Chemical pollution of water has raised great concerns among citizens, lawmakers, and nearly all manufacturing industries. As the legislation addressing liquid effluents becomes more stringent, water companies are increasingly scrutinized for their environmental performance. In this context, emergent contaminants represent a major challenge, and the remediation of water bodies and wastewater demands alternative sorbent materials. One of the most promising adsorbing materials for micropolluted water environments involves cyclodextrin (CD) polymers and cyclodextrin-containing polysaccharides.
- cyclodextrins
- polysaccharides
1. Introduction
The exponential degradation of water quality is a major challenge facing both developing and developed countries, recently addressed in The Zero Pollution Ambition plan launched by the European Commission. This plan is a key pillar of the European Green Deal, which is committed to promoting initiatives in strategic fields of energy, industry, mobility, agriculture, biodiversity, and climate towards a zero-pollution strategy by better preventing, remedying, monitoring, and reporting on pollution [1]. These actions have been strongly reiterated by experts in environmental agencies: “Something has really changed in the past year. Everyone is talking about water quality. Local interest groups are attracting not just local media interest but national media interest. Rivers and beaches are all our shop windows. People notice and people care.”[2].
Extensive research has been performed to develop new nanotechnologies for remediating water pollution by inorganic and organic contaminants[3]. These include advanced materials, such as those of a polymeric nature, which have been explored with great success for remediation purposes at different functional scales [4][5][6][7][8][9][10]. The most recent contributions have emphasized the use of nanobiomaterials to improve the detection, capture, and degradation of emergent and persistent contaminants [11][12][13].
14]. Lately, the number of contributions addressing CD-based polymers and CD-containing polysaccharides, in particular, and CD-grafted and/or CD-coated materials to deal with the remediation of micropolluted water environments has grown exponentially. However, the formulation and understanding of a complete, integrated picture of the major targeted environmental needs and research objectives and the spotting of cutting-edge synthetic routes, analytical techniques, and scaling-up trends in the design of multifunctional CD materials are still critical tasks[4][14][15][16][17][18][19].
By assessing the multiple facets of CDs and CD-based polymers in environmental remediation, considering specific target contaminants (e.g., dyes, pesticides, pharmaceutical compounds, heavy metals) and target matrices (e.g., water and wastewater), it is possible to identify the most relevant types of pollutants, the removal of which has benefited from the application of CD-based adsorbents. This is particularly important to (i) understanding the dynamics of persistent and emerging contaminants, (ii) developing effective remediation strategies based on CD polymers and CD-containing polysaccharides, and (iii) giving rise to promising research directions. Some reviews have already been published on this matter, targeting a specific kind of CD polymer, such as those crosslinked with epichlorohydrin [4]. However, the present review seeks to provide a comprehensive view, addressing the potential and limitations of CDs, both as backbone constituents and as pendant groups, and the role of different crosslinkers, anchoring groups, and polysaccharides attached to these cyclic oligosaccharides.
2. Cyclodextrin-Based Polymers in Pollutant Removal
Bibliometric indicators have shown an increasing interest in polymers of natural origin as an alternative to conventional adsorbents such as activated carbon, silica gels, and zeolites[20][18][21]. More specifically, oligo-and polysaccharides have been effectively used for pollution remediation on the basis of their high availability, biodegradability, biocompatibility, nontoxicity, and high chemical functionality, allowing researchers to synthesize sustainable, green, and, if desired, selective adsorbents[20][18][21].
In general, polysaccharides are highly hydrophilic polymers whose structure (fundamentally containing hydroxyl groups and, in some cases, acetamido and primary amino groups) confers not only preferential adsorption sites but also reactive sites for the development of new materials. On the other hand, certain oligosaccharides, in particular cyclodextrins, offer more sites for pollutant molecule adsorption. Due to the hydrophobic cavity of cyclodextrins, host–guest assemblies, mainly based on hydrophobic interactions, may occur[20][21][22][23]. It should, however, be stressed that not only amphiphilic or hydrophobic compounds interact with cyclodextrins. As will be discussed below, metal ions also interact with the protonated or deprotonated hydroxyl groups of CDs, located at both rims of the CD structure, thus allowing their removal from the environment. Therefore, the synergy obtained by the use of CD-containing polysaccharides enlarges their effectiveness in water remediation. It should be expected that the sorption of pollutants on CD-based materials benefits from the formation of the CD–pollutant complex and from other interactions external to the cavity, which are influenced by the contact time, the type of sorbent, and the chemical structure of the pollutant.
Another strategy that takes advantage of the versatile properties of CDs is based on the polymerization or copolymerization of the CDs by using crosslinkers and other grafting agents[9][24][25][26][27][28][29][30][31][32][33][34][35]. Furthermore, the alkylation, hydroxyalkylation, and carboxymethylation of natural CDs help broaden the spectrum of guest molecules that can be effectively bound and, thus, the variety of pollutants to be removed[36][37].
Recently, Valente and coworkers[20] provided a summary of the key progress in developing CD-based materials, which include polymers, hydrogels, and nanocomposites, used as sorbents for removing toxic compounds from wastewater. CDs have been modified in different ways to produce derivatives with high affinity towards targeted contaminants in aqueous solutions. Modification relies, e.g., on the direct linkage of β-CD by crosslinking with a coupling agent or on the covalent binding of β-CD molecules to a pre-existing insoluble matrix, including polysaccharides such as chitosan and alginates, nanofibers, textiles, silica beads, and zeolites[20].
One of the most popular crosslinkers is epichlorohydrin (EPI). The existence of two reactive groups in EPI allows its bivalent binding to cyclodextrins, generating hydrophilic polymeric networks (hydrogels) with multiple CD molecules linked by repeating glyceryl moieties[24][25][28][35][36][37][38][39][40]. These materials display high adsorption capability, are efficient in the removal of water contaminants present at trace levels, and show relatively fast kinetics [9][25][26][29][30][41][42][43][44][45][46][47].
2.1. Synthesis and Properties of CD-Based Materials
CDs can be considered polyfunctional monomers since they possess several reactive hydroxyl groups at the 2, 3, and 6 positions of each anhydroglucose unit, susceptible to substitution and elimination. The primary hydroxyl groups at the C6 position are usually more reactive than the secondary ones (C2 and C3 positions), although this reactivity order can be reversed by manipulating reaction conditions such as temperature and alkalinity[48]. Due to these intrinsic characteristics, CDs can be directly copolymerized with other monomers or attached to a myriad of materials. Different types of architecture can thus be obtained, including linear, branched, and crosslinked networks. Moreover, CDs can be modified in order to obtain derivatives with other functionalities, from amino to carboxyl groups.
Two main types of CD-based polymers can be prepared: the first involves the use of CDs or CD derivatives such as monomers and their reaction with a coupling agent in order to obtain linear polymers or crosslinked structures; in the second type, CDs are attached to a matrix (organic or inorganic) via chemical or physical interaction[49][50]. It should be noted that in this work, only polysaccharides are surveyed. Consequently, in this work, CD-based materials will be divided into two main groups, depending on whether CDs constitute or coconstitute the backbone of the polymer (polycyclodextrins) or are bound to polymeric matrices.
2.1.1. Polycyclodextrins
N
-
2
CD crosslinked polymers are particularly important in the context of water remediation due to the cooperative effect between the CD cavity and the polymeric network. Depending on the type of CD, the crosslinking agent, and the reaction conditions, materials with different characteristics can be prepared [14]. Among the diverse choices of crosslinker, epichlorohydrin (EPI) clearly stands out. In fact, CD–EPI polymers were one of the first to be used for pollutant removal [51][52][53]. These polymers are easily prepared by reacting CDs with EPI under heating, catalyzed by NaOH in a one-pot reaction (
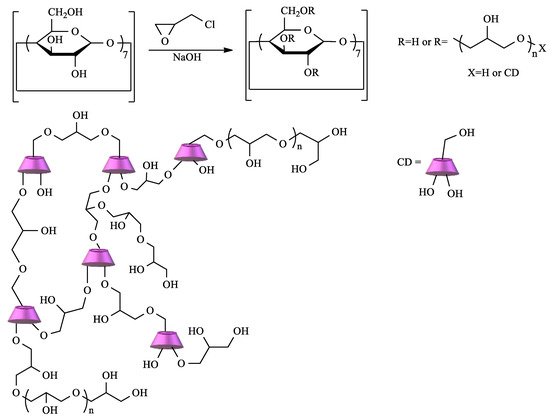
Scheme 1.
The mechanism of this reaction is well-known, and, depending on the conditions, polymers with different degrees of crosslinking can be obtained. Thus, soluble or insoluble polymers can be prepared, and, in the latter case, it is possible to obtain the polymer as gels, fine particles, or nanoparticles [51][53].
Since the first papers reporting the use of CD–EPI in the removal of dyes and aromatic pollutants, several other publications have appeared in the literature, using these polymers for the removal of contaminants, namely, metals, pesticides, surfactants, aromatic pollutants (including PHAs and PCBs), and pharmaceuticals, among others [54][55][56][57][58][59][60][61]. The sorption mechanism is, in general, of chemisorption, essentially based on host–guest interactions, but physisorption interaction also occurs once the swelling degree/crosslinking degree has an influence on the sorption removal[4]. The success of EPI–CD materials as adsorbents is embodied in the number of cyclodextrin derivatives, such as hydroxypropyl, carboxymethyl, aminoethyl, methyl, and sulfonyl, used to polymerize with EPI and evaluated for pollutant removal[61][62][63][64].
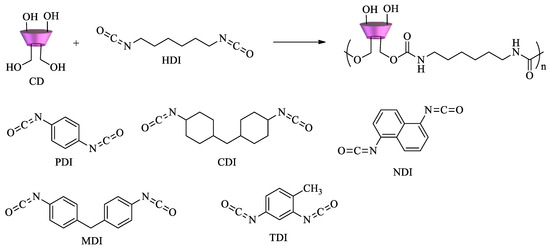
Scheme 2.
If an excess of diisocyanate is used, highly crosslinked polymers can be obtained. Nanosponge CD polyurethanes have proven to be effective in the removal of several pollutants. Dyes and aromatic amines[67][68], organic matter,
p-nitrophenol, pentachlorophenol and 2-methylisoborneol [69][70], perfluorinated compounds (PFCs) and pesticides[71][72]are among the pollutants tested.
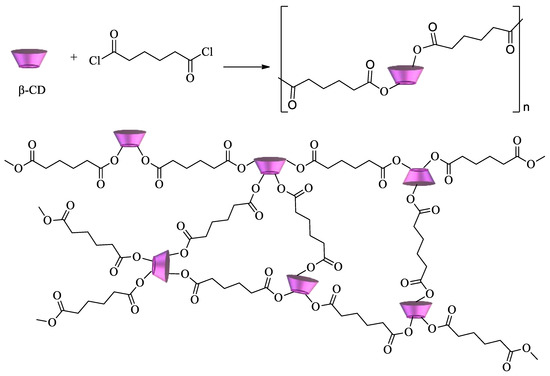
Scheme 3.
Another approach for the synthesis of cyclodextrin copolymers consists of using polycarboxylic acids (e.g., citric acid, succinic acid, 1,2,3,4-butanetetracarboxylic acid, and poly(acrylic acid)) as crosslinking agents[75][76][77].
Scheme 4 shows the synthesis of CD–polycarboxylic polymers using ethylenediamine tetraacetic acid (EDTA)[41][78]. The obtained polymer shows a significant number of sites available to interact with a multitude of pollutants, from metal ions to dyes and herbicides, regardless of their hydrophilic/hydrophobic character.
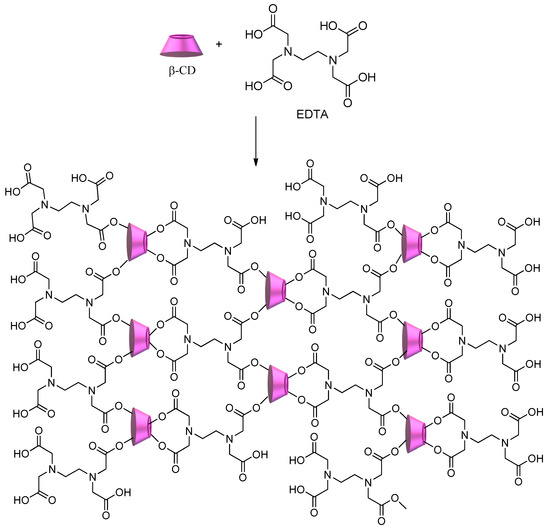
Scheme 4.
Due to the success of this strategy for the synthesis of amphiphilic polymers, other crosslinkers like 4,4-difluorodiphenylsulfone, tetrafluoroterephthalonitrile, 4,4′-bipyridine, decafluorobiphenyl, bis(4-hydroxyphenyl)sulfone, and 4,4′-bis(chloromethyl)biphenyl have been tested [44][79][80].
2.1.2. Polysaccharides Having CDs as Pendant Groups
The immobilization of CDs onto natural polymers has been equally described for remediation purposes. Usually, low-cost natural polymers like cellulose, starch, or chitosan are chosen. Several synthetic procedures can be used with this objective, including the previous functionalization of CDs with appropriate reactive groups and the use of linkers. The former is achieved by substituting at least one hydroxyl group of CD with groups such as amino, tosyl, carboxyl, and carbonyl. The latter involves using coupling agents like the ones described above for CD–CD binding, including polyacids, diisocyanates, and, once again, epichlorohydrin [81].
O-formylmethyl-α-CD in the presence of sodium cyanoborohydride[82][83].
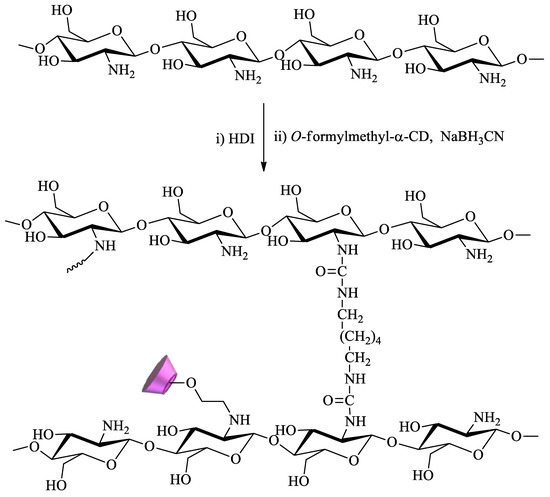
Scheme 5.
N,N′
3
4
Scheme 6). The obtained polymer provides an enhanced surface area, leading to higher removal efficiency of pollutants [84]. Graphene oxide (GO) has also been commonly used for the synthesis of new materials, taking advantage of its properties, which include high surface area, a negatively charged surface, and water solubility[85]. Having that in mind, based on the previous description, new GO adsorbents containing CD–chitosan have also been synthesized using the route described in
β
N
β
3
4 [86].
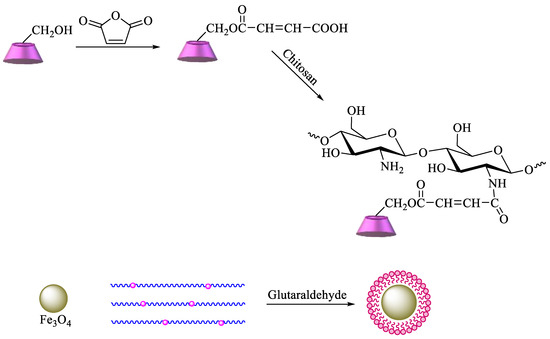
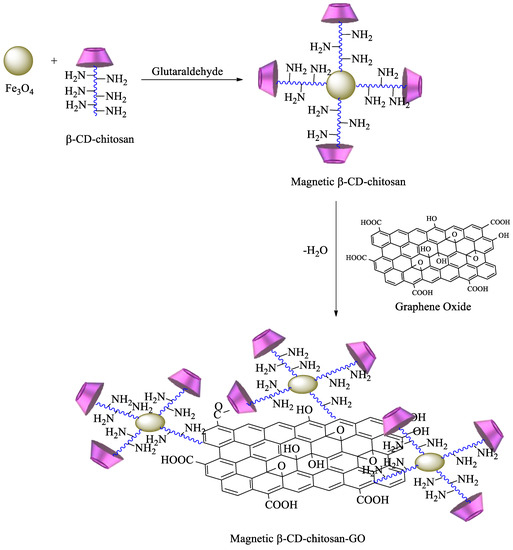
Scheme 7.
Other strategies have been used for similar objectives. For instance, β-CD can be functionalized with monochlorotriazinyl groups and then reacted with chitosan via a substitution reaction [87]. In another study, Aoki et al. prepared crosslinked chitosan modified with β-CD by amidation of the former with succinic anhydride, followed by reaction with mono-6-amino-mono-6-deoxy-β-CD and a carbodiimide[88].
−1 for copper(II) ions[89].
94]. Recently, we published a review on the ways to combine CDs and cellulose, with emphasis on pharmaceutical technology, textiles, and sensors, and, consequently, this topic will not be mentioned here[20][90].
