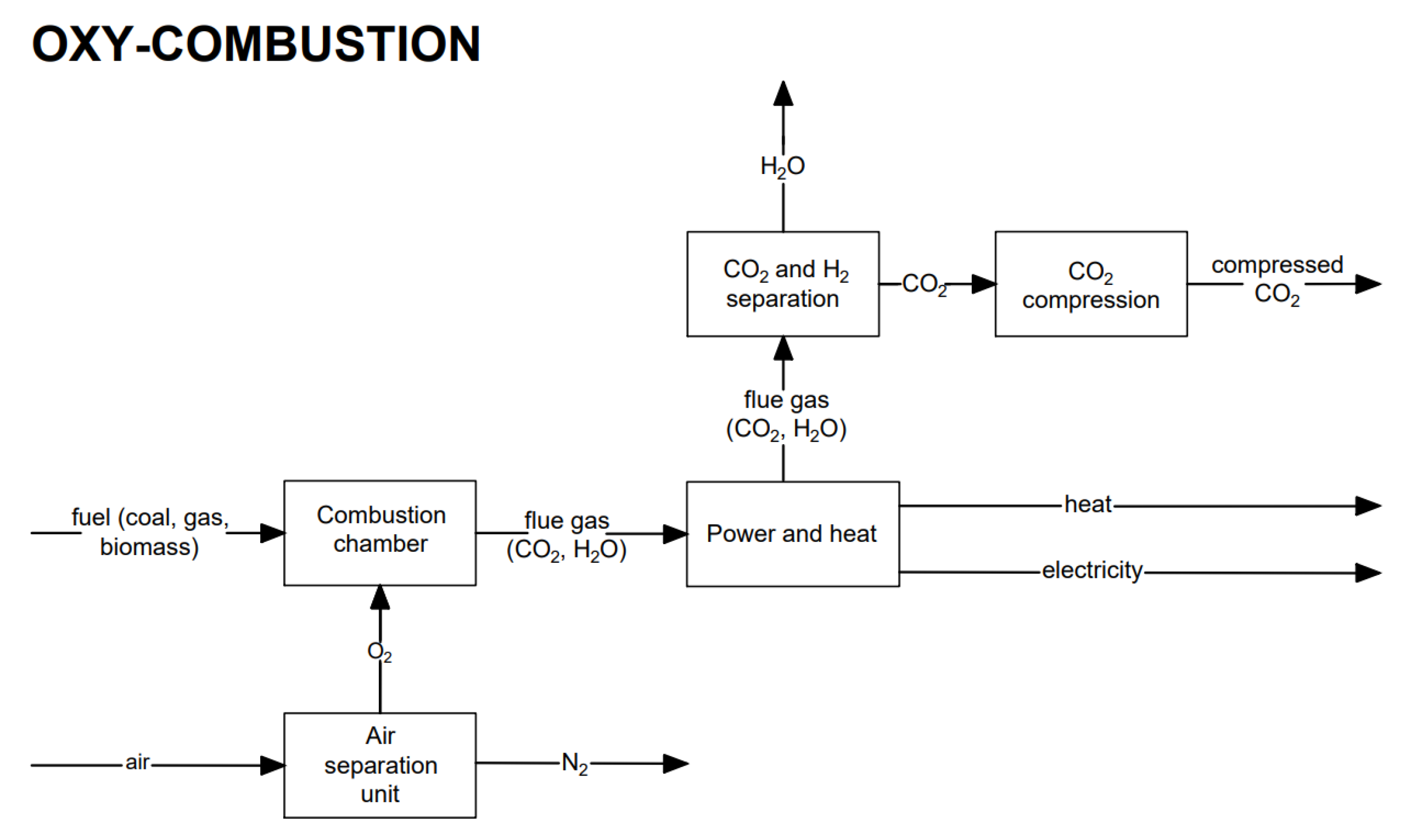
4. Oxy-Combustion CO2 Capture
The exhaust gas from combustion in an oxygen-enriched atmosphere (oxy-combustion) consists mainly of carbon dioxide and water vapor (nitrogen content is minimized). From the condensation of water vapor, CO2 separation is possible. The condensation temperature is higher than ambient conditions, except for very low partial pressures during the condensation process. The oxygen for combustion is produced using the air separation process, which gives an oxygen purity of about 95%. The general scheme for the process using the oxy-combustion method is presented in Figure 9.
Figure 9. Block diagram of electricity generation heat production with the oxy-combustion CO2 capture method.
Application of oxy-combustion technology mainly concerns solid fuel-fired boilers, including pulverized coal boilers (PCs) or circulating fluidized bed boilers (CFBs), but more and more consideration is being given to the possibility of using oxy-combustion in energy systems with gas turbines. In oxy-combustion technologies, low-temperature and high-temperature boilers can be distinguished. The combustion process in low-temperature boilers usually takes place in oxygen mixed with recirculated exhaust gases. The flame temperatures are similar to those of air-powered units. In the case of high-temperature boilers, the temperature can exceed 2400 °C.
The strengths of oxy-combustion are nitrogen oxide (NOx) reduction, boiler dimension reductions, a simplified CO2 capture method compared to other technologies, the possibility of applying in existing technologies, and less mass flow rate of exhaust gases (about 75% less compared to combustion in air). The weaknesses of oxy-combustion are the high material requirements because of the high temperatures, an efficiency decrease (oxygen production process is energy-consuming), and a high capital cost.
Oxy-combustion methods are mainly used at the laboratory scale, and for pilot installations, among which are the Callide Power Station [
51] and Compostilla Thermal Power [
52]. A 30 MW
e experimental unit in the Callide Power Station started operation in 2012. The nominal flow of 98% pure oxygen, which was supplied to the boiler, was 19,200 m
n3/h. Various types of fuel have been the object of investigation (Callide coal, Minerva coal, etc.). Daily production of CO
2 based on cryogenic capture technology was 75 t. The unit was closed after a successful demonstration in 2016. A pilot power plant unit with CO
2 capture, based on oxy-combustion technology, was developed as part of the OXYCFB 300 project at Compostilla Thermal Power. The CO
2 capture installation was equipped with a circulating fluidized bed boiler with thermal power of 30 MW
th, and a pulverized coal-fired boiler with thermal power of 20 MW
th. The flue gas stream was 800 m
3/h. The daily capacity of the separation process, which was carried out using the cryogenic method, was 3–5 tons of CO
2.
The development of oxy-combustion technology is not only connected with solid-fired fuel units. It also concerns systems equipped with gas turbines, where combustion in an oxygen-enriched atmosphere takes place. Within the framework of the project Negative CO
2 Emission Gas Power Plant [
53,
54], the concept of a negative CO
2 emission gas power plant based on oxy-combustion combined with CO
2 capture from flue gas was developed. Application of CO
2 neutral fuel, such as sewage sludge in combination with oxy-combustion and CO
2 capture, will allow negative emission levels [
55]. The CO
2 capture process will be possible, among other things, through the use of a prototype spray-ejector condenser (SEC). The main task of the SEC will be to condense the water vapor from the exhaust gases.
Among other CO
2 capture technologies, oxy-combustion carbon capture does not require many process modifications. Oxygen is used for the combustion process instead of air to eliminate the nitrogen content in flue gas, which leaves flue gas with carbon dioxide and water vapor as the major contents. This flue gas does not require high energy consumption for CO
2 separation. The boiler’s temperature is controlled by again sending part of the flue gas (about 70%) to the boiler. The air separation unit (ASU), which separates oxygen from air, is the most energy-consuming part of the oxyfuel combustion process. An industrial-scale cryogenic ASU consumes up to 200–225 kWh/t on an industrial scale [
56]. To improve efficiency and CO
2 capture, oxyfuel combustion carbon capture is combined with moderate and intense low-oxygen dilution (MILD). MILD oxy-combustion carbon capture (MOFC) holds many benefits, such as improving the efficiency of the plant, improving the purity of the CO
2, and reducing energy consumption [
12]. The oxy-fuel combustion CCS technology used in the Allam cycle shows a higher efficiency of 55–59%, which is higher when compared to that of a combined cycle power plant with a carbon capture unit. In the Allam cycle, the heat generated from the ASU is sent to the regenerator to heat up the CO
2 to 400 °C, which is then reused in the combustion chamber, improving the cycle efficiency [
57].