CDs can be considered polyfunctional monomers since they possess several reactive hydroxyl groups at the 2, 3, and 6 positions of each anhydroglucose unit, susceptible to substitution and elimination. The primary hydroxyl groups at the C6 position are usually more reactive than the secondary ones (C2 and C3 positions), although this reactivity order can be reversed by manipulating reaction conditions such as temperature and alkalinity [
53]. Due to these intrinsic characteristics, CDs can be directly copolymerized with other monomers or attached to a myriad of materials. Different types of architecture can thus be obtained, including linear, branched, and crosslinked networks. Moreover, CDs can be modified in order to obtain derivatives with other functionalities, from amino to carboxyl groups.
Two main types of CD-based polymers can be prepared: the first involves the use of CDs or CD derivatives such as monomers and their reaction with a coupling agent in order to obtain linear polymers or crosslinked structures; in the second type, CDs are attached to a matrix (organic or inorganic) via chemical or physical interaction [
54,
55]. It should be noted that in this work, only polysaccharides are surveyed. Consequently, in this work, CD-based materials will be divided into two main groups, depending on whether CDs constitute or coconstitute the backbone of the polymer (polycyclodextrins) or are bound to polymeric matrices.
2.1.1. Polycyclodextrins
As it was discussed before, the reactivity of CDs is mainly based on their hydroxyl groups. They can behave as nucleophiles or electrophiles and react with a variety of other functional groups, resulting in, e.g., ethers, esters, and halides. CD hydroxyl groups can be deprotonated using strong bases, thus originating alkoxide ions, strong nucleophiles that can readily undergo SN2 reaction. On the other hand, CD ethers can be prepared by protonation of the hydroxyl groups using acids, converting the poor leaving group OH- to H2O, a better leaving group, and thus acting as electrophiles in the reaction with other alcohols.
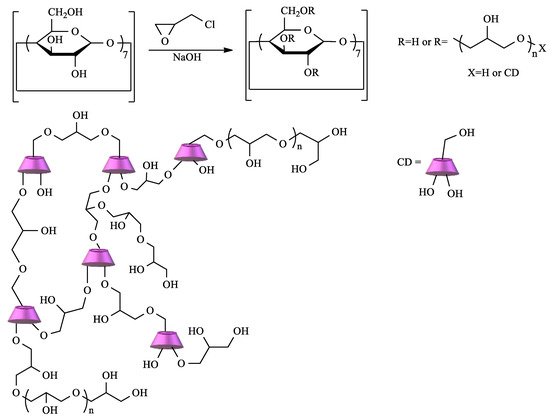
CD crosslinked polymers are particularly important in the context of water remediation due to the cooperative effect between the CD cavity and the polymeric network. Depending on the type of CD, the crosslinking agent, and the reaction conditions, materials with different characteristics can be prepared [
15]. Among the diverse choices of crosslinker, epichlorohydrin (EPI) clearly stands out. In fact, CD–EPI polymers were one of the first to be used for pollutant removal [
56,
57,
58]. These polymers are easily prepared by reacting CDs with EPI under heating, catalyzed by NaOH in a one-pot reaction (
Scheme 1).
Scheme 1. Polymerization of cyclodextrin (CD) by crosslinking with epichlorohydrin (EPI).
The mechanism of this reaction is well-known, and, depending on the conditions, polymers with different degrees of crosslinking can be obtained. Thus, soluble or insoluble polymers can be prepared, and, in the latter case, it is possible to obtain the polymer as gels, fine particles, or nanoparticles [
56,
58].
Since the first papers reporting the use of CD–EPI in the removal of dyes and aromatic pollutants, several other publications have appeared in the literature, using these polymers for the removal of contaminants, namely, metals, pesticides, surfactants, aromatic pollutants (including PHAs and PCBs), and pharmaceuticals, among others [
44,
59,
60,
61,
62,
63,
64,
65]. The sorption mechanism is, in general, of chemisorption, essentially based on host–guest interactions, but physisorption interaction also occurs once the swelling degree/crosslinking degree has an influence on the sorption removal [
4]. The success of EPI–CD materials as adsorbents is embodied in the number of cyclodextrin derivatives, such as hydroxypropyl, carboxymethyl, aminoethyl, methyl, and sulfonyl, used to polymerize with EPI and evaluated for pollutant removal [
65,
66,
67,
68].
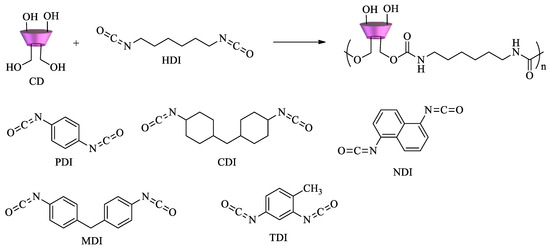
However, EPI is not the only crosslinker used to polymerize cyclodextrins. Diisocyanates can react with CDs to form polyurethanes (
Scheme 2). Depending on the structure and the relative molar ratio of the diisocyanate, materials with different surface areas, pore size distribution, mechanical properties, and sorption ability can be obtained. Various diisocyanates can be used as crosslinking agents, namely, 1,6-hexamethylene diisocyanate (HDI), 2,4-toluene diisocyanate (TDI), 1,4-phenylene diisocyanate (PDI), 4,4′-dicyclohexylmethane diisocyanate (CDI), 4,4′-diphenylmethane diisocyanate (MDI), and 1,5-naphtalene diisocyanate (NDI), as shown in
Scheme 2 [
69,
70].
Scheme 2. Diisocyanates for the synthesis of CD–polyurethane copolymers.
If an excess of diisocyanate is used, highly crosslinked polymers can be obtained. Nanosponge CD polyurethanes have proven to be effective in the removal of several pollutants. Dyes and aromatic amines [
71,
72], organic matter,
p-nitrophenol, pentachlorophenol and 2-methylisoborneol [
73,
74], perfluorinated compounds (PFCs) and pesticides [
75,
76] are among the pollutants tested.
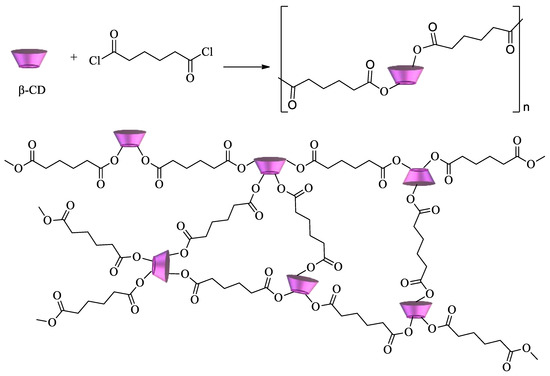
Cyclodextrin polyesters constitute another important class of CD polymers. They can be obtained by reaction of the oligosaccharides with diacids, diacid chlorides, or dianhydrides. Some of the first examples of these polymers were developed by reacting CDs with succinyl chloride, glutaryl chloride, and adipoyl chloride (
Scheme 3) [
77,
78].
Scheme 3. β-CD polyester using adipoyl chloride as a crosslinking agent.
Another approach for the synthesis of cyclodextrin copolymers consists of using polycarboxylic acids (e.g., citric acid, succinic acid, 1,2,3,4-butanetetracarboxylic acid, and poly(acrylic acid)) as crosslinking agents [
79,
80,
81].
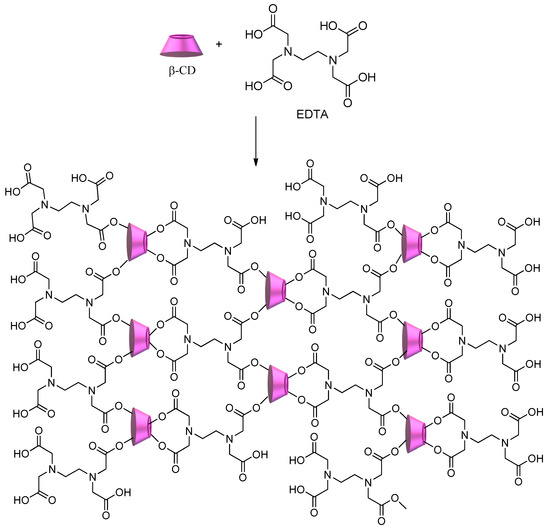
Scheme 4 shows the synthesis of CD–polycarboxylic polymers using ethylenediamine tetraacetic acid (EDTA) [
46,
82]. The obtained polymer shows a significant number of sites available to interact with a multitude of pollutants, from metal ions to dyes and herbicides, regardless of their hydrophilic/hydrophobic character.
Scheme 4. CD–polycarboxylic polymer using ethylenediamine tetraacetic acid (EDTA) as a crosslinker.
Due to the success of this strategy for the synthesis of amphiphilic polymers, other crosslinkers like 4,4-difluorodiphenylsulfone, tetrafluoroterephthalonitrile, 4,4′-bipyridine, decafluorobiphenyl, bis(4-hydroxyphenyl)sulfone, and 4,4′-bis(chloromethyl)biphenyl have been tested [
49,
83,
84].
2.1.2. Polysaccharides Having CDs as Pendant Groups
The immobilization of CDs onto natural polymers has been equally described for remediation purposes. Usually, low-cost natural polymers like cellulose, starch, or chitosan are chosen. Several synthetic procedures can be used with this objective, including the previous functionalization of CDs with appropriate reactive groups and the use of linkers. The former is achieved by substituting at least one hydroxyl group of CD with groups such as amino, tosyl, carboxyl, and carbonyl. The latter involves using coupling agents like the ones described above for CD–CD binding, including polyacids, diisocyanates, and, once again, epichlorohydrin [
85].
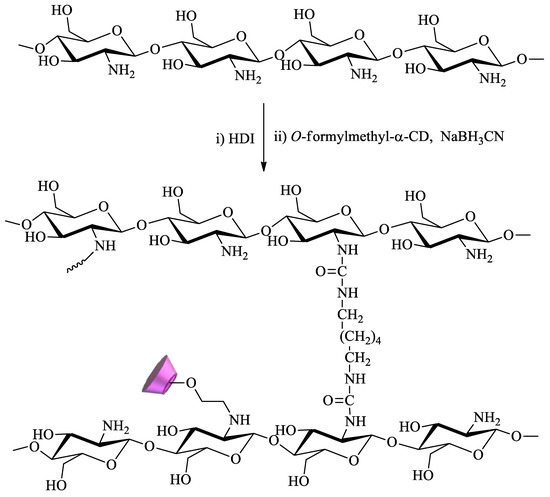
Chitosan, a cationic biopolymer, can be easily obtained through the deacetylation of chitin, a natural polymer found in crustaceans. The presence in this compound of primary amine groups, which exhibit extensive reactivity, is a determining factor for its wide range of applications, including remediation. Owing to this, chitosan is probably the polysaccharide most commonly functionalized with CDs for water remediation. For example, Tojima et al. prepared water-insoluble chitosan beads using 1,6-hexamethylene diisocyanate as a crosslinker (
Scheme 5). To this polymer, α-CD was anchored through reaction with 2-
O-formylmethyl-α-CD in the presence of sodium cyanoborohydride [
86,
87].
Scheme 5. Chitosan crosslinked with 1,6-hexamethylene diisocyanate (HDI) and modified with α-CD.
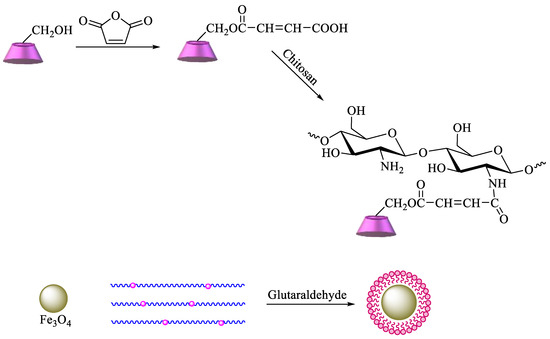
A more complex strategy involves the use of magnetic nanoparticles to improve the reuse of adsorbent materials and the recycling of pollutants. Based on these advantages, chitosan functionalized with β-CD can be prepared by reaction of maleoyl-β-CD with chitosan via activation with 3-(ethyliminomethyleneamino)-
N,N′-dimethylpropan-1-amine (EDC) and 4-dimethylaminopyridine. Then, β-CD–chitosan is linked to magnetic nanoparticles, namely, Fe
3O
4, using glutaraldehyde (
Scheme 6). The obtained polymer provides an enhanced surface area, leading to higher removal efficiency of pollutants [
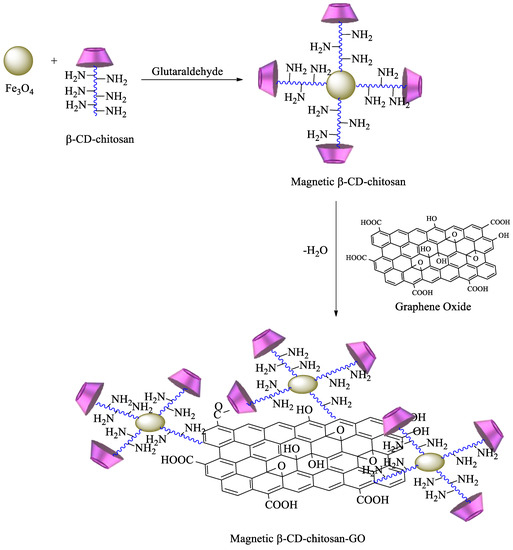
88]. Graphene oxide (GO) has also been commonly used for the synthesis of new materials, taking advantage of its properties, which include high surface area, a negatively charged surface, and water solubility [
89]. Having that in mind, based on the previous description, new GO adsorbents containing CD–chitosan have also been synthesized using the route described in
Scheme 7. In brief, maleoyl-
β–CD reacts initially with chitosan and subsequently with magnetic particles (pH 8.0–9.0, 55 °C, 1.5 h) in the presence of glutaraldehyde. The carboxylic groups of GO are then reacted with EDC/
N-hydroxysuccinimide, and the generated amide groups are crosslinked once again by using glutaraldehyde with
β-CD–chitosan–Fe
3O
4 [
90].
Scheme 6. Synthesis of β-CD–chitosan–Fe3O4.
Scheme 7. Synthesis of magnetic β-CD–chitosan–graphene oxide (GO).
Other strategies have been used for similar objectives. For instance, β-CD can be functionalized with monochlorotriazinyl groups and then reacted with chitosan via a substitution reaction [
91]. In another study, Aoki et al. prepared crosslinked chitosan modified with β-CD by amidation of the former with succinic anhydride, followed by reaction with mono-6-amino-mono-6-deoxy-β-CD and a carbodiimide [
92].
The availability of cellulose is much higher than that of chitin, but the lack of functional groups other than hydroxyl limits its usefulness. Crosslinked networks of carboxymethyl cellulose (CMC), one of the most common and easily synthesized cellulose derivatives, have been widely used as an absorbent in remediation. The grafting of CDs on CMC, achieved with epichlorohydrin in a basic medium, offers even greater versatility, reaching adsorption capacities of 8.55 mg g
−1 for copper(II) ions [
93].
Although chitosan and CMC are among the most cited polysaccharides, reference is also made in the literature to the use of other natural materials such as starch, wood flour, sawdust, and cotton for the removal of various pollutants [
94]. Recently, we published a review on the ways to combine CDs and cellulose, with emphasis on pharmaceutical technology, textiles, and sensors, and, consequently, this topic will not be mentioned here [
14,
19,
21].