
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Artur Valente | + 2515 word(s) | 2515 | 2021-01-22 06:57:28 | | | |
| 2 | Lily Guo | Meta information modification | 2515 | 2021-02-22 09:42:19 | | |
Video Upload Options
Chemical pollution of water has raised great concerns among citizens, lawmakers, and nearly all manufacturing industries. As the legislation addressing liquid effluents becomes more stringent, water companies are increasingly scrutinized for their environmental performance. In this context, emergent contaminants represent a major challenge, and the remediation of water bodies and wastewater demands alternative sorbent materials. One of the most promising adsorbing materials for micropolluted water environments involves cyclodextrin (CD) polymers and cyclodextrin-containing polysaccharides.
1. Introduction
The exponential degradation of water quality is a major challenge facing both developing and developed countries, recently addressed in The Zero Pollution Ambition plan launched by the European Commission. This plan is a key pillar of the European Green Deal, which is committed to promoting initiatives in strategic fields of energy, industry, mobility, agriculture, biodiversity, and climate towards a zero-pollution strategy by better preventing, remedying, monitoring, and reporting on pollution [1]. These actions have been strongly reiterated by experts in environmental agencies: “Something has really changed in the past year. Everyone is talking about water quality. Local interest groups are attracting not just local media interest but national media interest. Rivers and beaches are all our shop windows. People notice and people care.”[2].
Currently, researchers working in different areas of chemistry, materials science, and engineering are focusing on developing greener, safer, and more cost-effective materials for water remediation. Moreover, an assessment of energy efficiency and the respective environmental benefits and risks are being considered.
Extensive research has been performed to develop new nanotechnologies for remediating water pollution by inorganic and organic contaminants[3]. These include advanced materials, such as those of a polymeric nature, which have been explored with great success for remediation purposes at different functional scales [4][5][6][7][8][9][10]. The most recent contributions have emphasized the use of nanobiomaterials to improve the detection, capture, and degradation of emergent and persistent contaminants [11][12][13].
Specifically, the adsorption of toxic compounds by modified polysaccharides and cyclodextrins (CDs) has evidenced an increasing interest in the synthesis of new low-cost adsorbents for removing contaminants from natural water and wastewater [8,14]. Lately, the number of contributions addressing CD-based polymers and CD-containing polysaccharides, in particular, and CD-grafted and/or CD-coated materials to deal with the remediation of micropolluted water environments has grown exponentially. However, the formulation and understanding of a complete, integrated picture of the major targeted environmental needs and research objectives and the spotting of cutting-edge synthetic routes, analytical techniques, and scaling-up trends in the design of multifunctional CD materials are still critical tasks[4][14][15][16][17][18][19].
By assessing the multiple facets of CDs and CD-based polymers in environmental remediation, considering specific target contaminants (e.g., dyes, pesticides, pharmaceutical compounds, heavy metals) and target matrices (e.g., water and wastewater), it is possible to identify the most relevant types of pollutants, the removal of which has benefited from the application of CD-based adsorbents. This is particularly important to (i) understanding the dynamics of persistent and emerging contaminants, (ii) developing effective remediation strategies based on CD polymers and CD-containing polysaccharides, and (iii) giving rise to promising research directions. Some reviews have already been published on this matter, targeting a specific kind of CD polymer, such as those crosslinked with epichlorohydrin [4]. However, the present review seeks to provide a comprehensive view, addressing the potential and limitations of CDs, both as backbone constituents and as pendant groups, and the role of different crosslinkers, anchoring groups, and polysaccharides attached to these cyclic oligosaccharides.
2. Cyclodextrin-Based Polymers in Pollutant Removal
Bibliometric indicators have shown an increasing interest in polymers of natural origin as an alternative to conventional adsorbents such as activated carbon, silica gels, and zeolites[20][18][21]. More specifically, oligo-and polysaccharides have been effectively used for pollution remediation on the basis of their high availability, biodegradability, biocompatibility, nontoxicity, and high chemical functionality, allowing researchers to synthesize sustainable, green, and, if desired, selective adsorbents[20][18][21].
In general, polysaccharides are highly hydrophilic polymers whose structure (fundamentally containing hydroxyl groups and, in some cases, acetamido and primary amino groups) confers not only preferential adsorption sites but also reactive sites for the development of new materials. On the other hand, certain oligosaccharides, in particular cyclodextrins, offer more sites for pollutant molecule adsorption. Due to the hydrophobic cavity of cyclodextrins, host–guest assemblies, mainly based on hydrophobic interactions, may occur[20][21][22][23]. It should, however, be stressed that not only amphiphilic or hydrophobic compounds interact with cyclodextrins. As will be discussed below, metal ions also interact with the protonated or deprotonated hydroxyl groups of CDs, located at both rims of the CD structure, thus allowing their removal from the environment. Therefore, the synergy obtained by the use of CD-containing polysaccharides enlarges their effectiveness in water remediation. It should be expected that the sorption of pollutants on CD-based materials benefits from the formation of the CD–pollutant complex and from other interactions external to the cavity, which are influenced by the contact time, the type of sorbent, and the chemical structure of the pollutant.
Another strategy that takes advantage of the versatile properties of CDs is based on the polymerization or copolymerization of the CDs by using crosslinkers and other grafting agents[9][24][25][26][27][28][29][30][31][32][33][34][35]. Furthermore, the alkylation, hydroxyalkylation, and carboxymethylation of natural CDs help broaden the spectrum of guest molecules that can be effectively bound and, thus, the variety of pollutants to be removed[36][37].
Recently, Valente and coworkers[20] provided a summary of the key progress in developing CD-based materials, which include polymers, hydrogels, and nanocomposites, used as sorbents for removing toxic compounds from wastewater. CDs have been modified in different ways to produce derivatives with high affinity towards targeted contaminants in aqueous solutions. Modification relies, e.g., on the direct linkage of β-CD by crosslinking with a coupling agent or on the covalent binding of β-CD molecules to a pre-existing insoluble matrix, including polysaccharides such as chitosan and alginates, nanofibers, textiles, silica beads, and zeolites[20].
One of the most popular crosslinkers is epichlorohydrin (EPI). The existence of two reactive groups in EPI allows its bivalent binding to cyclodextrins, generating hydrophilic polymeric networks (hydrogels) with multiple CD molecules linked by repeating glyceryl moieties[24][25][28][35][36][37][38][39][40]. These materials display high adsorption capability, are efficient in the removal of water contaminants present at trace levels, and show relatively fast kinetics [9][25][26][29][30][41][42][43][44][45][46][47].
2.1. Synthesis and Properties of CD-Based Materials
CDs can be considered polyfunctional monomers since they possess several reactive hydroxyl groups at the 2, 3, and 6 positions of each anhydroglucose unit, susceptible to substitution and elimination. The primary hydroxyl groups at the C6 position are usually more reactive than the secondary ones (C2 and C3 positions), although this reactivity order can be reversed by manipulating reaction conditions such as temperature and alkalinity[48]. Due to these intrinsic characteristics, CDs can be directly copolymerized with other monomers or attached to a myriad of materials. Different types of architecture can thus be obtained, including linear, branched, and crosslinked networks. Moreover, CDs can be modified in order to obtain derivatives with other functionalities, from amino to carboxyl groups.
Two main types of CD-based polymers can be prepared: the first involves the use of CDs or CD derivatives such as monomers and their reaction with a coupling agent in order to obtain linear polymers or crosslinked structures; in the second type, CDs are attached to a matrix (organic or inorganic) via chemical or physical interaction[49][50]. It should be noted that in this work, only polysaccharides are surveyed. Consequently, in this work, CD-based materials will be divided into two main groups, depending on whether CDs constitute or coconstitute the backbone of the polymer (polycyclodextrins) or are bound to polymeric matrices.
2.1.1. Polycyclodextrins
As it was discussed before, the reactivity of CDs is mainly based on their hydroxyl groups. They can behave as nucleophiles or electrophiles and react with a variety of other functional groups, resulting in, e.g., ethers, esters, and halides. CD hydroxyl groups can be deprotonated using strong bases, thus originating alkoxide ions, strong nucleophiles that can readily undergo SN2 reaction. On the other hand, CD ethers can be prepared by protonation of the hydroxyl groups using acids, converting the poor leaving group OH- to H2O, a better leaving group, and thus acting as electrophiles in the reaction with other alcohols.
CD crosslinked polymers are particularly important in the context of water remediation due to the cooperative effect between the CD cavity and the polymeric network. Depending on the type of CD, the crosslinking agent, and the reaction conditions, materials with different characteristics can be prepared [14]. Among the diverse choices of crosslinker, epichlorohydrin (EPI) clearly stands out. In fact, CD–EPI polymers were one of the first to be used for pollutant removal [51][52][53]. These polymers are easily prepared by reacting CDs with EPI under heating, catalyzed by NaOH in a one-pot reaction (Scheme 1).
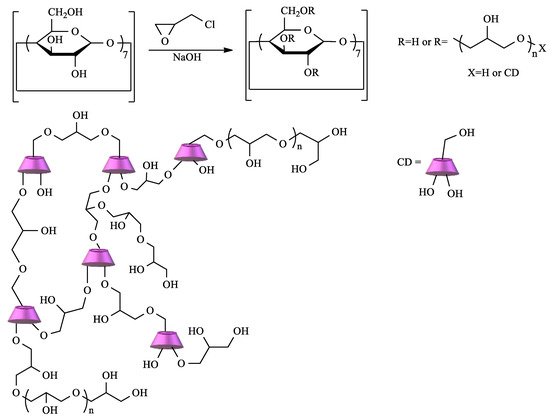
Scheme 1. Polymerization of cyclodextrin (CD) by crosslinking with epichlorohydrin (EPI).
The mechanism of this reaction is well-known, and, depending on the conditions, polymers with different degrees of crosslinking can be obtained. Thus, soluble or insoluble polymers can be prepared, and, in the latter case, it is possible to obtain the polymer as gels, fine particles, or nanoparticles [51][53].
Since the first papers reporting the use of CD–EPI in the removal of dyes and aromatic pollutants, several other publications have appeared in the literature, using these polymers for the removal of contaminants, namely, metals, pesticides, surfactants, aromatic pollutants (including PHAs and PCBs), and pharmaceuticals, among others [54][55][56][57][58][59][60][61]. The sorption mechanism is, in general, of chemisorption, essentially based on host–guest interactions, but physisorption interaction also occurs once the swelling degree/crosslinking degree has an influence on the sorption removal[4]. The success of EPI–CD materials as adsorbents is embodied in the number of cyclodextrin derivatives, such as hydroxypropyl, carboxymethyl, aminoethyl, methyl, and sulfonyl, used to polymerize with EPI and evaluated for pollutant removal[61][62][63][64].
However, EPI is not the only crosslinker used to polymerize cyclodextrins. Diisocyanates can react with CDs to form polyurethanes (Scheme 2). Depending on the structure and the relative molar ratio of the diisocyanate, materials with different surface areas, pore size distribution, mechanical properties, and sorption ability can be obtained. Various diisocyanates can be used as crosslinking agents, namely, 1,6-hexamethylene diisocyanate (HDI), 2,4-toluene diisocyanate (TDI), 1,4-phenylene diisocyanate (PDI), 4,4′-dicyclohexylmethane diisocyanate (CDI), 4,4′-diphenylmethane diisocyanate (MDI), and 1,5-naphtalene diisocyanate (NDI), as shown in Scheme 2 [65][66].
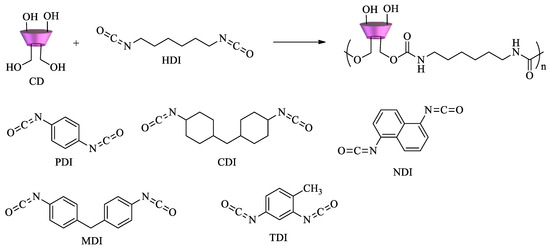
Scheme 2. Diisocyanates for the synthesis of CD–polyurethane copolymers.
If an excess of diisocyanate is used, highly crosslinked polymers can be obtained. Nanosponge CD polyurethanes have proven to be effective in the removal of several pollutants. Dyes and aromatic amines[67][68], organic matter, p-nitrophenol, pentachlorophenol and 2-methylisoborneol [69][70], perfluorinated compounds (PFCs) and pesticides[71][72]are among the pollutants tested.
Cyclodextrin polyesters constitute another important class of CD polymers. They can be obtained by reaction of the oligosaccharides with diacids, diacid chlorides, or dianhydrides. Some of the first examples of these polymers were developed by reacting CDs with succinyl chloride, glutaryl chloride, and adipoyl chloride (Scheme 3)[73][74].
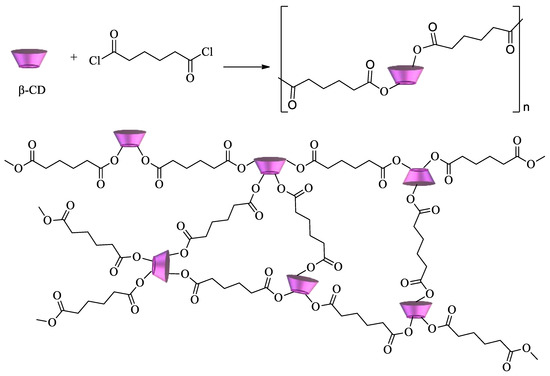
Scheme 3. β-CD polyester using adipoyl chloride as a crosslinking agent.
Another approach for the synthesis of cyclodextrin copolymers consists of using polycarboxylic acids (e.g., citric acid, succinic acid, 1,2,3,4-butanetetracarboxylic acid, and poly(acrylic acid)) as crosslinking agents[75][76][77]. Scheme 4 shows the synthesis of CD–polycarboxylic polymers using ethylenediamine tetraacetic acid (EDTA)[41][78]. The obtained polymer shows a significant number of sites available to interact with a multitude of pollutants, from metal ions to dyes and herbicides, regardless of their hydrophilic/hydrophobic character.
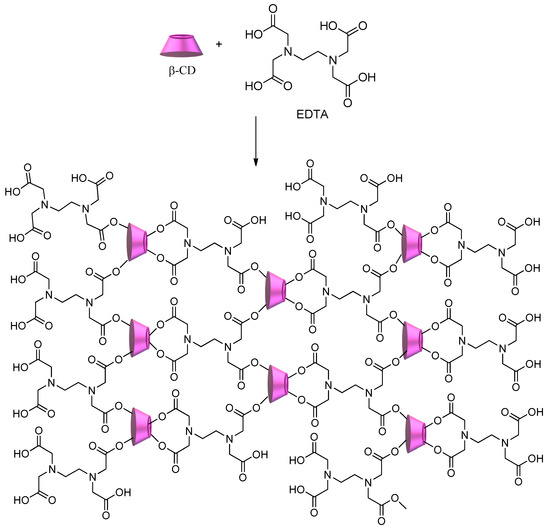
Scheme 4. CD–polycarboxylic polymer using ethylenediamine tetraacetic acid (EDTA) as a crosslinker.
Due to the success of this strategy for the synthesis of amphiphilic polymers, other crosslinkers like 4,4-difluorodiphenylsulfone, tetrafluoroterephthalonitrile, 4,4′-bipyridine, decafluorobiphenyl, bis(4-hydroxyphenyl)sulfone, and 4,4′-bis(chloromethyl)biphenyl have been tested [44][79][80].
2.1.2. Polysaccharides Having CDs as Pendant Groups
The immobilization of CDs onto natural polymers has been equally described for remediation purposes. Usually, low-cost natural polymers like cellulose, starch, or chitosan are chosen. Several synthetic procedures can be used with this objective, including the previous functionalization of CDs with appropriate reactive groups and the use of linkers. The former is achieved by substituting at least one hydroxyl group of CD with groups such as amino, tosyl, carboxyl, and carbonyl. The latter involves using coupling agents like the ones described above for CD–CD binding, including polyacids, diisocyanates, and, once again, epichlorohydrin [81].
Chitosan, a cationic biopolymer, can be easily obtained through the deacetylation of chitin, a natural polymer found in crustaceans. The presence in this compound of primary amine groups, which exhibit extensive reactivity, is a determining factor for its wide range of applications, including remediation. Owing to this, chitosan is probably the polysaccharide most commonly functionalized with CDs for water remediation. For example, Tojima et al. prepared water-insoluble chitosan beads using 1,6-hexamethylene diisocyanate as a crosslinker (Scheme 5). To this polymer, α-CD was anchored through reaction with 2-O-formylmethyl-α-CD in the presence of sodium cyanoborohydride[82][83].
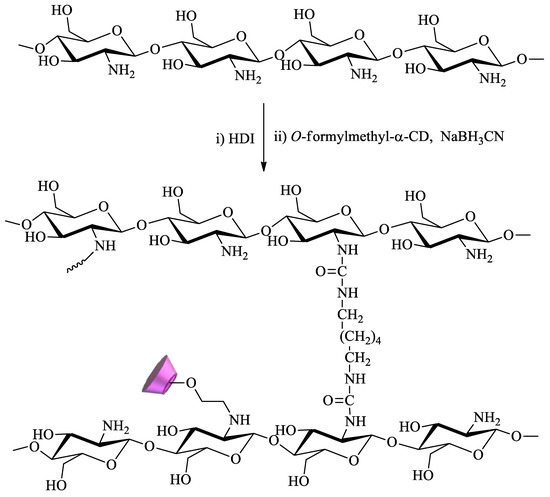
Scheme 5. Chitosan crosslinked with 1,6-hexamethylene diisocyanate (HDI) and modified with α-CD.
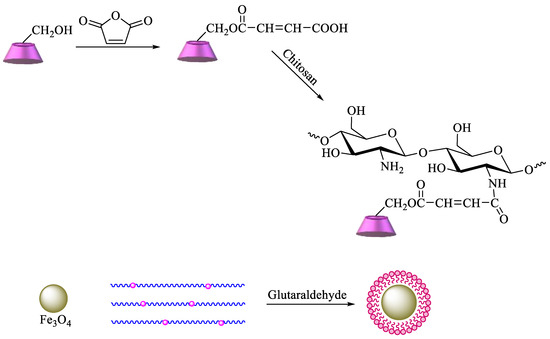
A more complex strategy involves the use of magnetic nanoparticles to improve the reuse of adsorbent materials and the recycling of pollutants. Based on these advantages, chitosan functionalized with β-CD can be prepared by reaction of maleoyl-β-CD with chitosan via activation with 3-(ethyliminomethyleneamino)-N,N′-dimethylpropan-1-amine (EDC) and 4-dimethylaminopyridine. Then, β-CD–chitosan is linked to magnetic nanoparticles, namely, Fe3O4, using glutaraldehyde (Scheme 6). The obtained polymer provides an enhanced surface area, leading to higher removal efficiency of pollutants [84]. Graphene oxide (GO) has also been commonly used for the synthesis of new materials, taking advantage of its properties, which include high surface area, a negatively charged surface, and water solubility[85]. Having that in mind, based on the previous description, new GO adsorbents containing CD–chitosan have also been synthesized using the route described in Scheme 7. In brief, maleoyl-β–CD reacts initially with chitosan and subsequently with magnetic particles (pH 8.0–9.0, 55 °C, 1.5 h) in the presence of glutaraldehyde. The carboxylic groups of GO are then reacted with EDC/N-hydroxysuccinimide, and the generated amide groups are crosslinked once again by using glutaraldehyde with β-CD–chitosan–Fe3O4 [86].
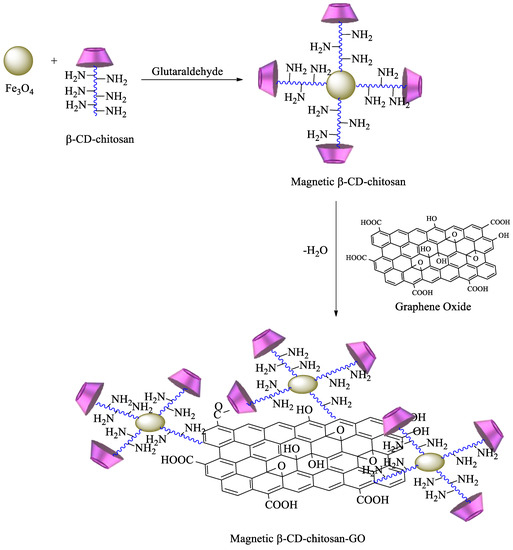
Scheme 7. Synthesis of magnetic β-CD–chitosan–graphene oxide (GO).
Other strategies have been used for similar objectives. For instance, β-CD can be functionalized with monochlorotriazinyl groups and then reacted with chitosan via a substitution reaction [87]. In another study, Aoki et al. prepared crosslinked chitosan modified with β-CD by amidation of the former with succinic anhydride, followed by reaction with mono-6-amino-mono-6-deoxy-β-CD and a carbodiimide[88].
The availability of cellulose is much higher than that of chitin, but the lack of functional groups other than hydroxyl limits its usefulness. Crosslinked networks of carboxymethyl cellulose (CMC), one of the most common and easily synthesized cellulose derivatives, have been widely used as an absorbent in remediation. The grafting of CDs on CMC, achieved with epichlorohydrin in a basic medium, offers even greater versatility, reaching adsorption capacities of 8.55 mg g−1 for copper(II) ions[89].
Although chitosan and CMC are among the most cited polysaccharides, reference is also made in the literature to the use of other natural materials such as starch, wood flour, sawdust, and cotton for the removal of various pollutants [94]. Recently, we published a review on the ways to combine CDs and cellulose, with emphasis on pharmaceutical technology, textiles, and sensors, and, consequently, this topic will not be mentioned here[20][90].
References
- European Commission. Commission Outlines Road to Zero Pollution Action Plan; European Commission: Brussels, Belgium, 2020.
- Wakeham, H. Zero Pollutions Conference 2020. Available online: https://wwtonline.co.uk/news/collaborative-approach-needed-to-achieve-zero-pollution (accessed on 2 November 2020).
- Corsi, I.; Fiorati, A.; Grassi, G.; Bartolozzi, I.; Daddi, T.; Melone, L.; Punta, C. Environmentally sustainable and ecosafe pol-ysaccharide-based materials for water nano-treatment: An eco-design study. Materials 2018, 11, 1228.
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23, doi:10.1016/j.progpolymsci.2017.07.004.
- Morin-Crini, N.; Fourmentin, M.; Fourmentin, S.; Torri, G.; Crini, G. Synthesis of silica materials containing cyclodextrin and their applications in wastewater treatment. Environ. Chem. Lett. 2019, 17, 683–696, doi:10.1007/s10311-018-00818-0.
- Alaba, P.A.; Oladoja, N.A.; Sani, Y.M.; Ayodele, O.B.; Mohammed, I.Y.; Olupinla, S.F.; Daud, W.M.W. Insight into wastewater decontamination using polymeric adsorbents. J. Environ. Chem. Eng. 2018, 6, 1651–1672, doi:10.1016/j.jece.2018.02.019.
- Marican, A.; Durán-Lara, E.F. A review on pesticide removal through different processes. Environ. Sci. Pollut. Res. 2018, 25, 2051–2064, doi:10.1007/s11356-017-0796-2.
- Wang, Z.; Cui, F.; Pan, Y.; Hou, L.; Zhang, B.; Li, Y.; Zhu, L. Hierarchically micro-mesoporous β-cyclodextrin polymers used for ultrafast removal of micropollutants from water. Carbohydr. Polym. 2019, 213, 352–360, doi:10.1016/j.carbpol.2019.03.021.
- Yang, C.; Huang, H.; Ji, T.; Zhang, K.; Yuan, L.; Zhou, C.; Tang, K.; Yi, J.; Chen, X. A cost-effective crosslinked β-cyclodextrin polymer for the rapid and efficient removal of micropollutants from wastewater. Polym. Int. 2019, 68, 805–811, doi:10.1002/pi.5771.
- Zhang, S.; He, Y.; Wu, L.; Wan, J.; Ye, M.; Long, T.; Yan, Z.; Jiang, X.; Lin, Y.; Lu, X. Remediation of Organochlorine Pesti-cide-Contaminated Soils by Surfactant-Enhanced Washing Combined with Activated Carbon Selective Adsorption. Pe-dosphere 2019, 29, 400–408, doi:10.1016/S1002-0160(17)60328-X.
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Ap-plications. Molecules 2018, 23, 1760, doi:10.3390/molecules23071760.
- Salazar, S.; Guerra, D.; Yutronic, N.; Jara, P. Removal of aromatic chlorinated pesticides from aqueous solution using β-cyclodextrin polymers decorated with Fe3O4 nanoparticles. Polymers 2018, 10, 1038.
- Liu, Y.; Liu, M.; Jia, J.; Wu, D.; Gao, T.; Wang, X.; Yu, J.; Li, F. β-Cyclodextrin-based hollow nanoparticles with excellent ad-sorption performance towards organic and inorganic pollutants. Nanoscale 2019, 11, 18653–18661, doi:10.1039/C9NR07342F.
- Landy, D.; Mallard, I.; Ponchel, A.; Monflier, E.; Fourmentin, S. Remediation technologies using cyclodextrins: An overview. Environ. Chem. Lett. 2012, 10, 225–237, doi:10.1007/s10311-011-0351-1.
- Murcia-Salvador, A.; Pellicer, J.A.; Fortea, M.I.; Gómez-López, V.M.; Rodríguez-López, M.I.; Núñez-Delicado, E.; Gabaldón, J.A. Adsorption of Direct Blue 78 Using Chitosan and Cyclodextrins as Adsorbents. Polymers 2019, 11, 1003, doi:10.3390/polym11061003.
- Pellicer, J.; Rodríguez-López, M.; Fortea, M.; Lucas-Abellán, C.; Mercader-Ros, M.; López-Miranda, S.; Gómez-López, V.; Semeraro, P.; Cosma, P.; Fini, P.; et al. Adsorption Properties of β- and Hydroxypropyl-β-Cyclodextrins Cross-Linked with Epichlorohydrin in Aqueous Solution. A Sustainable Recycling Strategy in Textile Dyeing Process. Polymers 2019, 11, 252, doi:10.3390/polym11020252.
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin–epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368, doi:10.1016/j.progpolymsci.2012.06.005.
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Fundamentals and applications of cy-clodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–55.
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70, doi:10.1016/j.progpolymsci.2004.11.002.
- Cova, T.F.G.G.; Murtinho, D.; Pais, A.A.C.C.; Valente, A.J.M. Cyclodextrin-based Materials for Removing Micropollutants From Wastewater. Curr. Org. Chem. 2018, 22, 2150–2181, doi:10.2174/1385272822666181019125315.
- Cova, T.F.; Murtinho, D.; Pais, A.A.C.C.; Valente, A.J.M. Combining Cellulose and Cyclodextrins: Fascinating Designs for Materials and Pharmaceutics. Front. Chem. 2018, 6, doi:10.3389/fchem.2018.00271.
- Cova, T.F.; Milne, B.F.; Pais, A.A.C.C. Host flexibility and space filling in supramolecular complexation of cyclodextrins: A free-energy-oriented approach. Carbohydr. Polym. 2019, 205, 42–54, doi:10.1016/j.carbpol.2018.10.009.
- Cova, T.F.G.G.; Milne, B.F.; Nunes, S.C.C.; Pais, A.A.C.C. Drastic Stabilization of Junction Nodes in Supramolecular Struc-tures Based on Host–Guest Complexes. Macromolecules 2018, 51, 2732–2741, doi:10.1021/acs.macromol.8b00154.
- Kono, H.; Onishi, K.; Nakamura, T. Characterization and bisphenol A adsorption capacity of be-ta-cyclodextrin-carboxymethylcellulose-based hydrogels. Carbohydr. Polym. 2013, 98, 784–792, doi:10.1016/j.carbpol.2013.06.065.
- Wang, Z.; Zhang, P.; Hu, F.; Zhao, Y.; Zhu, L. A crosslinked beta-cyclodextrin polymer used for rapid removal of a broad-spectrum of organic micropollutants from water. Carbohydr. Polym. 2017, 177, 224–231, doi:10.1016/j.carbpol.2017.08.059.
- Jiang, Y.; Liu, B.; Xu, J.; Pan, K.; Hou, H.; Hu, J.; Yang, J. Cross-linked chitosan/beta-cyclodextrin composite for selective removal of methyl orange: Adsorption performance and mechanism. Carbohydr. Polym. 2018, 182, 106–114, doi:10.1016/j.carbpol.2017.10.097.
- Wycisk, A.; Döring, A.; Schneider, M.; Schönhoff, M.; Kuckling, D. Synthesis of β-cyclodextrin-based star block copoly-mers with thermo-responsive behavior. Polymers 2015, 7, 921-938, doi:10.3390/polym7050921.
- Bhattarai, B.; Muruganandham, M.; Suri, R.P.S. Development of high efficiency silica coated beta-cyclodextrin polymeric adsorbent for the removal of emerging contaminants of concern from water. J. Hazard. Mater. 2014, 273, 146–154, doi:10.1016/j.jhazmat.2014.03.044.
- Morales-Sanfrutos, J.; Javier Lopez-Jaramillo, F.; Elremaily, M.A.A.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Divinyl Sulfone Cross-Linked Cyclodextrin-Based Polymeric Materials: Synthesis and Applications as Sorbents and Encapsulating Agents. Molecules 2015, 20, 3565–3581, doi:10.3390/molecules20033565.
- Wang, H.; Wang, Y.; Zhou, Y.; Han, P.; Lu, X. A Facile Removal of Phenol in Wastewater Using Crosslinked be-ta-Cyclodextrin Particles with Ultrasonic Treatment. Clean-Soil Air Water 2014, 42, 51–55, doi:10.1002/clen.201200605.
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature 2016, 529, 190-U146, doi:10.1038/nature16185.
- Kayaci, F.; Aytac, Z.; Uyar, T. Surface modification of electrospun polyester nanofibers with cyclodextrin polymer for the removal of phenanthrene from aqueous solution. J. Hazard. Mater. 2013, 261, 286–294, doi:10.1016/j.jhazmat.2013.07.041.
- Li, C.; Klemes, M.J.; Dichtel, W.R.; Helbling, D.E. Tetrafluorotereplithalomtrile-crosslinked beta-cyclodexfrin polymers for efficient extraction and recovery of organic micropollutants from water. J. Chromatogr. A 2018, 1541, 52–56, doi:10.1016/j.chroma.2018.02.012.
- Zhao, F.; Repo, E.; Yin, D.; Meng, Y.; Jafari, S.; Sillanpää, M. EDTA-Cross-Linked β-Cyclodextrin: An Environmentally Friendly Bifunctional Adsorbent for Simultaneous Adsorption of Metals and Cationic Dyes. Environ. Sci. Technol. 2015, 49, 10570–10580, doi:10.1021/acs.est.5b02227.
- Sikder, M.T.; Rahman, M.M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chem. Eng. J. 2019, 355, 920–941, doi:10.1016/j.cej.2018.08.218.
- Skold, M.E.; Thyne, G.D.; Drexler, J.W.; McCray, J.E. Solubility enhancement of seven metal contaminants using carbox-ymethyl-β-cyclodextrin (CMCD). J. Contam. Hydrol. 2009, 107, 108–113, doi:10.1016/j.jconhyd.2009.04.006.
- Badruddoza, A.Z.M.; Hazel, G.S.S.; Hidajat, K.; Uddin, M.S. Synthesis of carboxymethyl-β-cyclodextrin conjugated magnetic nano-adsorbent for removal of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2010, 367, 85–95, doi:10.1016/j.colsurfa.2010.06.018.
- Olteanu, A.A.; Arama, C.-C.; Bleotu, C.; Lupuleasa, D.; Monciu, C.M. Investigation of Cyclodextrin based Nanosponges Complexes with angiotensin I converting enzyme inhibitors (enalapril, captopril, cilazapril). Farmacia 2015, 63, 492–503.
- Okoli, C.P.; Adewuyi, G.O.; Zhang, Q.; Diagboya, P.N.; Guo, Q. Mechanism of dialkyl phthalates removal from aqueous solution using gamma-cyclodextrin and starch based polyurethane polymer adsorbents. Carbohydr. Polym. 2014, 114, 440–449, doi:10.1016/j.carbpol.2014.08.016.
- Nojavan, S.; Yazdanpanah, M. Micro-solid phase extraction of benzene, toluene, ethylbenzene and xylenes from aqueous solutions using water-insoluble beta-cyclodextrin polymer as sorbent. J. Chromatogr. A 2017, 1525, 51–59, doi:10.1016/j.chroma.2017.10.027.
- Huang, W.; Hu, Y.; Li, Y.; Zhou, Y.; Niu, D.; Lei, Z.; Zhang, Z. Citric acid-crosslinked β-cyclodextrin for simultaneous re-moval of bisphenol A, methylene blue and copper: The roles of cavity and surface functional groups. J. Taiwan Inst. Chem. Eng. 2018, 82, 189–197, doi:10.1016/j.jtice.2017.11.021.
- Tu, Y.; Xu, G.; Jiang, L.; Hu, X.; Xie, X.; Li, A. Amphiphilic hyper-crosslinked porous cyclodextrin polymer with high specific surface area for rapid removal of organic micropollutants. Chem Eng. J. 2020, 382, 123015, doi: 10.1016/j.cej.2019.123015.
- Trotta, F.; Tumiatti, W. Cross-Linked Cyclodextrins for Decontamination of Liquid, Gaseous or Solid Matrices, Is Obtaina-ble by Reacting Cyclodextrin with Carbonyl Compound. US2005154198-A1, 1 July 2005.
- Nagy, Z.,M.; Molnár, M.; Fekete-Kertész, I.; Molnár-Perl, I.; Fenyvesi, E.; Gruiz, K. Removal of emerging micropollu-tants from water using cyclodextrin. Sci. Total Environ. 2014, 485-486, 711-719, doi:10.1016/j.scitotenv.2014.04.003.
- Mak, Y.W.; Leung, W.W.-F. Crosslinking of genipin and autoclaving in chitosan-based nanofibrous scaffolds: Structural and physiochemical properties. J. Mater. Sci. 2019, 54, 10941–10962, doi:10.1007/s10853-019-03649-8.
- Qin, X.; Bai, L.; Tan, Y.; Li, L.; Song, F.; Wang, Y. β-Cyclodextrin-crosslinked polymeric adsorbent for simultaneous removal and stepwise recovery of organic dyes and heavy metal ions: Fabrication, performance and mechanisms. Chem. Eng. J. 2019, 372, 1007–1018, doi:10.1016/j.cej.2019.05.006.
- Bezerra, F.M.; Lis, M.J.; Firmino, H.B.; Silva, J.G.D.; Valle, R.C.S.C.; Valle, J.A.B.; Scacchetti, F.A.P.; Tessaro, A.L. The role of β-cyclodextrin in the textile industry-review. Molecules 2020, 25, 3624, doi:10.3390/molecules25163624.
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and functionalization of polymers with cyclodextrins: current applications and future prospects. Molecules 2014, 19, 14066-14079, doi: 10.3390/molecules190914066.
- Krause, R.W.; Mamba, B.B.; Bambo, F.M.; Malefetse, T.J. Cyclodextrin polymers: Synthesis and application in water treat-ment. In Cyclodextrins: Chemistry and Physic; Transworld Research Network: Trivandrum, India, 2010; pp. 1–25.
- Mocanu, G.; Vizitiu, D.; Carpov, A. Cyclodextrin polymers. J. Bioact. Compat. Polym. 2001, 16, 315–342.
- Renard, E.; Sebille, B.; Barnathan, G.; Deratani, A. Polycondensation of cyclodextrins with epichlorohydrin. Influence of reaction conditions on the polymer structure. Macromol. Symp. 1997, 122, 229–234, doi:10.1002/masy.19971220136.
- Renard, E.; Deratani, A.; Volet, G.; Sebille, B. Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 1997, 33, 49–57, doi:10.1016/S0014-3057(96)00123-1.
- Gidwani, B.; Vyas, A. Synthesis, characterization and application of Epichlorohydrin-β-cyclodextrin polymer. Colloids Surf. B: Biointerfaces 2014, 114, 130–137, doi:10.1016/j.colsurfb.2013.09.035.
- Liu, H.; Cai, X.; Wang, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511, doi:10.1016/j.watres.2011.04.004.
- Crini, G.; Exposito Saintemarie, A.; Rocchi, S.; Fourmentin, M.; Jeanvoine, A.; Millon, L.; Morin-Crini, N. Simultaneous removal of five triazole fungicides from synthetic solutions on activated carbons and cyclodextrin-based adsorbents. Heli-yon 2017, 3, e00380, doi:10.1016/j.heliyon.2017.e00380.
- Chen, Q.; Wen, Y.; Cang, Y.; Li, L.; Guo, X.; Zhang, R. Selective removal of phenol by spherical particles of α-, β- and γ-cyclodextrin polymers: Kinetics and isothermal equilibrium. Front. Chem. Sci. Eng. 2013, 7, 162–169, doi:10.1007/s11705-013-1318-5.
- Pratt, D.Y.; Wilson, L.D.; Kozinski, J.A.; Mohart, A.M. Preparation and sorption studies of β-cyclodextrin/epichlorohydrin copolymers. J. Appl. Polym. Sci. 2010, 116, 2982–2989, doi:10.1002/app.31824.
- Orprecio, R.; Evans, C.H. Polymer-immobilized cyclodextrin trapping of model organic pollutants in flowing water streams. J. Appl. Polym. Sci. 2003, 90, 2103–2110, doi:10.1002/app.12818.
- Zhang, K.-D.; Tsai, F.-C.; Ma, N.; Xia, Y.; Liu, H.-L.; Guo, X.; Yu, X.-Y.; Jiang, T.; Chiang, T.-C.; Chang, C.C.-J. Removal of azo dye from aqueous solution by host-guest interaction with β-cyclodextrin. Desalination Water Treat. 2017, 86, doi:10.5004/dwt.2017.21187.
- Hu, X.; Hu, Y.; Xu, G.; Li, M.; Zhu, Y.; Jiang, L.; Tu, Y.; Zhu, X.; Xie, X.; Li, A. Green synthesis of a magnetic β-cyclodextrin polymer for rapid removal of organic micro-pollutants and heavy metals from dyeing wastewater. En-viron. Res. 2020, 180, 108796, doi: 10.1016/j.envres.2019.108796 .
- Zhang, X.M.; Peng, C.S.; Xu, G.C. Synthesis of modified β-cyclodextrin polymers and characterization of their fuchsin ad-sorption. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 165–171, doi:10.1007/s10847-011-9956-z.
- Zhao, D.; Zhao, L.; Zhu, C.-S.; Wang, J.; Lv, X.-H. A novel β-cyclodextrin polymer modified by sulfonate groups. J. Incl. Phe-nom. Macrocycl. Chem. 2012, 73, 93–98, doi:10.1007/s10847-011-0024-5.
- Mallard Favier, I.; Baudelet, D.; Fourmentin, S. VOC trapping by new crosslinked cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 433–437, doi:10.1007/s10847-010-9776-6.
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Gabaldón Hernández, J.A.; Lucas-Abellán, C.; Mercader-Ros, M.T.; Serrano-Martínez, A.; Núñez-Delicado, E.; Cosma, P.; Fini, P.; et al. Removing of Direct Red 83:1 using α- and HP-α-CDs polymerized with epichlorohydrin: Kinetic and equilibrium studies. Dye. Pigment. 2018, 149, 736–746, doi:10.1016/j.dyepig.2017.11.032.
- Wilson, L.D.; Mohamed, M.H.; Headley, J.V. Surface area and pore structure properties of urethane-based copolymers con-taining β-cyclodextrin. J. Colloid Interface Sci. 2011, 357, 215–222, doi:10.1016/j.jcis.2011.01.081.
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Design and characterization of novel β-cyclodextrin based copolymer materi-als. Carbohydr. Res. 2011, 346, 219–229, doi:10.1016/j.carres.2010.11.022.
- Ozmen, E.Y.; Yilmaz, M. Use of β-cyclodextrin and starch based polymers for sorption of Congo red from aqueous solu-tions. J. Hazard. Mater. 2007, 148, 303–310, doi:10.1016/j.jhazmat.2007.02.042.
- Ozmen, E.Y.; Sirit, A.; Yilmaz, M. A Calix[4]arene Oligomer and Two Beta-cyclodextrin Polymers: Synthesis and Sorption Studies of Azo Dyes. J. Macromol. Sci. Part A 2007, 44, 167–173, doi:10.1080/10601320601031333.
- Nkambule, T.I.; Krause, R.W.; Mamba, B.B.; Haarhoff, J. Removal of natural organic matter from water using ion-exchange resins and cyclodextrin polyurethanes. Phys. Chem. EarthParts A/B/C 2009, 34, 812–818, doi:10.1016/j.pce.2009.07.013.
- Mamba, B.B.; Krause, R.W.; Malefetse, T.J.; Nxumalo, E.N. Monofunctionalized cyclodextrin polymers for the removal of organic pollutants from water. Environ. Chem. Lett. 2007, 5, 79–84, doi:10.1007/s10311-006-0082-x.
- Shabtai, I.A.; Mishael, Y.G. Polycyclodextrin–clay composites: regenerable dual-site sorbents for bisphenol A removal from treated wastewater. ACS Appl. Mater. Interfaces 2018, 10, 27088-27097, doi:10.1021/acsami.8b09715.
- Leudjo Taka, A.; Pillay, K.; Yangkou Mbianda, X. Nanosponge cyclodextrin polyurethanes and their modification with na-nomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107, doi:10.1016/j.carbpol.2016.12.027.
- Zemel, H.; Koch, M.B. Preparation of Crosslinked Cyclodextrin Resins with Enhanced Porosity. US4958015A, 18 September 1990.
- Flores, J.; Jiménez, V.; Belmar, J.; Mansilla, H.D.; Alderete, J.B. Inclusion Complexation of Phenol Derivatives with a β-Cyclodextrin Based Polymer. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 63–68, doi:10.1007/s10847-005-0994-2.
- Weltrowski, M.; Morcellet, M.; Martel, B. Cyclodextrin Polymers and/or Cyclodextrin Derivatives with Complexing Prop-erties and Ion-Exchange Properties and Method for the Production Thereof. US6660804B1, 9 December 2003.
- Martel, B.; Ruffin, D.; Weltrowski, M.; Lekchiri, Y.; Morcellet, M. Water-soluble polymers and gels from the polycondensa-tion between cyclodextrins and poly(carboxylic acid)s: A study of the preparation parameters. J. Appl. Polym. Sci. 2005, 97, 433–442, doi:10.1002/app.21391.
- Girek, T.; Kozlowski, C.A.; Koziol, J.J.; Walkowiak, W.; Korus, I. Polymerisation of β-cyclodextrin with succinic anhydride. Synthesis, characterisation, and ion flotation of transition metals. Carbohydr. Polym. 2005, 59, 211–215, doi:10.1016/j.carbpol.2004.09.011.
- Zhao, D.; Zhao, L.; Zhu, C.-S.; Huang, W.-Q.; Hu, J.-L. Water-insoluble β-cyclodextrin polymer crosslinked by citric acid: Synthesis and adsorption properties toward phenol and methylene blue. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 195–201, doi:10.1007/s10847-008-9507-4.
- Xiao, L.; Ling, Y.; Alsbaiee, A.; Li, C.; Helbling, D.E.; Dichtel, W.R. β-Cyclodextrin Polymer Network Sequesters Perfluo-rooctanoic Acid at Environmentally Relevant Concentrations. J. Am. Chem. Soc. 2017, 139, 7689–7692, doi:10.1021/jacs.7b02381.
- Li, X.; Zhou, M.; Jia, J.; Jia, Q. A water-insoluble viologen-based β-cyclodextrin polymer for selective adsorption toward anionic dyes. React. Funct. Polym. 2018, 126, 20–26, doi:10.1016/j.reactfunctpolym.2018.03.004.
- Yang, J.S.; Yang, L. Preparation and application of cyclodextrin immobilized polysaccharides. J. Mater. Chem. B 2013, 1, 909–918, doi:10.1039/C2TB00107A.
- Tojima, T.; Katsura, H.; Nishiki, M.; Nishi, N.; Tokura, S.; Sakairi, N. Chitosan beads with pendant α-cyclodextrin: Prepara-tion and inclusion property to nitrophenolates. Carbohydr. Polym. 1999, 40, 17–22, doi:10.1016/S0144-8617(99)00030-2.
- Chen, C.-Y.; Chen, C.-C.; Chung, Y.-C. Removal of phthalate esters by α-cyclodextrin-linked chitosan bead. Bioresour. Tech-nol. 2007, 98, 2578–2583, doi:10.1016/j.biortech.2006.09.009.
- Fan, L.; Zhang, Y.; Luo, C.; Lu, F.; Qiu, H.; Sun, M. Synthesis and characterization of magnetic β-cyclodextrin–chitosan na-noparticles as nano-adsorbents for removal of methyl blue. Int. J. Biol. Macromol. 2012, 50, 444–450, doi:10.1016/j.ijbiomac.2011.12.016.
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Gra-phene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180, doi:10.1016/j.envint.2019.03.029.
- Fan, L.; Luo, C.; Sun, M.; Qiu, H.; Li, X. Synthesis of magnetic β-cyclodextrin–chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surf. B: Biointerfaces 2013, 103, 601–607, doi:10.1016/j.colsurfb.2012.11.023.
- Martel, B.; Devassine, M.; Crini, G.; Weltrowski, M.; Bourdonneau, M.; Morcellet, M. Preparation and sorption properties of a β-cyclodextrin-linked chitosan derivative. J. Polym. Sci. Part. A Polym. Chem. 2001, 39, 169–176, doi:10.1002/1099-0518(20010101)39:1<169::aid-pola190>3.0.co;2-g.
- Aoki, N.; Nishikawa, M.; Hattori, K. Synthesis of chitosan derivatives bearing cyclodextrin and adsorption of p-nonylphenol and bisphenol A. Carbohydr. Polym. 2003, 52, 219–223.
- Xia, Y.; Wan, J. Preparation and adsorption of novel cellulosic fibers modified by β-cyclodextrin. Polym. Adv. Technol. 2008, 19, 270–275, doi:10.1002/pat.997.
- Sancey, B.; Trunfio, G.; Charles, J.; Badot, P.M.; Crini, G. Sorption onto crosslinked cyclodextrin polymers for industrial pollutants removal: An interesting environmental approach. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 315–320, doi:10.1007/s10847-010-9841-1.
- Cova, T.F.G.G.; Murtinho, D.; Pais, A.A.C.C.; Valente, A.J.M. Cyclodextrin-based Materials for Removing Micropollutants From Wastewater. Curr. Org. Chem. 2018, 22, 2150–2181, doi:10.2174/1385272822666181019125315.
- Cova, T.F.; Murtinho, D.; Pais, A.A.C.C.; Valente, A.J.M. Combining Cellulose and Cyclodextrins: Fascinating Designs for Materials and Pharmaceutics. Front. Chem. 2018, 6, doi:10.3389/fchem.2018.00271.
- Skold, M.E.; Thyne, G.D.; Drexler, J.W.; McCray, J.E. Solubility enhancement of seven metal contaminants using carbox-ymethyl-β-cyclodextrin (CMCD). J. Contam. Hydrol. 2009, 107, 108–113, doi:10.1016/j.jconhyd.2009.04.006.
- Badruddoza, A.Z.M.; Hazel, G.S.S.; Hidajat, K.; Uddin, M.S. Synthesis of carboxymethyl-β-cyclodextrin conjugated magnetic nano-adsorbent for removal of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2010, 367, 85–95, doi:10.1016/j.colsurfa.2010.06.018.




